Back to Journals » Journal of Multidisciplinary Healthcare » Volume 13
Torus Palatinus in Taiwan Patients Receiving Peritoneal Dialysis and Hemodialysis: A Prospective Observational Study
Authors Chang PC , Hsu CL , Tai SY, Tsai AI, Wang IK, Weng CH , Huang WH, Hsu CW, Yen TH
Received 1 March 2020
Accepted for publication 2 April 2020
Published 15 April 2020 Volume 2020:13 Pages 373—379
DOI https://doi.org/10.2147/JMDH.S252013
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Pei-Ching Chang,1 Chia-Lin Hsu,2 Shao-Yu Tai,2 Aileen I Tsai,2 I-Kuan Wang,3 Cheng-Hao Weng,4 Wen-Hung Huang,4 Ching-Wei Hsu,4 Tzung-Hai Yen4–6
1Department of Pediatric Dentistry, Chang Gung Memorial Hospital, Taoyuan, Taiwan; 2Department of Pediatric Dentistry, Chang Gung Memorial Hospital, Linkou, Taiwan; 3Department of Nephrology, China Medical University Hospital and College of Medicine, China Medical University, Taichung, Taiwan; 4Department of Nephrology and Clinical Poison Center, Chang Gung Memorial Hospital and College of Medicine, Chang Gung University, Linkou, Taiwan; 5Kidney Research Center, Chang Gung Memorial Hospital, Linkou, Taiwan; 6Center for Tissue Engineering, Chang Gung Memorial Hospital, Linkou, Taiwan
Correspondence: Tzung-Hai Yen
Department of Nephrology and Clinical Poison Center, Chang Gung Memorial Hospital and College of Medicine, Chang Gung University, 199 Tung Hwa North Road, Taipei 105, Taiwan
Tel +886 3 3281200 ext 8181
Fax +886 3 3282173
Email [email protected]
Purpose: A consensus about the pathogenesis of torus palatinus (TP) in patients receiving dialysis still eludes the scientific community. This prospective observational study investigated the epidemiology of TP in peritoneal dialysis and hemodialysis patients and analyzed the influences of multiple pathogenic factors such as mineral and bone disorders, genetic, environmental or nutritional triggers, progression of age, heredity, climatologic or biomechanical causes, and hyperparathyroidism on the formation of TP.
Methods: Between 2013 and 2016, a total of 575 chronic dialysis patients (441 on hemodialysis and 134 on peritoneal dialysis) were recruited from Chang Gung Memorial Hospital, Taiwan. Patients were stratified into two groups based on the presence (n = 179) or absence (n = 396) of TP. Demographic, oral examination, laboratory, and dialysis data were collected for analysis. Student’s t-test was used to analyze the quantitative variables and Chi-square or Fisher’s exact test for categorical variables. Univariate binary logistic regression analysis was conducted to determine the predictors for TP and multivariate binary logistic regression analysis to identify significant associated factors.
Results: The prevalence of TP in dialysis patients in this study was 31.1% (28.3% for hemodialysis and 40.3% for peritoneal dialysis). Patients with TP were younger (54.6 ± 13.4 versus 58.9 ± 14.7 years, P = 0.001) and mostly female (60.3 versus 41.2%, P < 0.001). Most TP cases (55.3%) were small in size (< 2 cm), with the flat shape (56.4%) being the most common followed by the spindle (17.9%), nodular (17.3%), and lobular (8.4%) shapes. A longer duration of dialysis was associated with TP ≥ 2 cm than with TP < 2 cm (94.4 ± 85.9 versus 72.8 ± 59.1 months, P = 0.048). Multivariate logistic regression revealed that female gender (odds ratio 2.108, 95% confidence interval 1.455– 3.055, P < 0.001) and younger age (odds ratio 0.982; 95% confidence interval 0.969– 0.994, P = 0.005) were significant predictors for TP.
Conclusion: The prevalence of TP in chronic dialysis patients is 31.1%, higher in patients receiving peritoneal dialysis (40.3%) than hemodialysis (28.3%). Female gender and younger age are significant predictors associated with TP.
