Back to Journals » Clinical Epidemiology » Volume 14
Time Trends in Incidence of Reported Memory Concerns and Cognitive Decline: A Cohort Study in UK Primary Care
Authors Hallam B , Petersen I , Cooper C, Avgerinou C, Walters K
Received 2 December 2021
Accepted for publication 11 February 2022
Published 24 March 2022 Volume 2022:14 Pages 395—408
DOI https://doi.org/10.2147/CLEP.S350396
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Henrik Sørensen
Brendan Hallam,1 Irene Petersen,1 Claudia Cooper,2 Christina Avgerinou,1 Kate Walters1
1UCL Research Department of Primary Care & Population Health, University College London, London, UK; 2Division of Psychiatry, University College London, London, UK
Correspondence: Brendan Hallam, UCL Research Department of Primary Care & Population Health, University College London, London, UK, Email [email protected]
Purpose: To investigate time trends in incidence of recorded memory concerns (MC) and cognitive decline (CD) in a UK older population presenting to primary care with no prior diagnosis of dementia. To determine the risk of developing dementia in people with recorded memory concern and cognitive decline.
Patients and methods: We included individuals aged 65– 99 years who contributed to data within the IQVIA medical research database from 1st January 2009 to 31st December 2018. We reported crude incidence rates for MC (study population n=1,310,838) and CD (n=1,348,796). We conducted survival analysis to estimate the risk of developing dementia using fine-grey sub-distribution hazard model with competing risk of death.
Results: We identified 55,941 individuals (4.3%) with a record of incident MC; rates were fairly stable over the decade of study. We identified 14,869 people (1.1%) with a record of incident CD, and these rates increased from 1.29/1000 PYAR (95% CI 1.21 to 1.38) in 2009 to 3.49/1000 PYAR (95% CI 3.30 to 3.68) in 2018. Within 3 years of follow up from the first record of MC, 45.5% of individuals received a diagnosis of dementia, while of those with a record of CD, 51.7% received a dementia diagnosis. Women, people in older age groups and those living in more deprived areas were more likely to have a record of MC or CD, and their symptoms were more likely to progress to a dementia diagnosis.
Conclusion: Incidence rates of MC and CD estimated from routinely collected primary care data are lower than those reported in community surveys, suggesting that a minority of people who experience memory loss consult their GP and have it recorded. Our findings indicate that those who do report concerns to primary care, especially women, those in older age groups and those in more deprived areas, are at a higher risk for developing dementia.
Keywords: primary care, memory, dementia, incidence rate, survival analysis
Introduction
Dementia is a syndrome with progressive deterioration in cognitive functioning, which can impact memory, judgement, language and other functions of cognition.1,2 Fifty million people are currently estimated to be living with dementia worldwide, and this is projected to rise to 152 million over the next 30 years. Many national dementia strategies3,4 acknowledge memory concerns that are not dementia as a point of intervention for primary care to deliver advice on dementia awareness and healthier lifestyle. The initial memory concerns, when not part of the presentation of dementia itself, can also be referred to as subjective memory complaints (SMC) (where no clear impairment is found by objective psychometric testing) or mild cognitive impairment (MCI), where there is objective evidence of decline, which is not severe enough to interfere with daily activities and be defined as dementia.5 Community-based studies have found that SMC affects approximately one-quarter of people 65–69 years old and rises to over half of people aged 90+.6,7 Cognitive impairment (including MCI) affects up to one in five people over 65.8,9 Mitchell10 published a meta-analysis of 28 studies and found that people with SMC (approximately 2.2–6.6%) are twice as likely to go on to develop dementia after 5 years, in comparison to cognitively healthy older adults.10,11
While there is some understanding of the characteristics of people with SMC and MCI in screened populations in the community, there have only been a small number of studies investigating people who present with these complaints to primary care. Juncos-Rabadan et al12 reported a prevalence of MCI of 31.4%, in a sample (n = 689) of people attending primary care with memory complaints. However, when Bohlken and Kostev13 investigated coded prevalence of MCI across a larger sample of approximately one million patients from 432 German primary care practices, they identified 818 patients coded with MCI (0.08%), which corresponds to less than 10% of its true community prevalence. They also noted a change in practice, with a 505% increase in the identification and coding of MCI from 2007 to 2017. Other research published on MCI incidence in primary care is limited14 and, to our knowledge, there are none exploring incidence of memory loss symptoms and cognitive decline.
