Back to Journals » Cancer Management and Research » Volume 12
Timing of Adjuvant Chemoradiation in pT1-3N1-2 or pT4aN1 Esophageal Squamous Cell Carcinoma After R0 Esophagectomy
Authors Wu L, Zhang Z, Li S, Ke L, Yu J, Meng X
Received 12 August 2020
Accepted for publication 3 October 2020
Published 27 October 2020 Volume 2020:12 Pages 10573—10585
DOI https://doi.org/10.2147/CMAR.S276426
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Leilei Wu,1,2 Zhenshan Zhang,1 Shuo Li,2 Linping Ke,2 Jinming Yu,2 Xue Meng2
1Department of Radiation Oncology, School of Medicine, Shandong University, Jinan, People’s Republic of China; 2Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, People’s Republic of China
Correspondence: Jinming Yu; Xue Meng
Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, No. 440 Jiyan Road, Jinan, Shandong, People’s Republic of China
Tel/ Fax +86 531-67627082
Email [email protected]; [email protected]
Introduction: Postoperative adjuvant radiation therapy (RT) and chemotherapy (aCRT) have been supposed to improve prognosis and outcomes in patients with node-positive thoracic esophageal squamous cell carcinoma (TESCC). Our aim was to analyze the impacts of interval between surgery and aCRT on prognosis, determining the optimal time interval.
Methods: We retrospectively reviewed 520 patients with TESCC between 2007 and 2015 treated with aCRT following radical esophagectomy without neoadjuvant chemotherapy and RT. These patients underwent RT (50– 60 Gy) combined with 2– 6 cycles chemotherapy after surgery. The time intervals were from 17 days to 145 days and divided into three groups: short interval group (≤ 28 days, S-Int group), medial interval group (≥ 29 and ≤ 56 days, M-Int group) and long interval group (≥ 57 days, L-Int group).
Results: Median follow-up was 35.6 months and the 3-, 5-year survival rates and median survival were 49.5%, 36.6% and 35.9 months. The duration of postoperative interval was a predictor of survival outcomes. The median survival and 5-year survival rates in S-Int, M-Int and L-Int groups were 23.6 (32.1%), 44.2 (43.3%) and 32.0 (31.5%) months (P=0.007). The difference was statistically significant between the M-Int and S-Int or L-Int group but was not between the S-Int and L-Int group. Besides, toxic reactions including early, late and adverse events (grade ≥ 3) in M-Int group were significantly less than S-Int and show no significant differences with L-Int group.
Conclusion: The optimal time interval was from 29 days to 56 days (5– 8 weeks) both in terms of survival outcomes and toxic reactions.
Keywords: thoracic esophageal squamous cell carcinoma, TESCC, adjuvant chemotherapy and radiation therapy, aCRT, time interval, survival, toxic reactions
Introduction
Esophagus cancer (EC) is a common malignant tumor of the digestive tract. In 2018, an estimated 572,034 new cases of EC were diagnosed all over the world, making it the seventh most common malignancy.1 In China, the incidence of EC in 2015 was higher, with men ranked third and women ranked fifth, and death from it ranked fourth among both men and women.2 Five-year survival rates for patients with locally advanced EC remains dismal after surgery alone as a result of a high incidence of local and systemic recurrence.3 Efforts on multimodality treatment have never stopped and a lot of inspiring achievements have been made during the last two decades.4 Although there is increasing evidence that neoadjuvant radiation therapy (RT) and chemotherapy (nCRT) can improve the prognosis of patients with EC,5,6 including esophageal squamous cell carcinoma (ESCC),7–9 postoperative chemotherapy and RT (CRT) are still a main choice for patients in China.
