Back to Journals » Journal of Multidisciplinary Healthcare » Volume 15
Time to Recovery of Severely Ill COVID-19 Patients and its Predictors: A Retrospective Cohort Study in Tigray, Ethiopia
Authors Abebe HT, Zelelow YB , Bezabih AM, Ashebir MM, Tafere GR , Wuneh AD , Araya MG, Kiros NK, Hiluf MK , Ebrahim MM , Gebrehiwot TG , Welderufael AL, Mohammed AH
Received 31 March 2022
Accepted for publication 26 July 2022
Published 11 August 2022 Volume 2022:15 Pages 1709—1718
DOI https://doi.org/10.2147/JMDH.S368755
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Haftom Temesgen Abebe,1,2 Yibrah Berhe Zelelow,3 Afework Mulugeta Bezabih,4 Mengistu Mitiku Ashebir,5 Getachew Redae Tafere,6 Alem Desta Wuneh,5 Medhanie Gebresilassie Araya,7 Nguse Kahsay Kiros,8 Molla Kahssay Hiluf,9 Mohamedawel Mohamedniguss Ebrahim,10 Tesfay Gebregzabher Gebrehiwot,5 Abadi Leul Welderufael,11 Abrahim Hassen Mohammed12
1Department of Biostatistics, College of Health Sciences, Mekelle University, Mekelle, Ethiopia; 2Laboratory Interdisciplinary Statistical Data Analysis, College of Health Sciences, Mekelle University, Mekelle, Ethiopia; 3Department of Obstetrics and Gynecology, College of Health Sciences, Mekelle University, Mekelle, Ethiopia; 4Department of Nutrition, College of Health Sciences, Mekelle University, Mekelle, Ethiopia; 5Department of Health System, College of Health Sciences, Mekelle University, Mekelle, Ethiopia; 6Department of Environmental and Behavioral Sciences, College of Health Sciences, Mekelle University, Mekelle, Ethiopia; 7Department of Ear, Nose and Throat, College of Health Sciences, Mekelle University, Mekelle, Ethiopia; 8Tigrai Institute of Policy Studies, Mekelle, Ethiopia; 9Department of Public Health, College of Medicine and Health Sciences, Samara University, Samara, Ethiopia; 10Department of Surgery, College of Health Sciences, Mekelle University, Mekelle, Ethiopia; 11Department of Pediatric and Child Health, College of Health Sciences, Mekelle University, Mekelle, Ethiopia; 12Tigrai Health Bureau, Mekelle, Ethiopia
Correspondence: Haftom Temesgen Abebe, Department of Biostatistics, College of Health Sciences, Mekelle University, P.O. Box 1871, Mekelle, Ethiopia, Email [email protected]
Background: COVID-19 is one of the leading causes of morbidity and mortality and is caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). A patient infected with SARS-CoV-2 is said to be recovered from the infection following negative test results and when signs and symptoms disappear. Different studies have shown different median recovery time of patients with COVID-19 and it varies across settings and disease status. This study aimed to assess time to recovery and its predictors among severely ill COVID-19 patients in Tigray.
Methods: A total of 139 severely ill COVID-19 patients who were hospitalized between May 7, 2020 and October 28, 2020 were retrospectively analyzed. Cox proportional hazard regression model was fitted to identify the risk factors associated with the time duration to recovery from severe COVID-19 illness.
Results: The median age of the patients was 35 years (IQR, 27– 60). Eighty-three (59.7%) patients recovered with a median time of 26 days (95% CI: 23– 27). The results from the multivariable analysis showed that the recovery time was lower for severely ill patients who had no underline comorbidity diseases (AHR=2.48, 95% CI: 1.18– 5.24), shortness of breath (AHR=2.08, 95% CI: 1.07– 3.98) and body weakness (AHR=2.62, 95% CI: 1.20– 5.72). Moreover, COVID-19 patients aged younger than 40 years had lower recovery time compared to patients aged 60 and above (AHR=4.09, 95% CI: 1.58– 10.61).
Conclusion: The median recovery time of severely ill COVID-19 patients was long, and older age, comorbidity, shortness of breath, and body weakness were significant factors related with the time to recovery among the severely ill COVID-19 patients. Therefore, we recommended that elders and individuals with at least one comorbidity disease have to get due attention to prevent infection by the virus. Moreover, attention should be given in the treatment practice for individuals who had shortness of breath and body weakness symptoms.