The current study aims to investigate, for the first time to our knowledge, the incidence of reported memory concern and cognitive decline events presenting to GPs in UK primary care; and to explore the prognostic significance of reports in terms of likelihood of subsequently developing dementia. The study will:
- Examine the incidence of recording of memory concern and cognitive decline in primary care records, between 2009–2018 by calendar year and by sociodemographic factors such as age, sex and social deprivation.
- Examine the survival time to dementia diagnosis from the date of either a memory concern or cognitive decline event being recorded on patients’ records.
Methods
For the STROBE criteria15 of the current study, please see Supplementary Table 1.
Study Design
A retrospective cohort study design was used.
Data Source
We used the IQVIA medical research database, previously known as The Health Improvement Network (THIN). IQVIA collects over 18 million anonymised patient records from over 790 practices across UK primary care. These longitudinal records include patients’ medical conditions, symptoms, diagnoses and prescriptions, which are recorded during their general practice consultations. Practices within IQVIA that met the quality criteria for acceptable mortality reporting (AMR)16 and acceptable computer usage (ACU) were included.17 The participating practices are largely representative of UK practices regarding patients’ demographics such as age, sex, practice size and geographical distribution.18 Social deprivation is measured using the Townsend Deprivation Score from linked UK 2011 census data.19 This is a composite score of levels of owner occupation, unemployment, car ownership and overcrowding in the local neighbourhood based on the patient’s postal (zip) code.20 Results are displayed in quintiles, with a score of 5 being from an area most deprived and 1 being from an area least deprived. The Read code system is used within THIN to record symptoms and diagnoses.21,22
Ethics Approval
Use of the IQVIA medical research database for scientific research was obtained and approved by IQVIA World Publications Scientific Review Committee in October 2020 (reference 20SRC058).
Study Population
Individuals aged 65–99 years who were registered with a GP practice contributing data to IQVIA medical research database from 1st January 2009 to 31st December 2018 were included. Individuals without a recorded memory concern or a diagnosis of dementia at baseline were included for the memory concern incidence cohort. For the cognitive decline cohort, individuals without a record of cognitive decline or dementia prior to baseline were included. We excluded those with recorded memory concern or cognitive decline within 6 months of registering with a practice. The 6-month threshold was selected based on Lewis plots to ensure only incident cases were captured.23 During the first few months after a patient’s registration with a GP, as a result of their past medical history notes being transferred to the new practice, they may appear as a new case of a condition or disease although they may have in fact been diagnosed with this condition in the past.23 This means that the perceived incidence rate of events is expected to be artificially high in electronic health data due to prevalent cases being incorrectly identified as incident cases during the first few months from registration. Therefore, we used Lewis plots to identify the point in time from which the incidence rate of an event stabilised, indicating a more reliable estimated incidence rate. The Lewis plot is available in Supplementary Figure 1.
Index Date for Study Observation Period
Incidence
The study was a dynamic cohort, meaning individuals could enter and leave the study cohort at any time point during the study period (1st January 2009 to 31st December 2018). Therefore, there was no single fixed time point on entry and the follow-up length for individuals varied. The index date (start of follow up) was defined as the latest date from: the start of the study period (1st January 2009), individual’s 65th birthday, date from when they permanently registered at general practice after 6 months, date when general practice met acceptable mortality reporting (AMR) criteria and date when general practice met acceptable computer usage (ACU) criteria.
Survival Analysis
The index date criteria for the time-to-dementia survival analysis were the same as for the incidence study, but also included the date of the first recorded memory concern or cognitive decline code.