The role of adjuvant RT is still debatable in ESCC with no establishment in previous randomized trials,10–14 and no survival benefit was found with the use of postoperative RT by a meta-analysis including 5 randomized trials.15 Several randomized trials have also evaluated adjuvant chemotherapy and have not demonstrated a survival benefit.16–18 As a result, only surveillance is recommended for patients with R0 resection by the NCCN clinical practice guidelines.19 However, different from western countries, ESCC exceeds 90% in China and adjuvant therapy is difficult to replace. Several recent studies reported the survival benefits with the use of postoperative RT or CRT, especially in the presence of some risk factors.20–28
Nevertheless, there is no clear consensus on the timing of adjuvant RT and chemotherapy (aCRT) and there is a paucity of research on the effects of time interval prior to aCRT on the prognosis of patients with locally advanced thoracic ESCC (TESCC). Based on this, we aimed to determine the optimal time after surgical procedures.
Patients and Methods
Patients
A retrospective study was conducted in patients with TESCC who had experienced radical esophagectomy with lymph node dissection from March 2007 to May 2015 at Shandong Cancer Hospital and Qilu Hospital of Shandong University. Patients who met all the following criteria were enrolled: 1) all experienced R0 resection and pathologically confirmed TESCC; 2) pathologic stage III or node-positive excluding lymph node metastases stage III (pN3) by postoperative pathology according to the 7th edition of the American Joint Committee on Cancer (AJCC) criteria; 3) receiving postoperative adjuvant intensity modulated radiation therapy (IMRT, 50–60Gy) and chemotherapy (2–6 regimens) without nCRT; 4) patients were 18 years of age or older with an Karnofsky Performance Score (KPS) ≥ 70; 5) no severe drug allergy history; 6) normal liver and kidney function; 7) blood tests: WBC ≥ 4.0 G/L, HGB ≥ 110 g/L, PLT ≥ 100 G/L; and 8) heart and lung function were not obviously impaired, and the patients were assessed to be able to tolerate CRT. The exclusion criteria: 1) lost follow-up in 3 months after aCRT; 2) progress before aCRT; 3) delay of more than 15 days during treatment; or 4) a history of cancer at any other site. (Figure 1) The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice.
 |
Figure 1 Flowchart of enrollment. |
Baseline characteristics were obtained from electronic records. All analyses were performed at the Shandong Cancer Hospital, Shandong, China. All patients were assessed by the 7th edition of AJCC in electronic records. We think that there may be some inaccuracies if we reassess stages according to electronic records as part of the surgery process is not very clear. Hence, we use the 7th edition of AJCC. This study was approved by the ethics committee of Shandong Cancer Hospital and written informed consent to use the clinical data for research was obtained from each participant before the medical intervention started.
Time Interval Designation
Time from surgery to aCRT was measured in days from the date of surgery to the first day of aCRT. The time interval was evaluated as a continuous variable. Receiver operator curve (ROC) analysis was used to identify time from surgery to aCRT which was associated with survival. An area under curve (AUC) of 0.566 was obtained and the curve crossed with the diagonal line taking 3-year overall survival as the state variable. Considering that postoperative recovery for too short of a time may indicate worse outcome, a result of 57 days was obtained excluding a small proportion of patients who initiated aCRT within 28 days. The ROC with an AUC of 0.653 is shown in Figure 2. Then all the patients were divided into 3 groups according to postoperative intervals: short interval group (≤28 days, S-Int group), medial interval group (≥29 and ≤56 days, M-Int group) and long interval group (≥57 days, L-Int group).
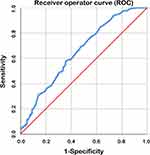 |
Figure 2 Receiver operator curve (ROC) excluding the patients who initiated aCRT within 28 days (4 weeks). The area under curve (AUC) was 0.653. |
The Procedures of Chemoradiation Following Surgery
Surgery
All patients received curative esophagectomy with radical lymph node dissection, without nCRT before surgery. According to the surgery options, patients were divided into two groups: trans-right-chest (IVOR-LEWIS and McKeown surgery, TRC) and trans-left-chest (Sweet surgery, TLC). At least 15 nodes in two- or three-field were removed in radical surgery. The two-field included thoracic and abdominal part and the three-field included thoracic, abdominal and cervical part. The mediastinal lymph nodes were dissected as much as possible to ensure accurate pN staging and radical resection of the disease. Regional lymph node metastases (N) were obtained as follows: N1 indicated metastasis in 1 to 2 regional lymph nodes; N2, metastasis in 3–6 regional lymph nodes.
