Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 11
Tolvaptan in the treatment of autosomal dominant polycystic kidney disease: patient selection and special considerations
Authors Sans-Atxer L, Joly D
Received 20 July 2017
Accepted for publication 11 November 2017
Published 31 January 2018 Volume 2018:11 Pages 41—51
DOI https://doi.org/10.2147/IJNRD.S125942
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Laia Sans-Atxer,1 Dominique Joly2
1Department of Nephrology, Hospital del Mar, Institut Mar for Medical Research, Barcelona, Spain; 2Faculty of Medicine, Université Paris-Descartes, Assistance Publique-Hôpitaux de Paris, Service de Néphrologie, Hôpital Necker-Enfants Malades, Paris, France
Abstract: Standard of care therapies for autosomal dominant polycystic kidney disease (ADPKD) may limit morbidity and mortality due to disease-related complications, but they do not delay disease progression. Tolvaptan, a selective vasopressin V2 receptor antagonist, delays the increase in kidney volume (a surrogate marker for disease progression), slows the decline in renal function, and reduces pain in ADPKD patients with relatively preserved renal function. The most common adverse events of tolvaptan are linked to its aquaretic effect, and rare cases of idiosyncratic hepatitis were observed. Additional ongoing studies will determine whether the benefits are sustained over time, whether they can be observed in patients with advanced kidney disease, and whether they can be translated in terms of quality of life and cost/effectiveness parameters. Tolvaptan is currently approved in Europe and several countries throughout the world. In real-life conditions, selection of patients that would be good theoretical candidates to tolvaptan is a key but complex question. Eligibility criteria slightly differ from one country to another, and several models (based on conventional data, genetics, renal volume) were recently proposed to identify patients with evidence or risk of rapid disease progression. Eligible patients will ultimately make the decision to start tolvaptan, after complete information, consideration, and balancing of benefits, adverse events, and risks.
Keywords: autosomal dominant polycystic kidney disease, ADPKD treatment, tolvaptan
Introduction
Autosomal dominant polycystic kidney disease (ADPKD), the most common cause of genetic kidney disease, accounts for 4.7%–10% of prevalent patients on renal replacement therapy.1,2 ADPKD is characterized by the new development and expansion of kidney cysts that cause a massive enlargement and distortion of the kidney architecture and ultimately lead to ESRD in most patients, but at very different ages.3 Despite improvements in blood pressure targets and control,4,5 conventional treatments for CKD6,7 do not seem to have any significant impact on the need for renal replacement therapy in ADPKD patients.1
Thus, great efforts were undertaken to understand the molecular mechanisms implicated in cystogenesis and to establish markers for fast progression, so that specific disease-modifying treatments could be designed and tested.8
Cysts develop from only a small fraction of nephrons (<1%) in whom a combination of somatic mutation and germline mutation9 initiates cystogenesis. ADPKD is genetically heterogeneous. Patients with germline PKD1 mutation have a much more severe course of the disease than patients with PKD2 mutations.10,11 Early disease-related symptoms such as hypertension, albuminuria, or urological complications have also been identified as markers of fast progression of ADPKD.12 In most kidney diseases, progression assessment is based on GFR evolution. In the early stages of ADPKD, however, renal function often seems normal, despite major cyst expansion,13 probably thanks to the hyperfiltration of unaffected nephrons.14 For this reason, renal volume and changes in renal volume have arisen as key parameters to predict and monitor evolution at the earliest stages of ADPKD.12,15
The main cellular events linked to cyst enlargement are cellular proliferation and intracystic fluid secretion. Several molecular pathways, including mTOR, Src, and cAMP, have been shown to modulate these elements within tubular epithelial cells and to reduce cystic growth.16 Unfortunately, despite promising results in animals, clinical studies conducted in ADPKD patients with mTOR inhibitors failed to show any benefit in terms of renal volume or renal function;17,18 bosutinib, an Src inhibitor, reduced the rate of kidney growth but had no significant effect on kidney function.19 Concerning the cAMP pathway, several trials comparing somatostatin analogues with placebo are in progress, following promising preliminary results.20 To date, the most significant results with the cAMP pathway were obtained by studying the link between arginine vasopressin (AVP), renal V2R, and cAMP.21,22 Inhibition of this pathway by tolvaptan was efficient in preclinical/animal studies and in clinical studies involving ADPKD patients; tolvaptan has been recently released in the market.
Preclinical studies of tolvaptan
Renal collecting ducts express V2R which, when bound by arginine vasopressin, increase secondary messenger cAMP, upregulate aquaporin-2 channels, and promote free water reabsorption. A V2R agonist was shown to increase renal cAMP and cystogenesis in an animal model of ADPKD.23 Conversely, suppression of vasopressin release (by either genetic manipulation or by high water intake) or suppression of vasopressin effect (by the use of V2R antagonists) reduced secondary messenger cAMP and decreased cyst cell proliferation, fluid secretion, cystogenesis, and renal enlargement.23–27
These preclinical studies provided the rationale for the investigation of tolvaptan in ADPKD. Tolvaptan, an orally active, non-peptide selective arginine vasopressin V2R antagonist, acts by binding to the V2R expressed by the distal parts of the nephron with a higher affinity than vasopressin.28 Tolvaptan was initially developed to induce free water excretion in patients with dilutional hyponatremia as well as in patients with heart failure. Dose–response effects were studied by urine osmolality measurements. Two split doses per day taken at 8-hour intervals lower urine osmolality (<300 mOsm/kg for 24 hours) in more than 50% of patients with a 45 mg+15 mg regimen and in 85% of patients with a 90 mg+30 mg regimen.29 Half life is about 12 hours. Dosage adjustments are not necessary for patients with renal dysfunction.30
Clinical efficiency of tolvaptan in ADPKD patients
Several clinical trials testing molecules involved in the different pathways leading to cystogenesis have been developed in APDKD patients. Because the greatest advantage of specific therapies should come from treating patients at early stages of the disease, when normal renal tissue is still preserved and not substituted by cysts and renal function is in its normal range, renal volume has been used as the primary outcome in all those clinical trials. In Table 1, the description and main results of clinical trials performed with tolvaptan in ADPKD patients (either completed or ongoing) are shown.