Exit Date for Study Observation Period
Incidence
For each individual, the date of exit from the study cohort was defined as the earliest date from: End of the study period (31st December 2018), the individual’s 99th birthday, death, leaving the practice, when general practice stopped contributing to IQVIA medical research database and date of first memory concern or cognitive decline event (if occurred).
Survival Analysis
The exit date is the same as for the incidence study, except the date of first memory concern or cognitive decline event is replaced with the date of first dementia event (if occurred).
Outcomes and Covariates
Main Outcomes
To identify cases of reported memory concern, cognitive decline and dementia, we used Read codes updated from a previous study investigating cognitive impairment.24 Read code selection was agreed by a panel including 2 GPs and 1 consultant old age psychiatrist.
Objectives 1 and 2: Memory Concern and Cognitive Decline
We defined “memory concern” codes as those that GPs might typically use to note that memory loss symptoms were discussed during a consultation, including subjective memory concerns (please see Supplementary Table 2). We defined “cognitive decline” as codes indicative of cognitive impairment (implying an informal or formal cognitive assessment has taken place by a clinician) that is below the expected decline of cognition for that age, including mild cognitive impairment (MCI) as a subset of these codes (please see Supplementary Table 3).
Objective 3: Dementia
The panel identified codes that were likely to be specific for diagnosis, for the prescription of anti-dementia drugs (Donepezil, Memantine, Rivastigmine and Galantamine) and codes for annual reviews or monitoring of dementia (see Supplementary Table 4 for dementia case definition).
Covariates
Age band, sex, Townsend Deprivation Index20 and calendar year were considered as potential covariates of interest. Age bands 65–69, 70–79 and 80–99 were selected, with a wider age band for the oldest group reflecting the relatively smaller sample and to provide more accurate and reliable results for the oldest old population. Townsend deprivation score is 5-point interval data.
Statistical Analysis
Incidence Study
We calculated crude incidence for memory concern and cognitive decline per 1000 person-years at risk (PYAR), for the whole population at risk and by age band, calendar year, sex and Townsend deprivation score. To further examine differences of incidence between groups, incidence rate ratios (IRR) were calculated by fitting a negative binomial regression model to account for overdispersion in count data. The model was adjusted for each of the covariates: age band, sex, calendar year and Townsend score. In addition, the model was adjusted for general practices to account for clustering by practice in recording of memory concern and cognitive decline.
Survival Analysis
Survival analysis of time to incident dementia from each recorded cognitive group was calculated using Cox proportional hazards model and adjusted for sex, age band, Townsend score and practice. The memory concern and cognitive decline cohorts were analysed in separate models. If individuals had both a memory concern event and cognitive decline event, they were categorised in both groups. Cognitive records that occurred in 2018 were excluded to allow at least one year follow up, as the study ended in December 2018. Since risk of mortality increases within each older age band, cumulative incidence rates (CIR) were estimated for incident dementia and competing risk of death to compare people with recorded memory concern vs people with recorded cognitive decline. CIR was calculated using Fine and Gray’s semiparametric proportional sub-distribution hazards competing risk model and adjusted for sex, age band, Townsend and practice. Interactions between memory concern and cognitive decline with covariates were checked and included in the model if clinically and statistically significant. Both the Cox proportional hazard model and the Fine and Grey competing risk model are presented in results. A 3-year follow up was selected based upon the maximum number of years in which there was a large enough sample to provide valid and meaningful results.
Results
Incidence Results
Memory Concern
Overall 1,440,906 patients fitted the study criteria and did not have recorded dementia or memory concern prior to start of study. Of this study population, 1,310,838 patients had complete data on sex, age, and Townsend deprivation score and were included in the primary analysis. Of these, 55,941 (4.3%) incident memory concern events were reported (see Table 1 and Supplementary Figure 2). This was equivalent to an overall incidence rate of 9.12/1000 PYAR (95% CI 9.05 to 9.20) for memory concern.