Radiotherapy
All patients enrolled received radiation by IMRT following surgery, who underwent initial CT simulation before RT. All macroscopic gross disease including primary tumor (GTV-T) and regional lymph node metastases (GTV-nd) were defined as gross tumor volume (GTV), which was determined by all available information from EUS, CT, endoscopy and FDG-PET. GTV-nd for postoperative radiotherapy is prophylactic field, including areas of positive pathological lymph nodes that could not be identified by imaging studies. After plus a 0.8–1.0 cm in all directions, the GTV-T plus a 2.0–3.0 cm and GTV-nd plus a 1.0–1.5 cm radial margin in head-to-fail direction was defined as clinical target volume (CTV). The CTV included subclinical lesions and scopes of possible tumor invasion. The CTV plus a 0.5–1.0 cm margin in all directions was defined as planning target volume (PTV). A total dose of 50–60 Gy was given to these patients in daily fractions of 1.8–2Gy 5 days a week.
Chemotherapy
The chemotherapy usage was grouped into sequential with radiation (SCR) and concomitant CRT (CCR). Both SCR group and CCR group received chemotherapy regimens based on platinum agents. Specifically, there were two main categories of regimens, one was platinum drugs combined with fluorouracil and the other was platinum in combination with paclitaxel or docetaxel. Platinum drugs used for treatment included cisplatin and oxaliplatin. All patients received 2–6 cycles of chemotherapy.
Follow-Up
We followed up by telephone and the telephone numbers were from electronic records. The follow-up continued until April 2020 and all surviving patients were followed up for more than 5 years by then.
Statistical Analysis
Descriptive statistics were calculated for the 3 groups above. Overall survival (OS) was defined as the period from the date of surgery to the date of death. Patients’ characteristics were compared using chi-square test. Survival was assessed by Kaplan-Meier curve and Log rank test. A Cox proportional hazards model was used for univariate and multivariate analyses. Toxic reactions were compared by chi-square test and Fischer exact test was used when necessary. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) software (IBM Corp, Armonk, NY, USA). P values of 0.05 or lower were considered statistically significant.
Results
Patient Characteristics
Overall, a total of 520 patients with pT1-3N1-2 or pT4aN1 TESCC after surgery (R0) meeting the inclusion criteria were enrolled. There were 86 patients (16.5%) receiving adjuvant therapy within 28 days (≤28 days, S-Int group), 223 (42.9%) between 29 days and 56 days (≥29 and ≤56 days, M-Int group) and 211 (40.6%) more than 57 days (≥57 days, L-Int group). The majority of patients were male (73.3%) and the median age was 60 years (range 46 to 78 years). Briefly, 83.8% had an KPS ≥ 80, 87.9% were located in middle and distal third of esophagus, 52.5% received TRC and 45.8% received CCR. The clinicopathologic characteristics in the 3 groups are presented in Table 1 and the numbers of patients in each stage are listed in Table 2.