Keywords: COVID-19, comorbidity, cox proportional hazard regression, adjusted hazard ratio
Introduction
COVID-19 is one of the leading causes of morbidity and mortality globally. It is caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and was first identified in Wuhan, China in early December 2019. It can cause fever, headache, shortness of breath, coughing, body weakness, sore throat, pain, and severe respiratory syndrome in human beings and is mainly transmitted by respiratory droplets and close contact with an infected human being.1 On January 30, 2020, the outbreak was declared a public health emergency of international concern by the WHO. According to the WHO daily situation report, currently the transmission is classified as community transmission and the total cases of COVID-19 are increasing worldwide; there were a total of 538,321,874 confirmed cases and 6,320,599 deaths as of June 22, 2022.2
In Ethiopia, the first case of COVID-19 was confirmed on March 13, 2020 and the infection has been spreading to all parts of the country, including Tigray region, wherein this research was conducted. According to the WHO, Ethiopia reported a total of 484,536 confirmed cases and 7524 deaths as of June 22, 2022.2
The infection fatality rates of COVID-19 patients, patient outcomes and related complications reported so far have varied considerably between countries. Previous studies showed that the overall mortality rate of COVID-19 patients is 3.77% −5.4%,3–5 and 41.1% - 61.5%6–8 among critical ill and severe patients. To reduce the infection fatality rate, understanding the factors associated with the duration of viral ribonucleic acid (RNA) shedding, the time from infection to viral RNA-negative conversion in COVID-19 patients is urgently needed.9 Moreover, it is also important to evaluate the testing time in order to reduce the infection fatality rate.10
Reports from previous studies indicated that the median duration of viral shedding in COVID-19 patients ranged from 8–47 days.9,11–17 Most of the studies conducted on the duration of SARS-COV-2 shedding among COVID-19 patients are from China and Europe. The recovery time among severely ill patients is limited and different factors might hasten recovery or delay of the disease. Evidences reveal that older age, the time lag from illness onset to hospital admission and underlying comorbidities are associated with prolonged duration of viral RNA shedding in COVID-19 patients.9,16–20 However, the epidemic in high income countries seems to be different from that of low and middle income countries in the risk factors, speed of the spread of the virus, and the record of the death toll related to the duration of SARS-COV-2 shedding.
Understanding the average time of recovery and its predictors is crucial for the decision making process at national and international levels in order to formulate preventive measures and optimize treatment options. Different studies have shown that the median time to recovery from COVID-19 patients varies across settings and disease status. The present study aimed to estimate the time to recovery and identify its associated factors among severely ill COVID-19 patients admitted to treatment centers in Tigray, Northern Ethiopia. This study provided useful information to predict the recovery time of severely ill COVID-19 patients through this retrospective cohort study.
Methods
Study Design and Settings
A retrospective cohort study was analyzed that involved 139 severely ill COVID-19 patients admitted to isolation and treatment centers in Tigray, northern Ethiopia. Regardless of signs or symptoms development, all individuals with laboratory confirmed SARS-CoV-2 infection were admitted to the isolation and treatment centers within 24 hours. Anyone who has contact with confirmed COVID-19 case was being isolated for 14 days. Persons who failed to develop symptoms within 14 days were discharged from the isolation centers. Cases were confirmed by polymerase chain reaction (PCR) in the treatment centers.
Study Participants and Study Period
All laboratory-confirmed positive COVID-19 severely ill patients admitted to treatment centers in the region between May 7 and October 28, 2020 with a definite outcome (recovered or dead) were designated as study participants.
Data Source and Sample
The data were collected using a standardized form from electronic medical records. The data set contains demographic characteristics such as age, gender and occupation, clinical information of the patients contains temperature at admission, sign and symptoms status (such as fever, cough, shortness of breath, pain, sore throat, headache, body weakness), and presence of comorbidity (such as cardiovascular disease, diabetes mellitus, renal disease, respiratory disease) and patient outcomes (recovered or died). All severely ill COVID-19 patients admitted to the treatment centers between May 7 and October 28, 2020 were included in this study. Those severely ill patients with incomplete demographic and treatment outcome were excluded from the study.