 |
Table 1 Summary of Variables in the Main Analysis for Memory Concern and Cognitive Decline |
Recorded memory concern remained fairly stable over time, from 2009 (8.43/1000 PYAR, 95% CI 8.21 to 8.64) to 2018 (7.63 /1000 PYAR 95% CI (7.36 to 7.92) (Table 2 and Figure 1). Memory concern records increased with age, from 65–69 years old (3.66/1000 PYAR, 95% CI 3.58 to 3.75) to the oldest age band of 80–99 (17.89/1000 PYAR, 95% CI 17.68 to 18.10) (see Figure 2). There was an increase in reporting memory concern in each Townsend quintile from the least (9.05/1000 PYAR, 95% CI 8.91 to 9.20) to the most deprived quintile (10.51/1000 PYAR, 95% CI 10.26 to 10.77). Females (9.72/1000 PYAR, 95% CI 9.61 to 9.82) had a higher incidence of recorded memory concern events compared to males (8.50/1000 PYAR, 95% CI 8.39 to 8.61).
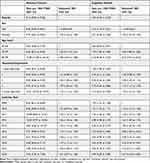 |
Table 2 Recording Rate of Individual Cognitive Groups by Socio-Demographics Factors (Complete Case) |
 |
Figure 1 Incidence Rate (1,000 PYAR) for Memory Concern and Cognitive Decline Between 2009 to 2018. |
 |
Figure 2 Incidence Rate (1,000 PYAR) for Memory Concern and Cognitive Decline by Ageband. |
Cognitive Decline
In total 1,468,975 patients fitted the study criteria, of whom 1,336,992 had complete data and were included in the analysis. Of these, 14,869 (1.1%) incident cognitive decline events were reported from 1,336,992 patients (see Table 1 and Supplementary Figure 3), equating to an incidence rate of 2.35/1000 PYAR (95% CI 2.32 to 2.39). Of the total 14,869 cognitive decline events, 5704 (38,4%) people with cognitive decline also had an overlapping recorded memory concern event (see Figure 3).
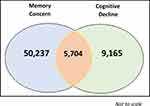 |
Figure 3 Venn Diagram of Number of People with Memory Concern and Cognitive Decline Records. |
Recorded cognitive decline increased with time, from 2009 (1.32/1000 PYAR, 95% CI 1.24–1.41) to 2018 (3.50/1000 PYAR 95% CI 3.32–3.69) (Table 2 and Figure 1). Cognitive decline records increased with age, from 65–69 years old (0.65/1000 PYAR, 95% CI 0.61–0.68) to the oldest age band of 80–99 (5.17/1000 PYAR, 95% CI 5.06–5.28) (see Figure 2). There was an increase in reporting cognitive decline in each Townsend quintile from the least (1.96/1000 PYAR, 95% CI 1.89 to 2.02) to the most deprived quintile (3.15/1000 PYAR, 95% CI 3.01 to 3.29). Females (2.51/1000 PYAR, 95% CI 2.46 to 2.56) had a higher incidence of recorded cognitive decline events compared to males (2.15/1000 PYAR, 95% CI 2.10 to 2.21).
Incidence Risk Ratio
Adjusted incidence risk ratios (IRR) from multivariable negative binomial regression are presented in Table 2.
Memory Concern
The risk increased with each age band from age 65–69 (reference 1) to 70–79 (IRR 2.25, 95% CI 2.19 to 2.31) and 80–99 (IRR 4.85 95% CI 4.72 to 4.98). Incidence for memory concern was stable across quintiles 2–3 compared to quintile 1 (least deprived area). However, incidence for memory concern in quintile 4 and quintile 5 (most deprived area) (IRR 1.16, 95% CI 1.12 to 1.20) increased and was higher than all other deprivation quintiles. Incidence was found to be slightly higher for women (IRR 1.04, 95% CI 1.02–1.06) compared to men but the difference is minimal and may not be clinically important.
Cognitive Decline
Reported cognitive decline incidence was higher from 65–69 (reference 1) to 70–79 (IRR 3.07, 95% CI 2.88 to 3.26) and 80–99 (IRR 8.23, 95% CI 7.74 to 8.74). There appeared to be a significant difference by deprivation quintile for quintile 3–5, rising to IRR 1.22 (95% CI 1.15 to 1.30) for those living in most deprived areas compared to least deprived. There was no difference in reporting cognitive decline between men (reference) and women (IRR 1.03, 95% CI 1.00–1.07).