 |
Table 1 Baseline Characteristics |
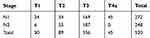 |
Table 2 The Numbers of Patients in Each Stage |
Overall Survival Rates
The median follow-up time was 35.6 months (range, 4.4–137.4months). The 1-, 3-, and 5-year OS rates were 89.8%, 49.5%, and 36.6% respectively. The median OS (mOS) was 35.9 months (95% CI 32.3–39.5 months). The 5-year OS rates of patients receiving aCRT within 28 days (≤28 days, S-Int group), between 29 days and 56 days (≥29 and ≤ 56 days, M-Int group) and more than 57 days (≥57 days, L-Int group) were 32.1%, 43.3% and 31.5% respectively. The mOS of the 3 groups above were 23.6 (95% CI 10.5–36.7), 44.2 (95% CI 34.7–53.7) and 32.0 (95% CI 27.5–36.5) months respectively (M-Int vs S-Int group P=0.020; M-Int vs L-Int P=0.002; S-Int vs L-Int P=0.787) (Figure 3A). Of all these patients, 272 were pathologic N1 (pN1) and 248 pN2. The 5-year OS rates in patients with pN1 and pN2 were 48.6% and 23.4% respectively (P<0.001, HR=0.535, 95% CI 0.436–0.658) (Figure 3B). 30, 89, 356 and 45 patients were pT1, pT2, pT3 and pT4a respectively and 5-year OS rates were 90.0%, 53.3%, 26.3% and 49.5% (P<0.001). There was no significant difference between pT2 and pT4a group (P=0.939) (Figure 3C). The 5-year OS rates in S-Int, M-Int and L-Int were 28.9%, 29.4%, and 15.2% in patients with pN2 (Figure 4B) and those were 34.4%, 55.7% and 47.2% with pN1 respectively (Figure 4A).
 |
Figure 4 (A) Kaplan–Meier Curve grouping by interval in pN1 patients. (B) Kaplan–Meier Curve grouping by interval in pN2 patients. |
Univariate and Multivariate Cox Analyses
Univariate analyses showed that age, KPS, histological grade, the pT stage, the pN stage, surgery option and postoperative interval were significantly associated with overall survival. Sex, tumor location and CRT sequence, number of chemotherapy cycles and hospital choice were variables without significant difference. A multivariate analysis obtained the same conclusion that variables above were independent prognostic factors. In addition, tumor location and CRT sequence were also significantly associated with the survival when the P value was relaxed to 0.1. Detailed data are listed in Table 3.
 |
Table 3 Univariate and Multivariate Cox Analysis of Covariables |
Toxicity
Grade 3 or 4 toxicities were rare and no grade 5 toxicities were observed. The most common RT-related toxicities were esophagitis and leukopenia. We found no significant differences in early or late toxic reactions between the M-Int and L-Int group. M-Int and L-Int groups were then lumped together as a whole to be compared to S-Int. Multiple toxic reactions including grade 1–2 esophagitis, grade 3–4 anemia and grade 1–2 thrombopenia happened obviously more in S-Int than the other two groups. More adverse events joined in including grade 3–4 leukopenia, grade 3–4 neutropenia, grade 1–2 anemia and anastomotic stenosis when the P value was relaxed to 0.1. No statistically significant difference was observed in some other side effects related to chemotherapy such as muscle or joint pain, hair loss, liver or kidney dysfunction or allergic reaction. All available worthy adverse events are listed in Tables 4 and 5.
 |
Table 4 Toxic Reactions by 3 Groups |
 |
Table 5 Toxic Reactions by 2 Groups |
Discussion
This study implies that starting aCRT between 29 and 56 days (5–8 weeks) is associated with significantly improved OS, which will not cause more serious complications. Furthermore, 5-year survival rate reached 44.2 months with such time interval.
To our knowledge, the current study is the first large-scale one to report the association between interval to adjuvant treatment and outcomes in stage III or node positive TESCC after R0 surgery. Our data demonstrated that too short or long a time interval between surgery and adjuvant therapy presented a worse prognosis. The difference was that S-Int produced a bad effect earlier as shown in Figure 3A. Survival is similar to the normal distribution with intervals and M-Int with intervals between 29 and 57 days showed the best outcome. By subgroup analysis, we observed that S-Int appeared to be worse than L-Int in pN1 group and the result was reversed in pN2 group. In terms of toxicity, S-Int presented worse toxicity than the other 2 groups, which may influence the survival finally. Except time interval, our multivariate analysis model displayed some variables such as pT, pN and surgical option were independent prognosis factors, which was broadly consistent with previous research.