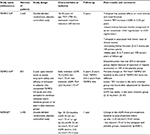  | Table 1 Interventional ADPKD human studies with tolvaptan Abbreviations: ADPKD, autosomal dominant polycystic kidney disease; TKV, total kidney volume; eGFR, estimated glomerular filtration rate. |
At the light of these results, tolvaptan is, to date, the only molecule with marketing authorization in Europe and in other several countries of the world, and the first disease-modifying treatment for ADPKD.
TEMPO 3:4 trial
Tolvaptan was tested in the TEMPO 3:4 trial.31 TEMPO 3:4 was a randomized, double-blind, controlled Phase III trial comparing tolvaptan versus placebo in adults aged 18–50 years with ADPKD and with a TKV ≥750 mL by MRI and a creatinine clearance ≥60 mL/min. A total of 1,445 patients were randomized (2:1) to receive either tolvaptan (n=961) or placebo (n=484) during 3-years follow-up. The initial daily dose of tolvaptan was 60 mg (45 mg in the morning and 15 mg in the afternoon), which was increased, if tolerated, to 90 mg (60 mg in the morning and 30 mg in the afternoon) to finally achieve a maximal dose of 120 mg (90 mg in the morning and 30 mg in the afternoon). The primary endpoint was the annual rate of change in TKV at 3 years. A total of 138 patients were excluded due to early withdrawal and or lack of MRI TKV measurements. The analysis included only patients with a TKV evaluation at the last scheduled visit (month 36): 88% (842/961) of patients taking tolvaptan and 96% (465/484) of placebo patients.
At 3 years, the rate of increase of TKV was lower in the tolvaptan group than in the placebo group (2.8% per year [95% CI]: 2.5–3.1] versus 5.5% [95% CI: 5.1–6.0]; p<0.001). The mean change in TKV over the 3-year period was 9.6% with tolvaptan versus 19% with placebo (p<0.001). That represents a 49% reduction in the rate of increase of TKV with tolvaptan versus placebo, with a greater effect in the first year than in the second and third years. This beneficial effect in tolvaptan’s arm was found for all prespecified stratification subgroups: sex, age <35 years or ≥35 years, TKV <1,500 mL or ≥1,500 mL, estimated creatinine clearance <80 mL/min or ≥80 mL/min, and hypertension (absent or present). A composite secondary endpoint that reflected the progression of the disease and which included four parameters – 1) worsening renal function (25% reduction in the reciprocal of serum creatinine), 2) clinically significant renal pain (requiring work interruption, opioid pharmacological therapy, or last resort analgesic or invasive procedure, 3) worsening hypertension, and 4) aggravating albuminuria – also favored the tolvaptan arm compared to placebo, with significantly fewer events related to clinical progression for 100 person-years of follow-up (44 versus 50 events; risk ratio [HR], 0.87; 95% CI: 0.78–0.97; p=0.01). However, the effects of tolvaptan on the four parameters of the composite score were highly variable: significant effect on aggravation of renal function and severe kidney pain, but no benefit for hypertension or albuminuria. Changes in renal function (measured by the reciprocal of the serum creatinine level between the end of the dose increase and month 36) were also measured as a secondary outcome regardless of the composite score. Tolvaptan slowed a loss of renal function by ~25% compared to placebo, with a reduction in the rate of deterioration of 1.20 mg/mL/year (95% CI: 0.62–1.78; p<0.001). Analysis of the estimated annual slope of eGFR using Chronic Kidney Disease Epidemiology Collaboration equation gave similar results, ie, a reduction in eGFR decline of 0.98 mL/min/1.73 m2 per year (95% CI: 0.60–1.36; p<0.001).
Adverse effects linked to the disease itself, such as pain or urologic events, also favored the tolvaptan arm. Otherwise, patients in tolvaptan’s arm experienced a significantly higher percentage of aquaretic adverse events related to the drug and a greater percentage of liver enzyme elevations. It is important to highlight the fact that two patients in the tolvaptan’s arm met with Hy’s law criteria, which led to a particular risk management plan, and will be discussed later.
The results of TEMPO 3:4 trial led to the approval of tolvaptan by the EMA in 2015 as the first treatment to delay the progression of ADPKD and it is already available in the market in several European countries, Japan, and Canada. Tolvaptan is the first specific drug available in the market to delay the progression of ADPKD for those patients with (or an expected) a fast progression of the disease and CKD stages 1–3, at the beginning of the treatment as indicated in its label.