Survival Analysis
A total of 47,246 memory concern and 11,373 cognitive decline events met the criteria for inclusion in the survival analysis (please see Supplementary Figure 4 for details). Table 3 outlines the social demographic characteristics for people with a record of memory concern and record of cognitive decline. After 3 years of follow up from first record of memory concern, 45.5% of individuals received a recorded diagnosis of dementia. For people with a record of cognitive decline, 51.7% received a recorded diagnosis of dementia within three years.
 |
Table 3 Baseline Summary of Complete Case Variables in the Survival Analysis for Cognitive Groups |
Table 4 shows two Cox multivariable regression models for risk of developing dementia, 3 years after the individuals’ first record of either memory concern or cognitive decline. Both models were adjusted for age, sex, Townsend deprivation score and practice clustering. Table 4 also shows the adjusted Fine and Gray competing risk model to account for competing risk of mortality and is the primary analysis. For individuals with a memory concern record, the likelihood of developing dementia increases with age (80–99; SHR 3.37 95% CI 3.14 to 3.63) (see Figure 4 for survival analysis graph). This is also the case for individuals with cognitive decline (80–99; SHR 1.75 95% CI 1.53 to 2.01). Across both memory concern and cognitive decline, females are at an increased risk of developing dementia (SHR 1.17, 95% CI 1.14 to 1.22) compared to males. The risk of developing dementia, from memory concern, increases in more deprived areas (Quintile 5, SHR 1.19, 95% CI 1.11 to 1.26) compared to the least deprived areas. However, when people receive a record of cognitive decline, there appears to be little difference in the risk of developing dementia between the least and most deprived areas (SHR 1.01 95% CI 0.91 to 1.11). Interactions between age band, Townsend and sex were tested but no clinically meaningful interactions were identified.
 |
Table 4 Separate Multivariate Regression Models of Risk of Dementia for People with Memory Concern and Cognitive Decline |
 |
Figure 4 Survival Analysis for Risk of Dementia in People with Memory Concern and Cognitive Decline. |
Discussion
The present study has demonstrated that recorded memory concerns have remained fairly stable over a 10-year period. Cognitive decline is recorded less often compared to memory concerns but has increased over the same 10-year period. After 3 years of follow-up from the first record of memory concern, nearly half of individuals got a diagnosis of dementia. For people with a record of cognitive decline, just over half went on to receive a diagnosis of dementia within a 3-year follow up. Therefore, whether an individual is coded as having memory concern or cognitive decline, it is important to highlight they are both at high risk of going on to receive a dementia diagnosis. For some of these it may be that they are already presenting with initial symptoms of dementia when recorded with memory concern or cognitive decline but are yet to receive a formal diagnosis. The gap between the first record of memory concern or cognitive decline and a dementia diagnosis may represent an opportunity for early intervention and support, if it has not already been provided. Advice on non-pharmacological interventions, such as aerobic exercise, could be delivered and has been highlighted to reduce cognitive decline in older people.25
The results of the present study illustrate that memory loss coding was stable over the last decade, whereas cognitive decline recording increased. The current incidence results contrast with previous literature investigating memory loss and cognitive decline recording. Iliffe and Wilcock illustrated that cognitive decline recordings remained fairly stable and memory concern recordings increased between 1995 and 2010.26 One potential explanation could be variation in use of the coding and case definition for each exposure over time. Secondly, the years examined are different and several important policies were implemented over the last decade that are important to consider. The Department of Health in the UK launched the National Dementia Strategy in 2009 to improve access to specialist assessment and highlighted the issue of under-diagnosis. In 2012, the Challenge on Dementia was published to build on the national dementia strategy with a focus on improving diagnosis rates of dementia. Three years later, in 2015, the government and NHS produced a mandate for England to be amongst the best in Europe for diagnosis of people with dementia by 2020. Publishing three key national dementia strategies within this 10-year period may have raised awareness of dementia and the need to assess and record cognitive function, and thus may explain the increase in use of recorded cognitive decline in primary care. With a financial incentive to identify and diagnose dementia,27 it is plausible that this would result in an increase in identification of mild cognitive impairment and impaired cognition during assessment. There is also an increasing recognition of cognitive decline over the past decade which may also explain the increase in recordings.28 However, the recording of memory concerns has remained stable and even declined slightly. Recording of memory concern is more in alignment with trends in dementia incidence, which has declined by 13% per decade over the past 25 years.29