The most common types of transthoracic methods are the Ivor Lewis, McKeown, and Sweet surgery and each method includes a thoracotomy.29 The transthoracic approach is a more complete oncologic operation, which provides direct visualization and greater exposure to achieve wider margins around the tumor and more extensive nodal dissection.30 Of course, direct visualization and greater exposure are also the key to correct pathological assessment. Our study revealed that TRC made significant benefits in survival than TLC, which was due to more thorough lymph node dissection in our view, confirming the conclusion of relevant literature.29
On account of poor prognosis by surgery alone, multimodal treatment including RT, chemotherapy and surgery is more required in TESCC. Although nCRT followed by esophagectomy has increasingly emerged as the standard of care for locally advanced ESCC,5–9 adjuvant therapy occupies an irreplaceable position, especially in China. To some degree, the combinations of treatment means are based more on the doctor’s preferences than on strong evidence. Patient selection also plays a key role. CRT prior to surgery is more common in the United States, with perioperative chemotherapy preferred in Europe. In China, patients often given priority for surgery and the complementary treatment are influenced by many factors.
Additional adjuvant therapy after radical esophagectomy has long been controversial with no large-sampled prospective study reported.10–18 Hence, adjuvant therapy is not recommended in the NCCN clinical practice guidelines in patients with R0 surgery.19 However, these aforementioned studies supporting no adjuvant therapy had their limitations: 1) compare surgery alone to addition of postoperative RT or chemotherapy, without further comparison between aCRT and surgery alone; 2) small-sampled, using old radiation techniques and chemotherapy regimens; and 3) confounding factors such as pathological type and R1-2 surgery. A lot of literature20–28 is available in favor of postoperative aCRT when there are high-risk factors such as surgical cut state, lymph node stage, primary tumor stage, low differentiation. In a prospective randomized study, Cao et al.27 evaluated patients with locally advanced esophageal cancer, 74 of whom received postoperative RT (dose, 50 Gy) plus chemotherapy (2 cycles) and 77 received surgery alone. They found that patients in the CRT group had a significantly longer median survival time (53.5 vs 37 months, P<0.05) and significantly lower rates of local recurrence and distant metastasis (P<0.05). Moreover, the complication rates of the 2 groups showed no significant difference.27 The largest study to date carried out by Wong et al.28 detected that OS improved under the use of postoperative aCRT (either sequentially or concomitantly) after esophagectomy for patients with positive margins or node-positive disease.
Based on these studies, postoperative adjuvant therapy may be mandatory following esophagectomy for those receiving surgery without neoadjuvant therapy, who are confirmed to have some risk factors. However, the optimal time from surgery to the adjuvant therapy is unknown so far. A number of studies31–35 reported the association between time intervals from the end of nCRT to surgery with survival and rates of pathologic complete response in patients with esophageal cancer, published evidence on which varied greatly. There are also some reports36–40 focusing on the time intervals from surgical procedure to aCRT in some other solid tumors. Eulenburgy et al.37 investigated the prognostic effect of the time to chemotherapy after surgery in patients with advanced ovarian cancer stratified by residual disease, and provided evidence that survival may slightly improve with early initiation of chemotherapy in patients who undergo complete cytoreduction in advanced ovarian cancer. Gao et al38 investigated how timing of adjuvant chemotherapy affected survival in stage III colon cancer and demonstrated that OS was significantly reduced with delayed adjuvant chemotherapy after 8 weeks but patients might still benefit from adjuvant chemotherapy even with a delay of approximately 5 months.