However, despite these positive results, several questions remain un answered. The TEMPO 3: 4 study included patients aged 18–50 years at the early stages of the disease (CKD stages 1–3). The lack of data on the later stages or the benefit beyond 50 years makes any extrapolation of the results of the study uncertain. Another trial (REPRISE trial) testing the effectivity of tolvaptan in advanced stages of the disease (CKD stages 3 and 4) is ongoing.33 Finally, the duration of the study (3 years) is too short to determine whether the treatment delayed dialysis or renal transplantation. Only a long-term follow-up of the first treated patients will make it possible to see if the initial results are maintained over time.
TEMPO 4:4 trial
Follow-up results were recently published in the TEMPO 4:4 trial, which is an open-label, 2-years extension trial following TEMPO 3:4. A total of 60% of the patients involved in TEMPO 3:4 participated in TEMPO 4:4. Patients treated with tolvaptan in TEMPO 3:4 continued tolvaptan and formed the “early treatment group”; patients treated with placebo in TEMPO 3:4 were proposed to start tolvaptan and formed the “late treatment group”. The aim of TEMPO 4:4 was to evaluate the long-term effect of tolvaptan on TKV and renal function.32 At the end of ≥5 years of follow-up, TKV changes between TEMPO 3:4 baseline and TEMPO 4:4 Month 24 were not statistically different (+29.9% in prior tolvaptan versus +31.6% in prior placebo). Two main factors may explain the absence of overall effect of tolvaptan on renal volume: 1) a larger effect of tolvaptan during the first year, ie, an acute reduction in cyst volume due to the antisecretory effect of tolvaptan,34,35 with less effect during subsequent years and 2) an imbalance across study arms due to the loss of randomization at the start of TEMPO 4:4, with notably a wide predominance of males in the early tolvaptan arm (it is well known that males have a faster TKV growth). Indeed, after adjustment by imbalanced baseline characteristics and other confounding factors, differences in TKV increased from 1.7% to 4.15% in favor of early treatment group and achieved statistical significance. Another analysis, focusing on patients with fast disease predictors (1C–1E categories of the Mayo Clinic model, truncating PKD1 mutations, CKD stages 2–3), showed a lower TKV increase in the early treated patients.
In terms of renal function, the absolute difference of eGFR between early and late treatment groups was maintained, which reached 3.15 mL/min/1.73 m2 (with statistical significance) at the end of TEMPO 4:4, whereas eGFR slopes were not statistically different across the two groups. However, at the end of the extension study, the hypothesis of a gradual decline of the benefit of tolvaptan over time cannot be totally ruled out.
REPRISE trial
While the TEMPO trial included patients with a relatively preserved renal function, the REPRISE trial included 18- to 55-year-old ADPKD patients with a baseline eGFR of 25–65 mL/min/1.73 m2 and 56- to 65-year-old patients with eGFR of 25–44 mL/min/1.73 m2 plus GFR decline >2.0 mL/min/1.73 m2/year. The results of this prospective double blinded, placebo-controlled trial, which included 1495 subjects, have been published recently.34 In patients with later stage ADPKD, the use of tolvaptan was associated with a slower eGFR decline (–2.34 mL/mn/1.73 m2 versus –3.6 mL/mn/1.73 m2). These results suggest that tolvaptan may be effective over a broad range of CKD stage. However, it must be underscored that the benefit was only apparent after treatment discontinuation, indicating a role for hemodynamic changes and possible confounding factors. Moreover, the duration of the trial was too short (1 year of treatment only) to address hard end points such as doubling of creatinine or ESRD.
ACQUIRE study
The impact of tolvaptan on patient’s QOL is largely unknown. The ACQUIRE study, which has been recently started in Europe, will hopefully address this question. This prospective non-interventional study will include 486 ADPKD patients with stages 1–3 CKD and rapidly progressing disease, either treated with or not treated with tolvaptan. This study includes two ADPKD-specific QOL evaluation scales, the ADPKD-impact scale and the ADPKD urinary impact scale. These recently validated scales were composed with the help of groups of patients. For patients not treated with tolvaptan, the ACQUIRE study will describe the effects of early stage ADPKD on QOL parameters, whereas most studies have focused on ADPKD with CKD stage 4/5. For patients treated with tolvaptan, the ACQUIRE study will report on QOL parameters satisfaction with treatment as well as the burden of increased aquaresis.
Adverse effects and precautions for use
The most important considerations to be taken with tolvaptan treatment are linked to its potential adverse effects.
Transient extracellular fluid volume contraction
There is a discrete volume contraction in the days following the initiation of tolvaptan.34,35 In TEMPO 3:4, uricemia increased and some patients had gout attacks. A reversible GFR reduction was observed in the first 2 weeks of treatment. This is why the evaluation of renal function in TEMPO 3:4 began only after the end of titration of tolvaptan. In practice, consideration should be given to reducing the dose or stopping diuretics before starting tolvaptan. Of note, the use of diuretics was authorized in TEMPO 3:4 but <1.5% of patients followed such treatment.