Study Variables
Dependent Variable
In this study, the dependent variable was time to recovery from COVID-19 among severely ill patients and recovery is the event of interest. The time was estimated in days and recovery time was defined as the number of days it takes from the day the PCR test was positive until the patient is diagnosed negative for COVID-19 and discharged from treatment centers. The confirmed COVID-19 patients in the treatment centers were retested when symptoms subside and the body temperature remains at the normal range for at least three days and they were considered as recovered only after receiving two consecutive laboratory tests negative.
Independent Variables
The independent variables considered in this study were sex, age, occupation, symptoms, comorbidity and type of comorbidity, temperature, travel history and source of infection.
Operational Definitions
The COVID-19 cases were all individuals tested positive for SARS-CoV-2 by PCR. Symptomatic cases were defined as any SARS-CoV-2 positive individual by PCR with at least one sign or symptom for COVID-19, including but not limited to: cough, fever, shortness of breath, headache, sore throat, and pain. Cases with comorbidity are COVID-19 patients with at least one known preexisting chronic medical illness. Severely ill COVID-19 patients: These patients with clinical signs of pneumonia and have at least one of the following conditions i) respiratory rate interval > 30 breaths/min; ii) SpO2 (saturation of peripheral oxygen) < 93% at rest; iii) severe respiratory distress, and iv) oxygenation index (artery partial pressure of oxygen/inspired oxygen fraction, PaO2/FiO2) < 300 mmHg.
Statistical Data Analysis
The data were coded, cleaned, and checked for inconsistencies and completeness. STATA version 16 software was used for data processing and data analysis. Summary measures such as counts, percentages, means, medians and IQRs were calculated. The Log rank test was applied to compare the survival time between different predictors. A cox proportional hazard regression model was used to determine the potential risk factors associated with the duration time to recovery among severely ill COVID-19 patients. Factors associated with outcome at p-value < 0.20 in bivariate Cox regression were selected for multivariable Cox regression analysis. An adjusted hazard ratio (AHR) with 95% confidence interval was computed and statistical significance was declared at p-value < 0.05. Cox proportional hazards assumption was checked using the Schoenfeld residual test.
Ethical Consideration
The ethics committee of Mekelle University, College of Health Sciences approved the current study with the ethical clearance registration number of IBR1826/2021. The study was conducted in accordance with the Declaration of Helsinki. Consent to participate was fully waived as the study participants were not directly involved in the study (ie an already existing data were utilized for analysis in the current study). The confidentiality of data was kept as there were no personal identifiers used and neither the raw data nor the extracted data were passed to a third person.
Results
Socio-Demographic Characteristics of Severely Ill COVID-19 Patients
A total of 139 severely ill COVID-19 patients were included in this study that was reported from May 7, 2020 to October 28, 2020. The median age of the patients was 35 years (IQR, 27–60). A total of 55.4% of patients were younger than 40 years, and 25.9% were older than 59 years. The patients in the non-survivor group were much older than those in the survivor group [median = 57 years (IQR, 29.5–72 years) versus median = 30 years (IQR, 25–40 years), p-value < 0.001]. Of the non-survival patients (56 (40.3%) of 139), 48.2% of the patients were older than 59 years. The majority of COVID-19 patients (77.0%) were males and 73.2% of COVID-19 deaths were in men. Majority (60.4%) of the severely ill COVID-19 patients their sources of infection were from the community and 18% were imported. Among the study patients in our study, 20.1% of the patients had travel history and 40.3% were died (Table 1).
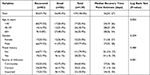 |
Table 1 Background Characteristics of 139 Severely Ill COVID-19 Patients Admitted to Treatment Centers of Tigray, Northern Ethiopia, 2020 |
Clinical Characteristic of Severely Ill COVID-19 Patients
Of the total study patients, 61.2% were symptomatic. The most common symptoms at the onset of disease reported were shortness of breath 57.5%, and cough 52.5%. This is followed by body weakness 36.7%, fever 32.4% and pain 28.1%. Among the non-survival patients (56 (40.3%) of 139), 78.6% had shortness of breath and 71.4% had cough symptoms. Moreover, 28.8% patients had one or more coexisting medical conditions alongside COVID-19. The comorbidity rate in the non-survivor group was higher than that of the survivor group (44.6% versus 18.1%, p-value < 0.001). Based on the body temperature on admission, 71.9% of the patients had temperature < 37.3°C and 28.1% had elevated temperature ≥37.3°C (Table 2). The most frequent comorbidities were cardiovascular diseases (50%), diabetes mellitus (20%) and respiratory diseases (18%) (Figure 1).