In contrast, there were hardly any reports on the time after surgery in patients with esophageal cancer. Yang et al.25 reported that radiotherapy of pT3N0M0 EC was initiated 4 to 10 weeks after surgery in their research. A study39 analyzed variables in localized gastric and gastroesophageal junction cancer and concluded that only a handful of patients received adjuvant within 56 days of surgery and delay in adjuvant therapy will not lead to inferior survival. The majority of these studies modeled dichotomous variable for interval due to small sample size or some limited knowledge. They tend to determine a cut-off point artificially and the results varied widely. Tiny differences in intervals could be compared and potential delay-related factors could also be adjusted with further grouping. Indeed, more precise results were reported in several such studies.35,36 In our view, postoperative RT can reduce the recurrence risk in regions that are difficult to clear completely by surgery alone and chemotherapy can reduce recurrence rates of subclinical lesions outside the radiation field and incidence of latent distant metastasis.20 Therefore, patients may benefit from aCRT under the control of toxic and side effects. Furthermore, animal models have shown that removal of the primary tumor can stimulate residual tumor growth and earlier initiation of chemotherapy can offer a significant advantage in preventing systemic relapse, as well as in growth suppression of the residual tumor.40–42 From this point of view, excessive delay of CRT is bound to cause a worse prognosis. Of course, adjuvant therapy may also do more harm than good on patients who receive too early aCRT to recover from surgery. The data available showed that those who started earlier treatment had no complications such as pneumonia but increased myelosuppression, which did not seem to have too many impacts on survival. Besides, the number of cycles of chemotherapy did not decrease in the group that started earlier. We speculate that late toxic reactions may not be accurately evaluated and some long-term, not-recorded effects may occur outside the hospitals. As for the chemotherapy cycles, there may be some delays during therapy but the regimens were achieved as far as possible through some supportive means. In our study, latency time was divided into 3 groups on the basis of a pre-analysis and some previous research to avoid rough segmentation. We found a prediction model similar to normal distribution which may provide some reference for clinical practice and future prospective control trials.
There are several limitations that should be acknowledged. Firstly, we did not collect more detailed information to understand the exact reason why adjuvant therapy was delayed. Secondly, chemotherapy regimens varied in these patients and there existed some delays adjustment in the implementation of aCRT after the initiation of it according to clinical practice, although patients with obviously different treatment were excluded. Moreover, we did not take the follow-up treatment after aCRT into account which may affect survival. Finally, a multi-centered, prospective, randomized study with unique chemotherapy regimen and target volume and dose of RT is warranted to verify the results given the natural limitations of retrospective analysis.
In conclusion, for patients whose pathological TNM stage are T1-3N1-2M0 or T4aN1M0 without neoadjuvant therapy should receive aCRT between 29 and 56 days (5–8 weeks) after esophagectomy with proper surgery option under normal physical conditions.
Funding
National Natural Science Foundation of China (81972864); Academic Promotion Program of Shandong First Medical University (2019RC002); Science and Technology Support Plan for Youth Innovation Teams of Universities in Shandong Province (2019KJL001); Science and Technology Plan of Jinan (201907113).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.21492
2. Chen W, Zheng R, Baade, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi:10.3322/caac.21338
3. Herskovic A, Russell W, Liptay M, et al. Esophageal carcinoma advances in treatment results for locally advanced disease: review. Ann Oncol. 2012;23:1095–1103. doi:10.1093/annonc/mdr433
4. Controversies in the multimodality management of locally advanced esophageal cancer: evidence-based review of surgery alone and combined-modality therapy. Ann Surg Oncol. 2004;11:665–673. doi:10.1245/ASO.2004.10.026
5. van Hagen P, Hulshof MCCM, van Lanschot JJB. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. doi:10.1056/NEJMoa1112088
6. Walsh TN, Grennell M, Mansoor S, Kelly A. Neoadjuvant treatment of advanced stage esophageal adenocarcinoma increases survival. Dis Esophagus. 2002;15(2):121–124. doi:10.1046/j.1442-2050.2002.00214.x
7. Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12(7):681–692. doi:10.1016/S1470-2045(11)70142-5
8. Bedenne L, Michel P, Bouche O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25(10):1160–1168. doi:10.1200/JCO.2005.04.7118
9. Yang H, Liu H, Chen Y, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a Phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. 2018;36(27):2796–2803. doi:10.1200/JCO.2018.79.1483
10. Teniere P, Hay JM, Fingerhut A, et al. Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle and lower esophagus as shown by a multicentered controlled trial. French University Association for Surgical Research. Surg Gynecol Obstet. 1991;173:123–130.