Sustained increased aquaresis
An aquaretic effect is expected due to the mode of action of tolvaptan.21 Taking a high dose in the morning (8 hours) and then a lower dose in the middle of the afternoon (16 hours) aims to maintain a desired biological effect without causing excessive nycturia. The aquaretic effect does not seem to diminish over time. In the TEMPO 3:4 study, patients receiving tolvaptan reported high rates of adverse events related to aquaresis: thirst (55% versus 20% of patients on placebo), polyuria (38% versus 17%), nycturia (29% versus 13%), and polydipsia (10% versus 3.5%). The proportion of patients who stopped taking the medication for these symptoms was 8.3% (80/961). Urine output and QOL were not reported in TEMPO 3:4. Patients should be advised of this undesirable effect and the need for good preventive hydration; but also and above all to be able to have free access to water to avoid dehydration. When access to water is not possible, treatment should be suspended temporarily. Patients may also be asked to reduce their osmotic load (sodium dietary intake and dietary protein) to limit the importance of aquaresis.
Tolvaptan-induced hepatitis
In TEMPO 3:4 trial, it appeared that the proportion of patients with ALT >3 times the upper limit of normal was higher in patients receiving tolvaptan (4.4% versus 1% on placebo).36 The Independent Data Monitoring Committee (IDMC) recommended to monitor more closely the liver function tests in the TEMPO 4:4 extension trial, and an independent Hepatic Adjudication Committee was set up to properly assess the risk of hepatoxicity in clinical trials including ADPKD and non-ADPKD patients under tolvaptan or placebo. In non-ADPKD patients (trials for hyponatremia, heart failure, or cirrhosis), no imbalance in hepatitis was observed. In ADPKD patients, ALT elevation was judged probably related to study drug in 16 patients under tolvaptan and 1 patient under placebo. Among them, three patients met the Hy’s law criteria (ALT >3× and total bilirubin >2× the upper limit normal) indicative of potentially severe liver injury. Hepatocellular injury occurred between 3 and 18 months after starting tolvaptan, with slow resolution after drug discontinuation. Liver biopsies (four cases) did not show any specific lesions. No liver failure was observed. Only half of the patients rechallenged with tolvaptan experienced ALT reelevation.30 The EMA has estimated that the number of patients currently exposed is insufficient to exclude rare but severe liver toxicity and that tolvaptan could potentially cause severe hepatitis in 1 in 4,000 patients. A risk management plan (included in the summary of product characteristics) has, therefore, been specifically set up. It includes recommendations for the initiation of treatment (liver function tests), monitoring (monthly biological checkup for 18 months and quarterly thereafter), and management of signs suggestive of hepatitis.
No pregnancy
Neither pregnancies nor breastfeeding are possible under tolvaptan. Effective contraception should be provided to patients treated in gestational age.
Interactions
Tolvaptan is a CYP3A substrate. Thus, attention must be paid at coadministration with CYP3A-inducing drugs (eg, rifampicin and St John’s wort) or CYP3A-inhibiting drugs (eg, ketoconazole, grapefruit juice, and pomegranate juice).29
Access to tolvaptan
In many countries throughout the world, tolvaptan is currently either not authorized on the market or not reimbursed. In the USA, the Food and Drug Administration requested in 2014 further data on side effects and the selection of patient cohorts who may benefit from tolvaptan. By opposition, the EMA approved in May 2015 the use of tolvaptan with a broad indication, ie, adult ADPKD patients with CKD stages 1–3 at initiation with evidence of rapidly progressing disease. Although there is currently no standard definition of rapidly progressing ADPKD, clinicians must have the ability to correctly identify patients, who would benefit from treatment initiation, and also avoid treatment initiation in those patients in whom the risks linked to treatment outweigh the benefits.12 Reimbursing criteria are supposed to correctly balance the patient selection to give a clear benefit to those patients who may have a fast progression and avoid risks in those whom treatment initiation will not change the course of the disease. These criteria, based on available evidence, should in theory be quite similar from one country to another. It is interesting to observe that in countries where tolvaptan is already in the market, national health authorities have 1) dictated different reimbursing criteria, as shown in Table 2, and 2) obtained different prices for the same treatment.
In France, the cost of one year of treatment with tolvaptan is around €15,000. The French reimbursement criteria do not take age into account: this allows the treatment of elderly patients with correct renal function, allows for a dubious long-term benefit, and allows for an unfavorable cost/benefit ratio. Worse, it does not make it possible to treat young patients who have an active disease but have not yet reached the threshold of renal volume required (600 mL/m). The benefit and safety of treatment in patients with advanced renal disease (stage 3b), which were not included in the TEMPO 3:4 study, may also be questioned. In England, as CKD stage 1 patients are not reimbursed, young patients with a fast progressing disease cannot benefit from early treatment initiation. In Spain, the criteria used allow treatment initiation in young patients expected to show a fast progression, even though they might still have normal renal function with modestly enlarged kidneys. These criteria are closely linked to the European recommendations, which will be discussed later.
Patient selection: who are the best candidates for tolvaptan treatment?
Boundaries for age and/or GFR
Tolvaptan is only indicated in adults. As a small percentage of polycystic patients older than 50 years have been treated with tolvaptan in clinical trials, caution should be taken at treatment initiation beyond this age. In initial studies, a small percentage of patients with CKD stage 3B (eGFR ≤45mL/min/1.73m2) have been treated with tolvaptan.31,38 Until recently, tolvaptan’s label indicated that in the absence of safety and efficacy data for patients with an eGFR ≤30 mL/min/1.73m2, treatment initiation should be avoided in CKD stage 4. However, the recently published REPRISE trial comprising patients with later-stage ADPKD (GFR 25 to 65 mL/min/1.73m2) shows that over a 1-year period, tolvaptan slightly reduced GFR decline, at the price of an elevation of transaminases in 5.6% of patients.34 Even if such evidence allows initiation of tolvaptan in patients with eGFR as low as 30 or maybe 25 ml/mn/1.73m2, long term data on safety for these patients are clearely needed. Also, when should we consider stopping treatment if renal function declines? There is no clear consensus pointing to a particular cut-off value. In our opinion, until further data is available, it may be reasonable to stop treatment when eGFR is lower than 20 mL/min/1.73m2.