The earlier that primary care can recognise and identify people with memory problems, the earlier dementia prevention strategies can be provided (eg aerobic exercise, diet, blood pressure, social stimulation etc.) within primary care.14,30 Whilst many of these strategies would be delivered to older people as general health advice, many people who experience memory concern want help to understand how lifestyle and other non-pharmacological interventions might help reduce cognitive decline.31 In turn, this can potentially increase motivation for behaviour change. The prevalence of memory problems recorded in primary care is lower than its true prevalence in the community.13,32,33 There are several potential explanations, including recognition of a problem by older people and decisions to seek help for memory concerns, and under-identification, acknowledgement and recording in primary care. Without a formal assessment, GPs may be reluctant to record a definitive diagnosis of dementia on patients’ records and may use cognitive decline Read codes instead. However, evidence suggests that some people recorded with memory loss and cognitive decline actually have dementia after a detailed records review.34 However, as Russell et al34 highlight, one factor that can be improved is the correct use of Read codes and continued monitoring thereafter. Memory problems in any patient over the age of 65, whether subjective or objective, should be coded. Improvement in the recording of memory problems can lead to an improvement in monitoring these people at high risk of dementia. These people at high risk include patients who present to primary care at an older age, female and living in areas of highest deprivation. However, these groups commonly miss out on services and so it is crucial to recognise the importance of addressing inequalities in care. This could in turn lead to enhanced dementia prevention strategies targeting populations at risk. Clinicians could also become more aware and proactive in facilitating a formal cognitive assessment so that affected individuals can receive a timely diagnosis and be managed appropriately as early as possible.
Strengths and Limitations
One potential limitation of the present study is the measurement error of GP recording for memory concerns and cognitive decline Read codes; however, the findings do reflect real world practice in the recording of these issues. Variation in which GPs record memory concerns means that not all people who present to GPs with memory concerns may be captured. Additionally, one GP may use a memory concern code and another GP may use a cognitive decline code for the same person. There is therefore a limitation of not knowing the reasoning or context behind GPs’ decisions to record the code. The code may be recorded where memory is the main concern or where it is raised in the context of a consultation for a different reason. It is possible that those who consult frequently for other reasons (eg multiple long-term conditions) have more opportunities to be coded by their GP with memory concerns. However, it is also possible that having memory concerns or mild cognitive impairment influences attendance at the GP. The current study addresses potential variation between GP practices by adjusting for GP practice clusters in the analysis. However, differences in individual-level variation in recording by doctors within practices could not be accounted for. Another limitation to consider is that 9% of the study population had missing data for Townsend deprivation, which was therefore not included in the incidence and survival analysis investigations. Townsend deprivation score is an area-level measure of deprivation and does not capture individual-level deprivation. A further limitation to consider is the potential risk of bias in underestimating incident dementia cases due to selective drop out from the electronic health database. However, research has highlighted that the IQVIA medical research database has a reliable estimate that is representative of dementia diagnoses within the general UK population.35 Additionally, there may be other factors (such as education) which would have been included but a limitation of the database is that it does not record educational history.
Future Research
Future research is needed to better understand the discrepancy between rates of memory symptoms and cognitive decline in the general population and those recorded in primary care. A better understanding of the characteristics of individuals who present to primary care with memory concern and cognitive decline is also needed. Further research could also explore the risk of dementia in those with cognitive decline and memory concerns versus those without in the general population, accounting for potential confounders such as other long-term health conditions that might influence coding of memory concerns.