Keywords: end-stage renal disease, hemodialysis, peritoneal dialysis, torus palatinus
Introduction
Torus palatinus (TP) is non-pathological bony deposition, which gradually develops along the midline of the palate and consists of cortical and trabecular bones. TP is often discovered incidentally after middle age due to the lack of symptoms that can be detected during routine dental examinations.1 Surgical removal of TP may be necessary when patients present the following: speech and masticatory disturbances, trauma and ulceration of the mucosa from wearing the dental prosthesis,2 prosthetic instability, intubation difficulties,3 obstructive sleep apnea,4 cancer phobia, or as TP becomes the potential source of autogenous cortical bone for grafting.
A consensus about the pathogenesis of TP in patients receiving dialysis still eludes the scientific community. Many associated factors have been proposed, such as: mineral and bone disorders;5 genetic;6 environmental or nutritional triggers; progression of age;7 heredity;8 climatologic9 or biomechanical (functional) causes. The prevalence of treated end-stage renal disease in Taiwan has been one of the highest in the world.10 Chronic renal failure alters bone metabolism through multiple mechanisms. For example, phosphate retention and decreased conversion of vitamin D lead to hypocalcemia and stimulate parathyroid chief cells to produce more parathyroid hormone. Padbury et al,11 have found decreased cortical density and increased likelihood of oral tori in patients with primary hyperparathyroidism. Long-term dialysis also inhibits bone homeostasis as a result of aluminum deposition that interferes with bone mineralization.12
In terms of prevalence, Sisman et al13 reported a TP prevalence of 41.7% in peritoneal dialysis patients. There were also studies reporting markedly different prevalence rates in different ethnic groups.6,14-16 According to Chiang et al,17 the prevalence of TP in the general population of Taiwan was 21.1%. Also in Taiwan, the prevalence of TP in hemodialysis patients was 23.5% as found by Chao et al5 and 28.9% reported by Tai et al,18 respectively, and 34.3% in peritoneal dialysis patients according to Hsu et al.19
These studies5,18,19 also concluded that neither hyperparathyroidism nor inflammation-malnutrition syndrome contributes to the risk of developing tori in dialysis patients. Meanwhile, Sisman et al13 found evidence for the association between the peritoneal dialysis duration and the TP size. Tai et al18 demonstrated that younger age, female gender, higher blood concentration of phosphate, and lower blood concentration of bicarbonate were significant predictors for TP in hemodialysis patients.
Therefore, the objective of this prospective observational study was to investigate the epidemiology of TP in peritoneal dialysis and hemodialysis patients, and analyzed the influences of multiple pathogenic factors such as mineral and bone disorders, genetic, environmental or nutritional triggers, progression of age, heredity, climatologic or biomechanical causes, hyperparathyroidism on the formation of TP.
Patients and Methods
Ethical Statement
This clinical study followed the Declaration of Helsinki for Human Experimentation and was approved by the Medical Ethics Committee of the Chang Gung Memorial Hospital. The Institutional Review Board numbers assigned to the study were 102-2761B and 104-6913C.
Sample Size
Sample size was determined using G*Power software version 3.1.9.7. An odds ratio of female gender to predict TP was 2.305 according to a pilot study. Therefore, the sample size as calculated by G*Power with odds ratio of 2.305, alpha error of 0.05 and power of 0.95 was 255.
Study Design and Setting
Between 2013 and 2016, a total of 575 chronic dialysis patients (441 on hemodialysis and 134 on peritoneal dialysis) were recruited from two branches of the Chang Gung Memorial Hospital located in Linkou and Taoyuan. All primary data were collected according to procedures outlined in strengthening the reporting of observational studies in epidemiology (STROBE).
Inclusion and Exclusion Criteria
All dialysis patients who agreed to participate were eligible for inclusion in this study. Patients undergoing hemodialysis or peritoneal dialysis for less than 6 months, having been hospitalized, operated on or received kidney transplant in the preceding 3 months as well as those with cancer were excluded from the study.