Patients at risk for rapid progression
European recommendations for the use of tolvaptan were published in 2016 by a group of experts.37 These recommendations have pointed some futile indications and tried to identify patients at risk for progression, including young patients with a predicted fast progression of the disease even if renal function is still normal. A multi-step decision tree is proposed. In the algorithm’s first step, patients are selected according to age and renal function, so that a very normal renal function at certain age makes unlikely a fast evolution of the disease with no benefits from treatment initiation (30–40 years and eGFR >90 mL/min/1.73 m2 or 40–50 years and eGFR >60 mL/min/1.73 m2).
The algorithm proposes, in descending order of reliability, a list of different situations that can drive to propose treatment initiation, based on four models: 1) GFR slope, 2) kidney growth rate, 3) predictive model based on volume or renal length, or 4) predictive model based on genetics.38–41
Rapid progression based on observed GFR decline or kidney growth
In long-term followed-up patients, the rate of renal function decline or the rate of volume growth might be used to evaluate the progression of the disease. Here, the difficulties might come in establishing a cut-off value to decide what is a fast progression. European experts have selected two criteria based on eGFR slope: 1) an average ≥2.5 mL/min/1.73 m2/yearly loss of renal function over a period of 5 years, based on the class 1C patients of the Mayo classification (seethe following text)39 and 2) an eGFR decline ≥5 mL/min/1.73 m2 within 1 year, based on 2012 KDIGO CKD Guidelines. However, caution should be taken in using a greater cut-off value (5 mL/min/1.73 m2) between only two measurements to diagnose a fast progression, as renal function variation between two different measurements can be influenced by other factors not related to disease progression.
Future studies should focus on GFR slope in young ADPKD patients. We observed that many young adults have a “normal” and “stable” estimated GFR (>90 mL/min/1.73 m2), but measured GFR experiences a progressive decline (personal unpublished data). These patients are unfortunately not identified by the proposals of the European expert group.42 Other teams have already reported a far better assessment of GFR slope in ADPKD by measurement over creatinine-based estimations.43
The rate of renal volume enlargement can also be used to retrospectively monitor disease progression. European experts and Japanese regulatory authorities define as fast progressors ADPKD patients with an increase of renal volume ≥5% per year during a certain period of time (ideally three MRI-based measurements, each at least 6 months apart). This criterion is mainly based on outcomes observed in class 1D and 1E patients of the Mayo classification (see the following text).39
Predicted progression based on renal volume: the Mayo Clinic model
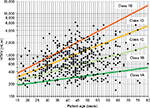
A large cohort (n=590) of ADPKD patients followed at the Mayo Clinic Translational PKD Center was used to construct the Mayo Clinic’s model39 necessitating optimal patient selection for enrollment into clinical trials. Patients from the Mayo Clinic Translational PKD Center with ADPKD (n=590 to predict ADPKD progression. This model uses height-adjusted total renal volume (expressed in mL/min) together with the age of patients to classify patients into five different categories (from class 1A to 1E) (Figure 1). Patients in 1A and 1B progression categories are considered slow progressors, while patients in class from 1C to 1E are considered fast progressors.
This model also allows a prediction of future renal function, according to the class of progression, age, and gender and considering eGFR at the time of evaluation. This model is freely available at the internet site http://www.mayo.edu/research/documents/pkd-center-adpkd-classification/doc-20094754. This model, which relies on an accurate kidney volume assessment, is only applicable to those patients with a typical (symmetric) imaging disease presentation. About 10% of patients who present with an atypical imaging presentation (for example renal asymmetry >30%) will not fit the model. Moreover, 12%–20% of patients evaluated by a new imaging study performed years later will display a different risk category. This model, exclusively created and externally validated within North-American populations, has not been validated in European population yet. On top of height-adjusted TKV, which is the best MRI-based biomarker for ADPKD, additional computing techniques such as “imaging texture analysis” may improve outcomes prediction.44
Predicted progression based on genetics: the PROPKD score
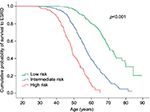
The PROPKD score, based on a large population of ADPKD French patients, is calculated with three individual characteristics of each patient: 1) gender (one point for males), 2) apparition of hypertension or urological complications before 35 years of age (two points), and 3) the type of mutation (PKD2 or PKD1 either truncating mutation – four points – or non-truncating mutation – two points). Patients with a PROPKD score >6 points are prone to experience a fast progression of the disease, while those with a score <3 are likely to have a slow progression and so should not be considered for treatment (Figure 2). The cons of this model are that a genetic study is mandatory, that a score between 3 and 6 does not guide us to clear conclusions, and that to be completely informative, you have to wait until the patient turns 35.