Conclusion
Primary care is in an optimal position to identify people with memory concerns and cognitive decline. The current study highlighted that the time trends for recorded memory concern stayed stable between 2009–2018 but recorded cognitive decline increased, particularly in the oldest old group. Memory concerns and cognitive decline not only are hallmark symptoms of dementia, but also predict a high risk of developing dementia, and around half of people with recorded memory concern and those with cognitive decline will develop dementia within three years of the memory concern or cognitive decline being recorded. Improved identification of first symptoms is necessary to allow timely diagnosis of dementia and deliver tailored interventions.
Acknowledgments
The authors would like to thank the researchers’ part of the THIN support group within the Department of Primary Care and Population Health at University College London for feedback on and support for the project.
Funding
Brendan Hallam is a PhD student funded by the Economic & Social Research Council’s London (UBEL) Doctoral Training Partnership, embedded within the APPLE-TREE programme (Project reference: ES/S010408/1).
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organisation. Dementia World Health Organisation. World Health Organisation; 2020 [updated September 21, 2020]. Available from: https://www.who.int/news-room/fact-sheets/detail/dementia.
2. Alzheimer’s Society. The dementia guide: living well after diagnosis. Alzheimer’s Society; 2017. Available from: https://www.alzheimers.org.uk/sites/default/files/pdf/the_dementia_guide.pdf.
3. Department of Health. Living well with dementia: a national dementia strategy. Department of Health; 2009.
4. World Health Organisation. Global status report on the public health response to dementia; 2021. Available from: https://www.who.int/publications/i/item/9789240033245.
5. Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367(9518):1262–1270. doi:10.1016/S0140-6736(06)68542-5
6. Montejo P, Montenegro M, Fernández MA, et al. Subjective memory complaints in the elderly: prevalence and influence of temporal orientation, depression and quality of life in a population-based study in the city of Madrid. Aging Ment Health. 2011;15(1):85–96. doi:10.1080/13607863.2010.501062
7. Luck T, Roehr S, Rodriguez FS, et al. Memory-related subjective cognitive symptoms in the adult population: prevalence and associated factors–results of the LIFE-Adult-Study. BMC Psychol. 2018;6(1):23. doi:10.1186/s40359-018-0236-1
8. Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med. 2013;29(4):753–772. doi:10.1016/j.cger.2013.07.003
9. Overton M, Pihlsgård M, Elmståhl S. Prevalence and incidence of Mild Cognitive Impairment across subtypes, age, and sex. Dement Geriatr Cogn Disord. 2019;47(4–6):219–232. doi:10.1159/000499763
10. Mitchell AJ. The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta‐analysis. Int J Geriatr Psychiatry. 2008;23(11):1191–1202. doi:10.1002/gps.2053
11. Mitchell A, Beaumont H, Ferguson D, et al. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta‐analysis. Acta Psychiatr Scand. 2014;130(6):439–451. doi:10.1111/acps.12336
12. Juncos‐Rabadán O, Pereiro AX, Facal D, et al. Prevalence and correlates of mild cognitive impairment in adults aged over 50 years with subjective cognitive complaints in primary care centers. Geriatr Gerontol Int. 2014;14(3):667–673. doi:10.1111/ggi.12157
13. Bohlken J, Kostev K. Coded prevalence of mild cognitive impairment in general and neuropsychiatrists practices in Germany between 2007 and 2017. J Alzheimer’s Dis. 2019;67(4):1313–1318. doi:10.3233/JAD-181180
14. Hallam B, Rees J, Petersen I, et al. How are people with mild cognitive impairment or subjective memory complaints managed in primary care? A systematic review. Fam Pract. 2021;38(5):669–683. doi:10.1093/fampra/cmab014
15. Elm E, Altman D, Egger M, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi:10.1136/bmj.39335.541782.AD
16. Maguire A, Blak BT, Thompson M. The importance of defining periods of complete mortality reporting for research using automated data from primary care. Pharmacoepidemiol Drug Saf. 2009;18(1):76–83. doi:10.1002/pds.1688
17. Horsfall L, Walters K, Petersen I. Identifying periods of acceptable computer usage in primary care research databases. Pharmacoepidemiol Drug Saf. 2013;22(1):64–69. doi:10.1002/pds.3368
18. Blak B, Thompson M, Dattani H, et al. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. J Innov Health Inform. 2011;19(4):251–255. doi:10.14236/jhi.v19i4.820