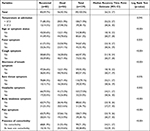 |
Table 2 Clinical Characteristics of 139 Severely Ill COVID-19 Patients in Tigray, Northern Ethiopia, 2020 |
Survival Estimates of Time to Recovery of Severely Ill COVID-19 Patients
A total of 139 severely ill COVID-19 patients were followed for a minimum of 1 and a maximum of 37 days with median follow-up of time 18 days (IQR: 11–27). Eighty-three patients were recovered with median time of 26 days (95% CI: 23–27 days). The overall incidence recovery rate was 3.1 (95% CI: 2.5 −3.9) per 100 person-days of observations. The incidence recovery rate among male and female severely ill patients was 3.1 per 100 person-day (95% CI: 2.5–4.0) and 3.2 per 100 person-day (95% CI: 2.0–5.1) respectively. In this study, the recovery rate from COVID-19 among severely ill patients with and without comorbidity was found to be 2.1 (95% CI: 1.3–3.5) and 3.5 (95% CI: 2.8–4.4) per 100 person-day respectively. Log Rank test was used to compare survival time between categories of different predictors. The survival estimates of severely ill patients varied in relation to age, fever, cough, shortness of breath, headache, body weakness, pain, and underline comorbidity (Tables 1 and 2). The survival status of severely ill COVID-19 patients was also estimated by the Kaplan–Meier survival curve. The overall graph of Kaplan–Meier survival function depicted that the curve tends to decrease rapidly in between 18 and 26 days indicating that most severely ill COVID-19 patients recovered within this time (Figure 2). A separate Kaplan–Meier survivor functions curve was constructed to estimates the survival time based on different covariates to see the existence of difference in recovery rate between categories of individual covariates. There was a significant difference in the time of recovery between patients with and without previous medical conditions or comorbidity, where patients without comorbidity recovered faster (Figure 3). A significant difference in the recovery rate among the two groups is also found by the Log rank test (Table 2). The median recovery times of the patients with and without comorbidity were 32 days and 24 days, respectively.
 |
Figure 2 Kaplan-Meier survival estimate for time to recovery among severely ill COVID-19 patients in Tigray, Northern Ethiopia. |
 |
Figure 3 Kaplan-Meier survival estimate for time to recovery among patients with and without comorbidity. |
Predictors of Recovery Time of Severely Ill COVID-19 Patients
Predictors that had association at a p-value of <0.20 in bivariate Cox regression were included in multivariable Cox regression. Age, fever, coughing, shortness of breath, sore throat, headache, body weakness, pain, comorbidity, and temperature were statistically significant at a p-value of < 0.20 level of significance. In the multivariable cox regression model only age, shortness of breath, body weakness and comorbidity were found to have statistically significant association with recovery time among the severely ill COVID-19 patients. Severely ill COVID-19 patients who were aged < 40 years had 4.1 times higher rate of recovery as compared to patients who were aged 60 and above years (AHR=4.09, 95% CI: 1.58–10.61). In addition, the recovery rate was higher for patients who had no underline comorbidity diseases (AHR = 2.48, 95% CI: 1.18–5.24), shortness of breath (AHR = 2.07, 95% CI: 1.08–3.98) and body weakness (AHR = 2.62, 95% CI: 1.20–5.72) (Table 3). The Schoenfeld residual test results confirmed that the proportional hazard assumption satisfies.