Simple tools to assess the risk of rapid progression
In daily practice, the prediction models cited above require information that is not always easily available for all patients, such as GFR slope, genetic analysis, or precise renal volumetric assessment by MRI. In such circumstances, two accessible criteria may help identify patients at risk for fast ADPKD progression. The first one is a single (ultrasound) renal length measurement higher than 16.5 cm, which was shown to predict CKD stage 3 in a 8-year-time frame.38 The second is the family history of ADPKD: any renal replacement before 58 years of age increases the risk to present a fast progression. However, waiting until this kidney length is reached might be linked to a late treatment initiation in patients prone to experience a fast disease progression; simple family history criteria are less reliable and its use to draw conclusions in terms of treatment is highly debatable.
Urine osmolality and other biological biomarkers
In the TEMPO 3:4 study, all patients were advised to drink large amounts of water. A recently published post hoc analysis of this study showed that tolvaptan reduced Uosm by an average of 300 mOsm/kg at the end of the drug titration period versus only –65 mOsm/kg in patients taking placebo.45 This result indicates that V2R blockade is far more efficient than primary water intake to increase aquaresis. Interestingly, best responses to tolvaptan (lower eGFR decline, lower incidence of cystic pain) were observed in patients with higher baseline Uosm. Higher baseline Uosm was independently predicted by male gender, lack of hypertension, lower age, lower TKV, and higher eGFR. It was also shown that patients achieving greatest Uosm reductions (mostly patients with high baseline Uosm and high baseline eGFR) had a slower eGFR decline throughout the study. Altogether, these data suggest that tolvaptan provided a higher benefit to patients with less advance disease at the start of the study. Uosm may well be a marker of disease severity (reflecting concentrating ability of kidneys) in ADPKD; however, two major determinants of Uosm (water intake and the daily amount of osmoles excreted) were not taken into account in the TEMPO cohort. It is thus conceivable that tolvaptan has also a wider effect in patients with low water and/or high salt+protein intake. In the same study, it was shown that there was no apparent benefit of reducing Uosm below 250 mOsm/kg.45 It remains to be seen if achieving an Usom of 250 mOsm/kg by increasing water intake and eating less salt and proteins has, or not, the same effect than reducing Uosm with tolvaptan.46
Noninvasive and simple biomarker(s) to predict early ADPKD progression and response to treatment are awaited. As shown earlier, Usom is promising. Other biomarkers are worth mentioning: plasma copeptin, a surrogate for plasma AVP,47 is another biomarker underlining the link between V2R signaling, Usom, and early ADPKD progression. Regarding urine, albuminuria,48,49 monocyte chemoattractant protein-1 (MCP-1), neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule 1 (KIM-1), tumor necrosis factor-alpha, and proteomic profile12,50 have shown good correlations with disease severity and disease progression.
Additional considerations before starting tolvaptan
Cost-effectiveness
In 2014, following the publication of the TEMPO 3:4 trial, a Markov-based cost-effectiveness analysis of tolvaptan was published.51 This study assumed that tolvaptan compared with standard care could increase age at ESRD by 6.5 years and life expectancy by 2.6 years, at an extra cost of US$744,100 per QALY gained. This analysis was probably premature. First, the yearly cost of tolvaptan used in this model was much higher than the price currently charged in most countries. Second, the model postulated that the yearly gain in eGFR between the tolvaptan and placebo groups in the TEMPO trial (0.98 mL/min/year) could be linearly extrapolated over decades, and ultimately delay progression to ESRD and reduce death rates, which is still currently speculative. Third, the results highlighted in this analysis were based on the whole TEMPO study population, comprising many patients with slowly progressive ADPKD, in which the cost per QALY was even higher.
In 2015, a British National Institute for Health and Care Excellence committee reviewed the economic model proposed by Otsuka and agreed to consider that in ADPKD adults with CKD stages 2–3 and rapidly progressing disease – the cost per QALY gained under tolvaptan was £23,500. According to this model, the use of health system resources to reimburse tolvaptan in a high-risk subgroup of patients seemed cost-effective. Obviously, new cost-effectiveness studies will have to be carried out. Such studies will take into account long-term effectiveness, QOL, drug price by country, and a sensitivity analysis by rate of disease progression before treatment.51
Patients information, choice, and motivation
Starting tolvaptan in a given ADPKD patient requires a convinced nephrologist (based on the available studies and on individual assessment of expected clinical benefit) as well as a properly informed and motivated patient. The information provided should relate to mechanism of drug action, proven benefits and personal expectations from treatment, expected adverse events (polyuria) with subsequent need for lifestyle modification, and potential risks (hepatitis) with subsequent need for close monitoring. Although patients who participated in the TEMPO study were all motivated to try a potentially disease-modifying drug, 23% discontinued the treatment (versus 14% in the placebo group), mainly because of aquaresis-related side effects.31 In real-life conditions, it is expected that a significant proportion of eligible patients will not accept to start the drug. The familial and individual burden of ADPKD, family plans, occupation, education, and several other factors probably modulate the motivation whether to start tolvaptan or not. The ACQUIRE study cited earlier will also try to identify the individual determinants of treatment choices, which are currently hypothetical.
Assessing individual response to tolvaptan
Monitoring individual treatment benefits once tolvaptan has been initiated should be foreseen. On top of routine analysis, one should follow several parameters to evaluate an individual change in disease progression after treatment initiation: GFR slope based on estimated (or measured) GFR, renal volume, urine osmolality, and QOL. In the absence of significant response to tolvaptan, a decision could be made to discontinue the drug or to add or to switch to another disease-modifying drug when available. In case of good response to tolvaptan, an objective measured parameter would help to maintain the patient’s motivation.