Consenting Process
All patients provided written informed consent prior to participating in this study.
Measures – Dialysis, Diagnosis of TP and Molar Relationship
Dialysis. Hemodialysis patients underwent treatment 3 times a week. Each treatment lasted approximately 4 hours, using a single-use hollow-fiber artificial kidney with modified cellulose-based polyamide or polysulfone membranes. The dialysate used was a standard ionic composition and bicarbonate-based buffer. Peritoneal dialysis prescriptions were based on the peritoneal membrane characteristics determined by the peritoneal equilibration test. Intermittent therapy was prescribed to patients with high membrane transport, and continuous therapy, to those with average or low membrane transport.
Diagnosis of TP. TP was diagnosed through clinical inspection and palpation (Figure 1). The size of TP was determined by the maximum elevation of the outgrowth of tori. TPs were characterized as ≥ 2 cm or < 2 cm using a periodontal probe, as described by Gorsky et al.20 The shapes of TP were grouped as flat, spindle, nodular, or lobular according to the criteria suggested by Jainkittivong et al.21
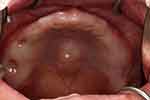 |
Figure 1 Torus palatinus. Intraoral view of the edentulous hemodialysis patient showed a flat torus palatinus (asterisk) along the midline of the hard palate. |
Molar relationship. The molar relationship was assessed and scored as none, Class I, II or III, based on Angle’s classification.22
Statistical Analysis
All data were tested for normality of distribution and equality of standard deviation before analysis. Student’s t-test was used to analyze the quantitative variables, and Chi-square or Fisher’s exact test, for categorical variables. Univariate binary logistic regression analysis was conducted to determine the predictors for TP, and multivariate binary logistic regression analysis, to identify significant associated factors. A P value of less than 0.05 was selected as the significance threshold to reject the null hypothesis. All analyses were performed using IBM SPSS Statistics Version 20.0.
Results
Table 1 presents the baseline characteristics of participating patients. The prevalence of TP was 31.1% (28.3% for hemodialysis and 40.3% for peritoneal dialysis). Patients with TP were younger (54.6 ± 13.4 versus 58.9 ± 14.7 years, P = 0.001) and mostly female (60.3 versus 41.2%, P < 0.001).
 |
Table 1 Baseline Demographics of Patients Receiving Dialysis (n = 575) |
As outlined in Table 2, the majority of TPs (55.3%) were of small size (< 2 cm). The flat-shaped TP (56.4%) was the most common, followed by the spindle- (17.9%), nodular- (17.3%), and lobular-shaped (8.4%) TPs.
 |
Table 2 Patterns of TP in Dialysis Patients (n = 179) |
The molar relationship could not be identified in 280 (48.7%) patients due to the loss of their first permanent molars (Table 3). There was no significant difference between the two study groups (P = 0.184).
 |
Table 3 Molar Relationships in Patients Receiving Dialysis (n = 575) |
Patients with TP had higher blood levels of phosphate (5.5 ± 1.3 versus 5.1 ± 1.4 mg/dL, P = 0.010) and bicarbonate (21.2 ± 3.7 versus 21.1 ± 3.4 mmol/L, P = 0.020), and lower residual renal function (4.4 ± 1.3 versus 4.6 ± 1.4 mL/min/1.73 m2, P = 0.012) than patients without TP (Table 4). Although patients with TP had lower blood concentrations of the intact parathyroid hormone than patients without TP, the difference was not significant (328.9 ± 340.4 versus 359.6 ± 364.2 pg/mL, P = 0.341). No significant differences in inflammatory variables such as high sensitivity C-reactive protein (7.6 ± 11.8 versus 8.7 ± 16.8mg/L, P = 0.433) and nutritional variables such as albumin (3.9 ± 0.3 versus 3.9 ± 0.4 g/dL, P = 0.220) were seen between the two groups. There were also no significant differences in dialysis parameters between the two groups, such as Kt/V (1.9 ± 0.4 versus 1.8 ± 0.4 g/dL, P = 0.071) and normalized protein catabolic rate (1.1 ± 0.5 versus 1.1 ± 0.4 g/dL, P = 0.470).