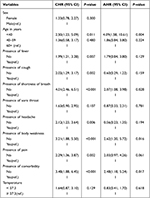 |
Table 3 Predictors of Time to Recovery Among Severely Ill COVID-19 Patients in Tigray Region, 2020 |
Discussion
This study comprised 139 severely ill COVID-19 patients who were admitted to treatment centers in Tigray region. Our study shows that the overall median time to recovery among severely ill COVID-19 patients was 26 days, which is consistent with some previous research study.11,21–23 However, the median recovery time was lower in many other previous studies. For instance, a study done in Eka Kotebe General Hospital, Ethiopia (19 days),20 Guangzhou Eighth People’s Hospital, China (12 days),9 Singapore (12 days),24 University of California San Diego (7 days)25 and Wollega Referral Hospital, Ethiopia (18 days).26 The discrepancy of the findings could be the differences in the composition of study participants, sample size and severity of the disease. In addition, the fact that the recovery time was shorter among patients treated in China, Singapore and the United States of America could be due to the availability of advanced medical technologies and medications and effective COVID-19 patients management approach and conducive hospital setting. The selected study participants in most of these studies were all COVID-19 cases, whereas our study was conducted among severely ill COVID-19 patients admitted to intensive care units. Evidences have shown that severely ill patients stay longer to recover from COVID-19.27 The time at which the first swab is taken and the criteria for considering patient recovered can also influence the recovery time. Moreover, in the current study, a significant number of the patients had previous medical conditions or comorbidity, which might have affected to delay recovery.
This study revealed that cardiovascular diseases and diabetes mellitus were the most common comorbidities, which is consistent with the previously reported research studies.5,28–34 Moreover, in this study 61% of the patients had signs and symptoms on admission. The most common symptoms were shortness of breath and cough. This finding is in line with most previous studies.35–38 The result of this study showed that there was no difference between males and females in the recovery period in bivariate analysis. This was consistent with some previous studies.39,40 However, other studies have found that male patients had longer duration of viral RNA shedding than female patients with COVID-19.17,41
The multivariable cox proportional hazard regression analysis demonstrated that age, comorbidity, shortness of breath and body weakness were risk factors for time to recovery among severely ill COVID-19 patients. We found that older age was associated with high risk of delayed viral clearance. The younger patient’s recovery rate is significantly higher than those older than 59 years. This finding was in line with previous studies.9,17,27,42–46 This might be attributed to the severity progression of COVID-19 among older age cases compared to the younger cases which in turn leads to either death or delayed duration of viral clearance in elderly patients.42 Moreover, it could be due to the life style of old aged patients in that they might not have a regular physical exercise or could have additional underlying disease conditions like chronic illnesses and may also have the habit of consuming lifesaving and life prolonging drugs.
The current study has demonstrated that patients with comorbidity condition had higher risk of delayed viral clearance from COVID-19 compared to their counterparts. The existing facts are supporting the present study finding that comorbidity conditions majorly cardiovascular diseases attributed to the high risk of delayed viral clearance from COVID-19 cases.17,37,42,47 Moreover, this study revealed that the recovery rate was higher for patients who had no shortness of breath and body weakness. The limitation of the present study is the retrospective study design and finding the whole array of COVID-19 patient information from the electronic medical record was a challenge.
Conclusion
The median of recovery time from severely ill COVID-19 patients was long. The study revealed that older age, having at least one comorbid condition, shortness of breath and body weakness were significant factors related with the time to recovery among the severely ill COVID-19 patients. Therefore, elders and individuals with at least one comorbid condition has to get due attention to prevent infection by the virus. Moreover, attention should be given in the treatment practice for individuals who had shortness of breath and body weakness symptoms.
Accessibility of Data and Materials
The findings of this research were extracted from the data gathered and analyzed based on the stated methods and materials. The dataset supporting this finding can be obtained from the corresponding author upon request.
Ethics Approval and Consent to Participant
Ethics approval to conduct this study was obtained from the institutional research review committee of College of Health Sciences, Mekelle University with the ethical clearance registration number of IBR1826/2021. The study was conducted in accordance with the Declaration of Helsinki. Consent to participate was fully waived as the study participants were not directly involved in the study (ie, already existing data were utilized for analysis in the current study).
Funding
No funding was obtained for this study.
Disclosure
The authors declare no conflicts of interest in relation to this work.