Conclusion
With tolvaptan available in the market, we have started a new era in the clinical management of ADPKD patients. A proper evaluation of each patient illegibility is essential to assure success in changing the course of the disease without exposing the patient to needlessly adverse effects. The earliest possible selection of the patients who are likely to benefit from treatment initiation remains the key point.
Abbreviations
ADPKD, autosomal dominant polycystic kidney disease; ESRD, end-stage renal disease; GFR, glomerular filtration rate; eGFR, estimated glomerular filtration rate; cAMP, cyclic 3′, 5′-monophosphate adenosine; TKV, total kidney volume; BP, blood pressure; RAS, renin–angiotensin system; MRI, magnetic resonance imaging; V2R, V2 receptor; CKD, chronic kidney disease; QOL, quality of life; ALT, alanine aminotransferase; EMA, European Medicines Agency; Uosm, urinary osmolality; QALY, quality-adjusted life-year.
Disclosure
LSA received honoraria from Otsuka (from lectures and for participating in Adivsory Boards). DJ received honoraria from Otsuka (from lectures and for participating in Adivsory Boards) and is the principal investigator for studies conducted by Otskua and Ipsen. The authors report no other conflicts of interest in this work.
References
Spithoven EM, Kramer A, Meijer E, et al. Renal replacement therapy for autosomal dominant polycystic kidney disease (ADPKD) in Europe: prevalence and survival – an analysis of data from the ERA-EDTA Registry. Nephrol Dial Transplant. 2014;29(Suppl 4):iv15–iv25. | ||
Saran R, Li Y, Robinson B, et al. US Renal Data System 2015 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2016;67(3):A7–A8. | ||
Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359(14):1477–1485. | ||
Schrier RW, Abebe KZ, Perrone RD, et al. Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med. 2014; 371(24):2255–2266. | ||
Torres VE, Abebe KZ, Chapman AB, et al. Angiotensin blockade in late autosomal dominant polycystic kidney disease. N Engl J Med. 2014; 371(24):2267–2276. | ||
Chapman AB, Devuyst O, Eckardt K-U, et al. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2015;88(1):17–27. | ||
Patch C, Charlton J, Roderick PJ, Gulliford MC. Use of antihypertensive medications and mortality of patients with autosomal dominant polycystic kidney disease: a population-based study. Am J Kidney Dis. 2011;57(6):856–862. | ||
Chang M-Y, Ong ACM. Mechanism-based therapeutics for autosomal dominant polycystic kidney disease: recent progress and future prospects. Nephron Clin Pract. 2012;120(1):c25–c34; discussion c35. | ||
Grantham JJ, Mulamalla S, Swenson-Fields KI. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2011;7(10):556–566. | ||
Hateboer N, v Dijk MA, Bogdanova N, et al. Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet. 1999;353(9147):103–107. | ||
Vujic M, Heyer CM, Ars E, et al. Incompletely penetrant PKD1 alleles mimic the renal manifestations of ARPKD. J Am Soc Nephrol. 2010;21(7):1097–1102. | ||
Schrier RW, Brosnahan G, Cadnapaphornchai MA, et al. Predictors of autosomal dominant polycystic kidney disease progression. J Am Soc Nephrol. 2014;25(11):2399–2418. | ||
Grantham JJ, Chapman AB, Torres VE. Volume progression in autosomal dominant polycystic kidney disease: the major factor determining clinical outcomes. Clin J Am Soc Nephrol. 2006;1(1):148–157. | ||
Wong H, Vivian L, Weiler G, Filler G. Patients with autosomal dominant polycystic kidney disease hyperfiltrate early in their disease. Am J Kidney Dis. 2004;43(4):624–628. | ||
Grantham JJ, Torres VE, Chapman AB, et al. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354(20):2122–2130. | ||
Chang M-Y, Ong ACM. Mechanism-based therapeutics for autosomal dominant polycystic kidney disease: recent progress and future prospects. Nephron Clin Pract. 2011;120(1):c25–c35. | ||
Walz G, Budde K, Mannaa M, et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010; 363(9):830–840. | ||
Serra AL, Poster D, Kistler AD, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010; 363(9):820–829. | ||
Tesar V, Ciechanowski K, Pei Y, et al. Bosutinib versus placebo for autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2017;28(11):3404–3413. | ||
Caroli A, Perico N, Perna A, et al. Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): a randomised, placebo-controlled, multicentre trial. Lancet. 2013;382(9903):1485–1495. | ||
Noda Y, Sohara E, Ohta E, Sasaki S. Aquaporins in kidney pathophysiology. Nat Rev Nephrol. 2010;6(3):168–178.22. | ||
Devuyst O, Torres VE. Osmoregulation, vasopressin, and cAMP signaling in autosomal dominant polycystic kidney disease. Curr Opin Nephrol Hypertens. 2013;22(4):459–470. | ||
Wang X, Wu Y, Ward CJ, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2008;19(1):102–108. | ||
Reif GA, Yamaguchi T, Nivens E, Fujiki H, Pinto CS, Wallace DP. Tolvaptan inhibits ERK-dependent cell proliferation, Cl-secretion, and in vitro cyst growth of human ADPKD cells stimulated by vasopressin. AJP Ren Physiol. 2011;301(5):F1005–F1013. | ||
Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med. 2004;10(4):363–364. | ||