 |
Table 4 Laboratory Findings and Dialysis Parameters in Patients Receiving Dialysis (n = 575) |
The multivariate logistic regression analysis revealed that female gender (odds ratio 2.108, 95% confidence interval 1.455–3.055, P < 0.001) and younger age (odds ratio 0.982; 95% confidence interval 0.969–0.994, P = 0.005) were significant predictors for TP (Table 5).
 |
Table 5 Logistic Regression Analysis of Risk Factors for TP (n = 575) |
As shown in Table 6, a longer duration of dialysis is associated with TP ≥ 2 cm than with TP < 2 cm (94.4 ± 85.9 versus 72.8 ± 59.1 months, P = 0.048).
 |
Table 6 Comparison of Dialysis Duration Between Patients with TP ≥ 2 Cm and TP < 2 Cm (n =179) |
Discussion
This study examined the largest series of TPs in the current dialysis literature. The prevalence of TP in chronic dialysis patients is 31.1%, higher in patients receiving peritoneal dialysis (40.3%) than hemodialysis (28.3%). Notably, female gender and younger age are significant predictors for TP in dialysis patients.
The mechanism for TP formation induced by female gender remains unclear. Nevertheless, the observed female predominance of TP may be related to genetic, environmental or functional factors. First, TP is transmitted as an autosomal dominant trait, and it is believed that there may be a dominant type linked to the X chromosome.6 Second, the different prevalence rates between females and males might be attributed to the same environmental factors, but exerted differently to women and men. Furthermore, differences in food consumption, oral pathology, and extra-masticatory dental activities could also produce a differential spreading of TPs.23 In Eskimo inhabitants, women exhibit TPs more frequently than men because they repeatedly use teeth for hide preparation. The condition is common in Eskimos who place great pressures on their masticatory system and upper jaw. These forces are medially directed towards the peak of the palatine vault, and creating thickening of the palatal roof along the median suture to limit this pressure.24 Dietary habits and nutritional disturbances are also considered by some researchers as potential causes.25 Eggen and Natvig suggested that the consumption of saltwater fish containing omega 3 unsaturated fatty acids and vitamin D could possibly encourage bone growth and increase the chances of TPs.26 Many previous studies have also found a higher prevalence of TP in females than males,9,27,28 as in the current study where 60.3% of patients with TP were females.
Similarly, the mechanism for TP formation induced by younger age also remains uncertain. Nevertheless, the more prevalence of TP in the younger age may be associated with nutritional and functional factor. First, a decreasing ability to chew and absorb food is likely in elderly people, which may result in malnutrition and, thereby, could possibly affect the occurrence of TP.29 Second, as progression of age there may be decrease in numbers of teeth as result to extraction of teeth and periodontal disease, this result in decrease of masticatory function as the persons become partially or completely edentulous, so less occlusal stress get and it can be consider as functional factors.9 In terms of the link between age and TP, the onset of tori can be found as early as in the prenatal period, newborns, and young children who have not been subjected to mechanical stimuli.30 Afterwards, tori tend to grow with age, possibly due to the continuous and increased functional occlusal stress from teeth to the underlying alveolar bone.31 Eggen et al also pointed out that TP seemed to be a dynamic phenomenon capable of bone growth and resorption remodeling.14 TP is usually discovered incidentally after middle age.1,4 In this study, patients with TP aged 54.6 ± 13.4 years. This finding was similar to those from previous studies.32–34 However, the prevalence of tori decreases especially after the age of 50. This drop in prevalence might be attributable to malnutrition32,34 and a decrease in the masticatory function and less occlusal stress35 due to loss of teeth from caries or periodontal diseases as the patient ages. Oral tori also subside in size after the fifth decade of life because of the reduced number of teeth as a result of bone resorption.36 In the current study, the average age of patients with TP was younger than those without (P = 0.001). This finding echoed that tori are more frequently observed during the middle age than at older ages.14,27
Different prevalence rates of TP have been reported for different population groups.14,15 Chiang et al17 found the TP prevalence to be 21.1% in the general population of Taiwan. In dialysis patients, Chao et al5 and Tai et al18 reported TP prevalence rates of 23.5% and 28.9%, respectively, among hemodialysis patients, and Hsu et al,19 a TP prevalence rate of 34.3% among peritoneal dialysis patients. In the current study, the prevalence of TP was 31.1% - 28.3% for hemodialysis and 40.3% for peritoneal dialysis.