References
1. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. doi:10.1016/j.jaut.2020.102433
2. World Health Organization. Global COVID-19 report; June, 2022. Available from: https://covid19.who.int.
3. Zhang J, Wang X, Jia X, et al. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26(6):767–772. doi:10.1016/j.cmi.2020.04.012
4. Zhang G, Hu C, Luo L, et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127:104364. doi:10.1016/j.jcv.2020
5. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi:10.1001/jama.2020.1585
6. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi:10.1016/S2213-26002030079-5
7. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi:10.1001/jamainternmed.2020.0994
8. Feng Y, Ling Y, Bai T, et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201(11):1380–1388. doi:10.1164/rccm.202002-0445OC
9. Chen X, Zhu B, Hong W, et al. Associations of clinical characteristics and treatment regimens with the duration of viral RNA shedding in patients with COVID-19. Int J Infect Dis. 2020;98:252–260. doi:10.1016/j.ijid.2020.06.091
10. Benoni R, Panunzi S, Campagna I, et al. The effect of test timing on the probability of positive SARS-CoV-2 swab test results: mixed model approach. JMIR Public Health Surveill. 2021;7(6):e27189. doi:10.2196/27189
11. Benoni R, Campagna I, Panunzi S, et al. Estimating COVID-19 recovery time in a cohort of Italian healthcare workers who underwent surveillance swab testing. Public Health. 2021;196:52–58. doi:10.1016/j.puhe.2021.05.014
12. Kampen van JJA, Vijver van de DAMC, Fraaij PLA, et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19). Nat Commun. 2021;12(1):267. doi:10.1038/s41467-020-20568-4
13. Zheng X, Chen J, Deng L, et al. Risk factors for the COVID-19 severity and its correlation with viral shedding: a retrospective cohort study. J Med Virol. 2020;93(2):952–961. doi:10.1002/jmv.26367
14. Qi L, Yang Y, Jiang D, et al. Factors associated with the duration of viral shedding in adults with COVID-19 outside of Wuhan, China: a retrospective cohort study. Int J Infect Dis. 2020;96:531–537. doi:10.1016/j.ijid.2020.05.045
15. Warabi Y, Tobisawa S, Kawazoe T, et al. Effects of oral care on prolonged viral shedding in coronavirus disease 2019 (COVID-19). Spec Care Dentist. 2020:1–5. doi:10.1111/scd.12498.
16. Shi D, Wu W, Wang Q, et al. Clinical characteristics and factors associated with long-term viral excretion in patients with severe acute respiratory syndrome coronavirus 2 infection: a single-center 28-day study. J Infect Dis. 2020;222(6):910–918. doi:10.1093/infdis/jiaa388
17. Xu K, Chen Y, Yuan J, et al. Factors associated with prolonged viral RNA shedding in patients with COVID-19. Clin Infect Dis. 2020;71(15):799–806. doi:10.1093/cid/ciaa351
18. Li TZ, Cao ZH, Chen Y, et al. Duration of SARS-CoV-2 RNA shedding and factors associated with prolonged viral shedding in patients with COVID-19. J Med Virol. 2021;93(1):506–512. doi:10.1002/jmv.26280
19. Moriconi D, Masi S, Rebelos E, et al. Obesity prolongs the hospital stay in patients affected by COVID-19, and may impact on SARS-COV-2 shedding. Obes Res Clin Pract. 2020;14(3):205–209. doi:10.1016/j.orcp.2020.05.009
20. Abrahim SA, Tessema M, Defar A, et al. Time to recovery and its predictors among adults hospitalized with COVID-19: a prospective cohort study in Ethiopia. PLoS One. 2020;15(12):e0244269. doi:10.1371/journal.pone.0244269
21. Bi Q, Wu Y, Mei S, et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;20(8):911–919. doi:10.1016/S1473-3099(20)30287-5
22. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi:10.1016/S0140-6736(20)30566-3
23. Voinsky I, Baristaite G, Gurwitz D. Effects of age and sex on recovery from COVID-19: analysis of 5769 Israeli patients. J Infect. 2020;81(2):e102–e103. doi:10.1016/j.jinf.2020.05.026
24. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323(15):1488–1494. doi:10.1001/jama.2020.3204
25. Daniels LB, Sitapati A, Zhang J, et al. Relation of statin use prior to admission to severity and recovery among COVID-19 inpatients. Am J Cardiol. 2020;136:149–155. doi:10.1016/j.amjcard.2020.09.012