11. Fok M, Sham JS, Choy D, et al. Postoperative radiotherapy for carcinoma of the esophagus: a prospective, randomized controlled study. Surgery. 1993;113:138–147.
12. Xiao ZF, Yang ZY, Liang J, et al. Value of radiotherapy after radical surgery for esophageal carcinoma: a report of 495 patients. Ann Thorac Surg. 2003;75:331–336.
13. Yamamoto M, Yamashita T, Matsubara T, et al. Reevaluation of postoperative radiotherapy for thoracic esophageal carcinoma. Int J Radiat Oncol Biol Phys. 1997;37:75–78. doi:10.1016/S0360-3016(96)00473-7
14. Zieren HU, Muller JM, Jacobi CA, et al. Adjuvant postoperative radiation therapy after curative resection of squamous cell carcinoma of the thoracic esophagus: a prospective randomized study. World J Surg. 1995;19:444–449. doi:10.1007/BF00299187
15. Malthaner RA, Wong RKS, Rumble RB, et al. Members of the gastrointestinal cancer disease site group of cancer care ontario’s program in evidence- based care. Neoadjuvant or adjuvant therapy for resectable esophageal cancer: a systematic review and meta-analysis. BMC Med. 2004;2::35.
16. Ando N, Iizuka T, Kakegawa T, et al. A randomized trial of surgery with and without chemotherapy for localized squamous carcinoma of the thoracic esophagus: the Japan Clinical Oncology Group Study. J Thorac Cardiovasc Surg. 1997;114:205–209. doi:10.1016/S0022-5223(97)70146-6
17. Pouliquen X, Levard H, Hay JM, et al. 5-Fluorouracil and cisplatin therapy after palliative surgical resection of squamous cell carcinoma of the esoph- agus. A multicenter randomized trial. French Associations for surgical research. Ann Surg. 1996;223:127–133.
18. Ando N, Iizuka T, Ide H, et al. Japan Clinical Oncology Group. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study—JCOG9204. J Clin Oncol. 2003;21:4592–4596. doi:10.1200/JCO.2003.12.095
19. Ajani JA, D’Amico TA, Almhanna K, et al. Esophageal and esophagogastric junction cancers, version 1.2017. J Natl Compr Canc Netw. 2017.
20. Chen J, Pan J, Zheng X, et al. Number and location of positive nodes, postoperative radiotherapy, and survival after esophagectomy with three- field lymph node dissection for thoracic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2012;82:475–482. doi:10.1016/j.ijrobp.2010.08.037
21. Schreiber D, Rineer J, Vongtama D, et al. Impact of postoperative radiation after esophagectomy for esophageal cancer. J Thorac Oncol. 2010;5:244–250.
22. Be´dard EL, Inculet RI, Malthaner RA, et al. The role of surgery and postoperative chemoradiation therapy in patients with lymph node positive esophageal carcinoma. Cancer. 2001;91:2423–2430. doi:10.1002/1097-0142(20010615)91:12<2423::AID-CNCR1277>3.0.CO;2-1
23. Rice TW, Adelstein DJ, Chidel MA, et al. Benefit of postoperative adjuvant chemoradiotherapy in locoregionally advanced esophageal carcinoma. J Thorac Cardiovasc Surg. 2003;126:1590–1596. doi:10.1016/S0022-5223(03)01025-0
24. Shridhar R, Weber J, Hoffe SE, et al. Adjuvant radiation therapy and lymphadenectomy in esophageal cancer: a SEER database analysis. J Gastrointestinal Surg. 2013;17:1339–1345. doi:10.1007/s11605-013-2192-7
25. Yang J, Zhang W, Xiao Z, et al. The impact of postoperative conformal radiotherapy after radical surgery on survival and recurrence in pathologic T3N0M0 esophageal carcinoma: a propensity score-matched analysis. J Thor Oncol. 2017;12(7):1143–1151.