A higher blood concentration of phosphate was also found in patients with TP than without (P = 0.010) in the current study. The different TP prevalence rates between the general population and chronic dialysis patients thus might be attributed to the existence of underlying disorders. Reduced renal function produces hypocalcemia by favoring phosphate retention. Low serum calcium leads to a compensatory parathyroid hyperactivity, resulting in elevated phosphate excretion, decrease in calcium excretion, and increased removal of calcium from bones by osteoclast activation.37 Hyperphosphatemia elevates the blood concentration of fibroblast growth factor 23, reduction in active vitamin D synthesis, and the tendency toward hypocalcemia - all are potent stimuli for secondary hyperparathyroidism.38–40 Hyperparathyroidism depletes cortical bone and stimulate formation of cancellous bone, resulting in changes in the contour of the affected bony structure. The preferential loss of cortical bone and increased formation of trabecular bone, particularly in the oral cavity, are usually the early signs of renal osteodystrophy.11
Our study also found that the number of patients with TP < 2 cm (55.3%) was greater than that with TP ≥ 2 cm (44.7%), which mirrors the study results by Jainkittivong et al,28 Reichart et al9 and Sisman et al.13 Furthermore, a longer duration of dialysis was associated with TP ≥ 2 cm than with TP < 2 cm (P = 0.048). This resonates with the observations by Sisman et al13 where a significant relationship existed between TP size and duration of peritoneal dialysis. They went on to suggest underlying disorders such as renal osteodystrophy as the likely culprit.
TP is a benign disease and most TP manifestations in this study are small in size. Many of our dialysis patients were often unaware of their presence before this study. Therefore, there is no major clinical significance of TP in dialysis patients. Nevertheless, the clinical observations of female and younger age predominance of TP are interesting, and warranted more researches in this area. Finally, the limitations of this study included small patient population, short follow up duration, and lacking pathological evaluation.
Conclusions
The prevalence of TP in chronic dialysis patients in Taiwan is 31.1%, higher in patients receiving peritoneal dialysis (40.3%) than hemodialysis (28.3%). Patients with TP are younger than patients without TP, and are mostly female. Most TP manifestations are small in size. The flat-shaped TP is the most common, followed by the spindle-, nodular-, and lobular-shaped. A longer duration of dialysis is associated with TP ≥ 2 cm than with TP < 2 cm. The female gender and younger age are significant predictors for TP in chronic dialysis patients.