Wang X, Gattone V, Harris PC, Torres VE. Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol. 2005;16(4):846–851. | ||
Nagao S, Nishii K, Katsuyama M, et al. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol. 2006;17(8):2220–2227. | ||
Bhatt PR, McNeely EB, Lin TE, Adams KF, Patterson JH. Review of tolvaptan’s pharmacokinetic and pharmacodynamic properties and drug interactions. J Clin Med. 2014;3(4):1276–1290. | ||
Shoaf SE, Chapman AB, Torres VE, Ouyang J, Czerwiec FS. Pharmacokinetics and pharmacodynamics of tolvaptan in autosomal dominant polycystic kidney disease: phase 2 trials for dose selection in the pivotal phase 3 trial. J Clin Pharmacol. 2017;57(7):906–917. | ||
Woodhead JL, Brock WJ, Roth SE, et al. Application of a mechanistic model to evaluate putative mechanisms of tolvaptan drug-induced liver injury and identify patient susceptibility factors. Toxicol Sci. 2017;155(1):61–74. | ||
Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367(25):2407–2418. | ||
Torres VE, Chapman AB, Devuyst O, et al. Multicenter, open-label, extension trial to evaluate the long-term efficacy and safety of early versus delayed treatment with tolvaptan in autosomal dominant polycystic kidney disease: the TEMPO 4:4 trial. Nephrol Dial Transplant. In press 2017. | ||
Torres VE, Devuyst O, Chapman AB, et al. Rationale and design of a clinical trial investigating tolvaptan safety and efficacy in autosomal dominant polycystic kidney disease. Am J Nephrol. 2017;45(3):257–266. | ||
Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan in Later-Stage Autosomal Dominant Polycystic Kidney Disease. N Engl J Med. 2017;377:1930–1942. | ||
Irazabal MV, Torres VE, Hogan MC, et al. Short-term effects of tolvaptan on renal function and volume in patients with autosomal dominant polycystic kidney disease. Kidney Int. 2011;80(3):295–301. | ||
Boertien WE, Meijer E, de Jong PE, et al. Short-term renal hemodynamic effects of tolvaptan in subjects with autosomal dominant polycystic kidney disease at various stages of chronic kidney disease. Kidney Int. 2013;84(6):1278–1286. | ||
Watkins PB, Lewis JH, Kaplowitz N, et al. Clinical pattern of tolvaptan-associated liver injury in subjects with autosomal dominant polycystic kidney disease: analysis of clinical trials database. Drug Saf. 2015;38(11):1103–1113. | ||
Gansevoort RT, Arici M, Benzing T, et al. Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice. Nephrol Dial Transplant. 2016;31(3):337–348. | ||
Bhutani H, Smith V, Rahbari-Oskoui F, et al. A comparison of ultrasound and magnetic resonance imaging shows that kidney length predicts chronic kidney disease in autosomal dominant polycystic kidney disease. Kidney Int. 2015;88(1):146–151. | ||
Irazabal MV, Rangel LJ, Bergstralh EJ, et al. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol. 2015;26(1):160–172. | ||
Cornec-Le Gall E, Audrezet M-P, Rousseau A, et al. The PROPKD score: a new algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2016;27(3):942–951. | ||
Helal I, Reed B, Schrier RW. Emergent early markers of renal progression in autosomal-dominant polycystic kidney disease patients: implications for prevention and treatment. Am J Nephrol. 2012;36(2):162–167. | ||
Gansevoort RT, Arici M, Benzing T, et al. Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice. Nephrol Dial Transplant. 2016;31(3):337–348. | ||
Ruggenenti P, Gaspari F, Cannata A, et al. Measuring and estimating GFR and treatment effect in ADPKD patients: results and implications of a longitudinal cohort study. PLoS One. 2012;7(2):e32533. | ||
Kline TL, Korfiatis P, Edwards ME, et al. Image texture features predict renal function decline in patients with autosomal dominant polycystic kidney disease. Kidney Int. 2017;92(%):1206–1216. | ||
Devuyst O, Chapman AB, Gansevoort RT, et al. Urine osmolality, response to tolvaptan, and outcome in autosomal dominant polycystic kidney disease: results from the TEMPO 3:4 trial. J Am Soc Nephrol. 2016;28(5):1592–1602. | ||
Torres VE, Bankir L, Grantham JJ. A case for water in the treatment of polycystic kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1140–1150. | ||
Boertien WE, Meijer E, Li J, et al. Relationship of copeptin, a surrogate marker for arginine vasopressin, with change in total kidney volume and GFR decline in autosomal dominant polycystic kidney disease: results from the CRISP cohort. Am J Kidney Dis. 2013;61(3):420–429. | ||
Chapman AB, Johnson AM, Gabow PA, Schrier RW. Overt proteinuria and microalbuminuria in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1994;5(6):1349–1354. | ||
Kistler AD, Poster D, Krauer F, et al. Increases in kidney volume in autosomal dominant polycystic kidney disease can be detected within 6 months. Kidney Int. 2009;75(2):235–241. | ||
Kistler AD, Serra AL, Siwy J, et al. Urinary proteomic biomarkers for diagnosis and risk stratification of autosomal dominant polycystic kidney disease: a multicentric study. PLoS One. 2013;8(1):e53016. | ||
Blanchette CM, Gutierrez B, Friend K. Cost-effectiveness of tolvaptan in autosomal dominant polycystic kidney disease. Ann Intern Med. 2014;160(2):142–143. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.