26. Tolossa T, Wakuma B, Seyoum Gebre D, et al. Time to recovery from COVID-19 and its predictors among patients admitted to treatment center of Wollega University Referral Hospital (WURH), Western Ethiopia: survival analysis of retrospective cohort study. PLoS One. 2021;16(6):e0252389. doi:10.1371/journal.pone.0252389
27. Xu J, Yang X, Yang L, et al. Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: a multicenter retrospective study from Wuhan, China. Crit Care. 2020;24(1):394. doi:10.1186/s13054-020-03098-9
28. Zhang X-B, Hu L, Ming Q, et al. Risk factors for mortality of coronavirus disease-2019 (COVID-19) patients in two centers of Hubei province, China: a retrospective analysis. PLoS One. 2021;16(1):e0246030. doi:10.1371/journal.pone.0246030
29. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
30. Vanhems P, Gustin M-P, Elias C, et al. Factors associated with admission to intensive care units in COVID-19 patients in Lyon-France. PLoS One. 2021;16(1):e0243709. doi:10.1371/journal.pone.0243709
31. Haas LEM, de Lange DW, van Dijk D, van Delden JJM. Should we deny ICU admission to the elderly? Ethical considerations in times of COVID-19. Crit Care. 2020;24(1):321. doi:10.1186/s13054-020-03050-x
32. Borges NIJ, Cacic N, Abdulazeem HM, et al. Novel coronavirus infection (COVID-19) in humans: a scoping review and meta-analysis. J Clin Med. 2020;9(4):941. doi:10.3390/jcm9040941
33. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. doi:10.1183/13993003.00547-2020
34. Zhu N, Zhang D, Wang W, et al. A novel Coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi:10.1056/NEJMoa2001017
35. Chen J. Pathogenicity and transmissibility of 2019-nCoV-A quick overview and comparison with other emerging viruses. Microbes Infect. 2020;22(2):69–71. doi:10.1016/j.micinf.2020.01.004
36. Wu J, Wu X, Zeng W, et al. Chest CT findings in patients with Corona virus disease 2019 and its relationship with clinical features. Investig Radiol. 2020;55(5):257–261. doi:10.1097/RLI.0000000000000670
37. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi:10.1007/s00134-020-05991-x
38. Reza S, Zohre K, Amirhossein E, et al. Epidemiological and clinical features of 2019 novel coronavirus diseases (COVID-19) in the South of Iran. BMC Infect Dis. 2020;20(1):427. doi:10.1186/s12879-020-05128-x
39. Barman MP, Rahman T, Bora K, Borgohain C. COVID-19 pandemic and its recovery time of patients in India: a pilot study. Diabetes Metab Syndr. 2020;14(5):1205–1211. doi:10.1016/j.dsx.2020.07.004
40. O’Brien J, Du KY, Peng C. Incidence, clinical features, and outcomes of COVID-19 in Canada: impact of sex and age. J Ovarian Res. 2020;13(1):137. doi:10.1186/s13048-020-00734-4
41. Ortolan A, Lorenzi M, Felicetti M, et al. Does gender influence clinical expression and disease outcomes in COVID-19? A systematic review and meta-analysis. Int J Infect Dis. 2020;99:496–504. doi:10.1016/j.ijid.2020.07.076
42. Du R-H, Liang L-R, Yang C-Q, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5):2000524. doi:10.1183/13993003.00524-2020
43. Hu X, Xing Y, Jia J, et al. Factors associated with negative conversion of viral RNA in patients hospitalized with COVID-19. Sci Total Environ. 2020;728:138812. doi:10.1016/j.scitotenv.2020.138812
44. Wu J, Li W, She X, et al. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19). J Intern Med. 2020;288(1):128–138. doi:10.1111/joim.13063
45. Das AK, Gopalan SS. Epidemiology of COVID-19 and predictors of recovery in the Republic of Korea. Pulm Med. 2020;2020:7291698. doi:10.1155/2020/7291698
46. Muhammad LJ, Islam MM, Usman SS, Ayon SI. Predictive data mining models for novel coronavirus (COVID-19) infected patients’ recovery. SN Comput Sci. 2020;1(4):206. doi:10.1007/s42979-020-00216-w
47. Chinnadurai R, Ogedengbe O, Agarwal P, et al. Older age and frailty are the chief predictors of mortality in COVID-19 patients admitted to an acute medical unit in a secondary care setting-a cohort study. BMC Geriatr. 2020;20(1):409. doi:10.1186/s12877-020-01803-5
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