Acknowledgment
Dr. Tzung-Hai Yen was supported by research grants from Chang Gung Memorial Hospital (CLRPG3D1116, CMRPG3F0601, CMRPG3F0602, and CMRPG3F0603).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Garcia-Garcia AS, Martinez-Gonzalez JM, Gomez-Font R, Soto-Rivadeneira A, Oviedo-Roldan L. Current status of the torus palatinus and torus mandibularis. Med Oral Patología Oral y Cirugia Bucal. 2010;e353–e360. doi:10.4317/medoral.15.e353
2. Morrison MD, Tamimi F. Oral tori are associated with local mechanical and systemic factors: a case-control study. J Oral Maxillofac Surg. 2013;71(1):14–22. doi:10.1016/j.joms.2012.08.005
3. Durrani MA, Barwise JA. Difficult endotracheal intubation associated with torus mandibularis. Anesth Analg. 2000;90(3):757–759. doi:10.1097/00000539-200003000-00045
4. Saffran AJ, Clark RF. Torus mandibularis: an unusual cause of obstructive sleep apnea. Ear Nose Throat J. 2004;83(5):324. doi:10.1177/014556130408300511
5. Chao PJ, Yang HY, Huang WH, et al. Oral tori in chronic hemodialysis patients. Biomed Res Int. 2015;2015:897674. doi:10.1155/2015/897674
6. Gorsky M, Bukai A, Shohat M. Genetic influence on the prevalence of torus palatinus. Am J Med Genet. 1998;75(2):138–140. doi:10.1002/(SICI)1096-8628(19980113)75:2<138::AID-AJMG3>3.0.CO;2-P
7. Ellertson CH. Continuous growth of the torus mandibularis. Oral Surg Oral Med Oral Pathol. 1969;27(6):786–789. doi:10.1016/0030-4220(69)90149-2
8. Eggen S. Torus mandibularis: an estimation of the degree of genetic determination. Acta Odontol Scand. 1989;47(6):409–415. doi:10.3109/00016358909004810
9. Reichart PA, Neuhaus F, Sookasem M. Prevalence of torus palatinus and torus mandibularis in Germans and Thai. Community Dent Oral Epidemiol. 1988;16(1):61–64. doi:10.1111/j.1600-0528.1988.tb00557.x
10. 2018 USRDS annual data report: executive summary. Am J Kidney Dis. 2019;73(3):A9–A22. doi:10.1053/j.ajkd.2019.01.002
11. Padbury AD
12. Chang JI, Som PM, Lawson W. Unique imaging findings in the facial bones of renal osteodystrophy. AJNR Am J Neuroradiol. 2007;28(4):608–609.
13. Sisman Y, Gokce C, Sipahioglu M, et al. Torus palatinus in end-stage renal disease patients receiving peritoneal dialysis: does renal osteodystrophy play a role? J Dent Sci. 2012;7(2):154–158. doi:10.1016/j.jds.2012.03.012
14. Eggen S, Natvig B, Gasemyr J. Variation in torus palatinus prevalence in Norway. Scand J Dent Res. 1994;102(1):54–59. doi:10.1111/j.1600-0722.1994.tb01153.x
15. Al Quran F, Al-Dwairi Z. Torus palatinus and torus mandibularis in edentulous patients. J Contemp Dent Pract. 2006;7(2):112–119. doi:10.5005/jcdp-7-2-112
16. Kolas S, Halperin V, Jefferis K, Huddleston S, Robinson HB. The occurrence of torus palatinus and torus mandibularis in 2478 dental patients. Oral Surg Oral Med Oral Pathol. 1953;6(9):1134–1141. doi:10.1016/0030-4220(53)90225-4
17. Chiang ML, Hsieh YJ, Tseng YL, Lin JR, Chiang CP. Oral mucosal lesions and developmental anomalies in dental patients of a teaching hospital in Northern Taiwan. J Dent Sci. 2014;9(1):69–77. doi:10.1016/j.jds.2013.06.004
18. Tai SY, Hsu CL, Tsai AI, et al. Survey of torus palatinus in patients with end-stage renal disease undergoing hemodialysis. Biomed Res Int. 2018;2018:1356910. doi:10.1155/2018/1356910
19. Hsu CL, Hsu CW, Chang PC, et al. Oral tori in chronic peritoneal dialysis patients. PLoS One. 2016;11(6):e0156988. doi:10.1371/journal.pone.0156988
20. Gorsky M, Raviv M, Kfir E, Moskona D. Prevalence of torus palatinus in a population of young and adult Israelis. Arch Oral Biol. 1996;41(6):623–625. doi:10.1016/0003-9969(96)00149-5