26. Hsu PK, Huang CS, Wang BY, et al. Survival benefits of postoperative chemoradiation for lymph node-positive esophageal squamous cell carcinoma. Ann Thorac Surg. 2014;97:1734–1741. doi:10.1016/j.athoracsur.2013.12.041
27. Cao XF, Lu¨ J, Zhu B, et al. A prospective comparison between surgery alone and postoperative chemoradiotherapy for locally advanced esophageal squaraous cell carcinoma. Zhonghua Zhong Liu Za Zhi. 2010;32:452–455.
28. Wong AT, Shao M, Rineer J, et al. The impact of adjuvant postoperative radiation therapy and chemotherapy on survival after esophagectomy for esophageal carcinoma. Ann Surg. 2017;265(6):1146–1151.
29. Lerut T, Coosemans W, Decker G, et al. Surgical techniques. J Surg Oncol. 2005;92(3):218–229.
30. Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347(21):1662–1669. doi:10.1056/NEJMoa022343
31. Haisley KR, Laird AE, Nabavizadeh N, et al. Association of intervals between neoadjuvant chemoradiation and surgical resection with pathologic complete response and survival in pa- tients with esophageal cancer. JAMA Surg. 2016;151:e162743. doi:10.1001/jamasurg.2016.2743
32. Lin G, Han SY, Xu YP, Mao WM. Increasing the interval between neoadjuvant chemoradiotherapy and surgery in esophageal cancer: a meta-analysis of published studies. Dis Esophagus. 2016;29:1107e14. doi:10.1111/dote.12432
33. Kathiravetpillai N, Koeter M, van der Sangen MJ, et al. Delaying surgery after neoadjuvant chemoradiotherapy does not significantly influence postoperative morbidity or oncological outcome in patients with oesophageal adenocarcinoma. Eur J Surg Oncol. 2016;42:1183e90. doi:10.1016/j.ejso.2016.03.033
34. Ruol A, Rizzetto C, Castoro C, et al. Interval between neo- adjuvant chemoradiotherapy and surgery for squamous cell carcinoma of the thoracic esophagus: does delayed surgery have an impact on outcome? Ann Surg. 2010;252:788e96. doi:10.1097/SLA.0b013e3181fc7f86
35. van der Werf LR, Dikken J, L van der Willik, et al. Time interval between neoadjuvant chemoradiotherapy and surgery for oesophageal or junctional cancer: a nationwide study. European. J Cancer. 2018;91:76–85.
36. Martella A, Hong JC, Foote J, Havrilesky L, Gaillard S, Chino JP. The impact of time to treatment initiation for adjuvant chemotherapy and radiation therapy in stage III endometrial cancer: a national cancer data base study. Int J Radiat Oncol Biol Phys. 2016;96:E287. doi:10.1016/j.ijrobp.2016.06.1346
37. Eulenburg MC, Staehle A, Wegscheider K, et al. Prognostic impact of the time interval between surgery and chemotherapy in advanced ovarian cancer: analysis of prospective randomised phase III trials. Eur J Cancer. 2013;49:142–149. doi:10.1016/j.ejca.2012.07.023
38. Gao P, Huang X-Z, Song Y-X, et al. Impact of timing of adjuvant chemotherapy on survival in stage III colon cancer: a population-based study. BMC Cancer. 2018;18::234.
39. Ahmed S, Iqbal N, Sunil Y, et al. Time to adjuvant therapy and other variables in localized gastric and gastroesophageal junction (GEJ) cancer (IJGC-D-13-00162). J Gastrointest Canc. 2014;45:284–290.
40. Bell RS, Roth YF, Gebhardt MC, et al. Timing of chemotherapy and surgery in a murine osteosarcoma model. Cancer Res. 1988;48:5533–5538.
41. Fisher B, Gunduz N, Saffer EA. Influence of the interval between primary tumor removal and chemotherapy on kinetics and growth of metastases. Cancer Res. 1983;43:1488–1492.
42. Gunduz N, Fisher B, Saffer EA. Effect of surgical removal on the growth and kinetics of residual tumor. Cancer Res. 1979;39:3861–3865.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