19. Statistics OfN. 2011 Census Data; 2011. Available from: https://www.ons.gov.uk/census/2011census/2011censusdata.
20. Townsend P, Phillimore P, Beattie A. Health and Deprivation: Inequality and the North. Routledge; 1988.
21. Chisholm J. The Read clinical classification. Br Med J. 1990;300(6732):1092. doi:10.1136/bmj.300.6732.1092
22. Davé S, Petersen I. Creating medical and drug code lists to identify cases in primary care databases. Pharmacoepidemiol Drug Saf. 2009;18(8):704–707. doi:10.1002/pds.1770
23. Lewis JD, Bilker WB, Weinstein RB, et al. The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Saf. 2005;14(7):443–451. doi:10.1002/pds.1115
24. Carreira H, Williams R, Strongman H, et al. Identification of mental health and quality of life outcomes in primary care databases in the UK: a systematic review. BMJ open. 2019;9(7):e029227. doi:10.1136/bmjopen-2019-029227
25. Whitty E, Mansour H, Aguirre E, et al. Efficacy of lifestyle and psychosocial interventions in reducing cognitive decline in older people: systematic review. Ageing Res Rev. 2020;62:101113. doi:10.1016/j.arr.2020.101113
26. Iliffe S, Wilcock J. The UK experience of promoting dementia recognition and management in primary care. Z Gerontol Geriatr. 2017;50(Suppl 2):63–67. doi:10.1007/s00391-016-1175-1
27. Liu D, Green E, Kasteridis P, et al. Incentive schemes to increase dementia diagnoses in primary care in England: a retrospective cohort study of unintended consequences. Br J Gen Pract. 2019;69(680):e154–e163. doi:10.3399/bjgp19X701513
28. Richardson C, Stephan BC, Robinson L, et al. Two-decade change in prevalence of cognitive impairment in the UK. Eur J Epidemiol. 2019;34(11):1085–1092. doi:10.1007/s10654-019-00554-x
29. Wolters FJ, Chibnik LB, Waziry R, et al. Twenty-seven-year time trends in dementia incidence in Europe and the United States: the Alzheimer Cohorts Consortium. Neurology. 2020;95(5):e519–e31. doi:10.1212/WNL.0000000000010022
30. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–2263. doi:10.1016/S0140-6736(15)60461-5
31. Poppe M, Mansour H, Rapaport P, et al. “Falling through the cracks”; Stakeholders’ views around the concept and diagnosis of mild cognitive impairment and their understanding of dementia prevention. Int J Geriatr Psychiatry. 2020;35(11):1349–1357. doi:10.1002/gps.5373
32. Bohlken J, von Stillfried D, Schulz M. Prevalence rates of mild cognitive impairment and of dementia in the German outpatient health care sector 2009–2016. Psychiatr Prax. 2019;47(1):16–21. doi:10.1055/a-1012-9502
33. Röhr S, Pabst A, Riedel-Heller SG, et al. Estimating prevalence of subjective cognitive decline in and across international cohort studies of aging: a COSMIC study. Alzheimer’s Res Ther. 2020;12(1):1–14.
34. Russell P, Banerjee S, Watt J, et al. Improving the identification of people with dementia in primary care: evaluation of the impact of primary care dementia coding guidance on identified prevalence. BMJ open. 2013;3:12. doi:10.1136/bmjopen-2013-004023
35. McGuinness LA, Warren‐Gash C, Moorhouse LR, et al. The validity of dementia diagnoses in routinely collected electronic health records in the United Kingdom: a systematic review. Pharmacoepidemiol Drug Saf. 2019;28(2):244–255. doi:10.1002/pds.4669
 © 2022 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2022 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