21. Jainkittivong A, Langlais RP. Buccal and palatal exostoses: prevalence and concurrence with tori. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(1):48–53. doi:10.1067/moe.2000.105905
22. Weinberger T. Angle classification. Am J Orthod Dentofacial Orthop. 1993;103(4):26A–28A. doi:10.1016/S0889-5406(05)80388-9
23. Pechenkina EA, Benfer RA. The role of occlusal stress and gingival infection in the formation of exostoses on mandible and maxilla from Neolithic China. Homo. 2002;53(2):112–130. doi:10.1078/0018-442X-00040
24. Hooton EA. On certain Eskimoid characters in Icelandic skulls. Am J Phys Anthrop. 1918;1(1):53–76. doi:10.1002/ajpa.1330010104
25. Seah YH. Torus palatinus and torus mandibularis: a review of the literature. Aust Dent J. 1995;40(5):318–321. doi:10.1111/j.1834-7819.1995.tb04820.x
26. Eggen S, Natvig B. Relationship between torus mandibularis and number of present teeth. Scand J Dent Res. 1986;94(3):233–240. doi:10.1111/j.1600-0722.1986.tb01758.x
27. Haugen LK. Palatine and mandibular tori. A morphologic study in the current Norwegian population. Acta Odontol Scand. 1992;50(2):65–77. doi:10.3109/00016359209012748
28. Jainkittivong A, Apinhasmit W, Swasdison S. Prevalence and clinical characteristics of oral tori in 1520 Chulalongkorn University Dental School patients. Surg Radiol Anat. 2007;29(2):125–131. doi:10.1007/s00276-007-0184-6
29. Kerdpon D, Sirirungrojying S. A clinical study of oral tori in southern Thailand: prevalence and the relation to parafunctional activity. Eur J Oral Sci. 1999;107(1):9–13. doi:10.1046/j.0909-8836.1999.eos107103.x
30. Woo JK. Torus palatinus. Am J Phys Anthropol. 1950;8(1):81–111. doi:10.1002/ajpa.1330080114
31. Sathya K, Kanneppady SK, Arishiya T. Prevalence and clinical characteristics of oral tori among outpatients in Northern Malaysia. J Oral Biol Craniofac Res. 2012;2(1):15–19. doi:10.1016/S2212-4268(12)60005-0
32. Hiremath V, Husein A, Mishra N. Prevalence of torus palatinus and torus mandibularis among Malay population. J Int Soc Prev Community Dent. 2011;1(2):60–64. doi:10.4103/2231-0762.97704
33. Apinhasmit W, Jainkittivong A, Swasdison S. Torus palatinus and torus mandibularis in a Thai population. Sci Asia. 2002;28:105–111.
34. Eggen S, Natvig B. Concurrence of torus mandibularis and torus palatinus. Scand J Dent Res. 1994;102(1):60–63. doi:10.1111/j.1600-0722.1994.tb01154.x
35. Yoshinaka M, Ikebe K, Furuya-Yoshinaka M, Hazeyama T, Maeda Y. Prevalence of torus palatinus among a group of Japanese elderly. J Oral Rehabil. 2010;37(11):848–853. doi:10.1111/j.1365-2842.2010.02100.x
36. AlZarea BK. Prevalence and pattern of torus palatinus and torus mandibularis among edentulous patients of Saudi Arabia. Clin Interv Aging. 2016;11:209–213. doi:10.2147/CIA.S100282
37. Choi YJ, Lee JY, Lee SJ, Chung CP, Park YJ. Alpha-adrenergic blocker mediated osteoblastic stem cell differentiation. Biochem Biophys Res Commun. 2011;416(3–4):232–238. doi:10.1016/j.bbrc.2011.09.095
38. Portillo MR, Rodriguez-Ortiz ME. Secondary hyperparthyroidism: pathogenesis, diagnosis, preventive and therapeutic strategies. Rev Endocr Metab Disord. 2017;18(1):79–95. doi:10.1007/s11154-017-9421-4
39. Hou YC, Lu CL, Zheng CM, et al. Emerging role of vitamins d and k in modulating uremic vascular calcification: the aspect of passive calcification. Nutrients. 2019;11(1):152. doi:10.3390/nu11010152
40. Hong HH, Hong A, Wang CC, et al. Calcitriol exerts a mineralization-inductive effect comparable to that of vitamin C in cultured human periodontium cells. Am J Transl Res. 2019;11(4):2304–2316.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
