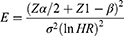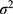Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 12
Time to Recovery from Severe Pneumonia and Its Predictors Among Children 2–59 Months of Age Admitted to Pediatric Ward of Nigist Eleni Mohammed Memorial Comprehensive Specialized Hospital, Hossana, Ethiopia: Retrospective Cohort Study
Authors Tirore LL , Abame DE , Sedoro T, Ermias D , Arega A , Tadesse T , Nadamo SA
Received 21 May 2021
Accepted for publication 6 July 2021
Published 21 July 2021 Volume 2021:12 Pages 347—357
DOI https://doi.org/10.2147/PHMT.S321184
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Roosy Aulakh
Lire Lemma Tirore,1 Desta Erkalo Abame,1 Tagesse Sedoro,1 Dejene Ermias,1 Abinet Arega,1 Tegegn Tadesse,1 Selamu Abose Nadamo2
1Department of Public Health, College of Medicine and Health Sciences, Wachemo University, Hossana, Ethiopia; 2Department of Midwifery, College of Medicine and Health Sciences, Wachemo University, Hossana, Ethiopia
Correspondence: Lire Lemma Tirore Tel +251 916281764
Email [email protected]
Background: Severe pneumonia is still the greatest infectious cause of morbidity and mortality in children under the age of five around the world. Each night spent in the hospital raises the chance of bad drug responses, infections, and ulcers by 0.5%, 1.6%, and 0.5%, respectively. In Southern Ethiopia, as well as the research area, little is known regarding death and recovery time from severe pneumonia and their determinants.
Objective: To determine time to recovery from severe pneumonia and its predictors among children 2– 59 months of age admitted to pediatric ward of Nigist Eleni Mohammed Memorial Comprehensive Specialized Hospital.
Methods: A facility-based retrospective cohort study was conducted among children 2– 59 months of age. Three years’ medical records, from January 2017 to December 2020, were reviewed. A total of 280 children with severe pneumonia were included. In the case of survival time, median was calculated. Kaplan Meier survival curve was used to estimate recovery time from severe pneumonia, and the independent effects of covariates on recovery time were analyzed using multivariable Cox-proportional hazard model.
Results: The median time to recovery was 4 days (interquartile range = 3, 5). The incidence rate of recovery was 24.16 per 100 person-days. Underweight (adjusted hazard ratio = 0.56, 95% CI = 0.38– 0.80), age group 12– 35 months (adjusted hazard ratio= 2.0, 95% CI=1.30– 3.30), treatment with ampicillin and gentamicin (adjusted hazard ratio= 0.35, 95% CI: 0.13– 0.80), and antibiotic change (adjusted hazard ratio= 0.34, 95% CI = 0.21– 0.53) were statistically significant predictors of time to recovery from severe pneumonia.
Conclusion: The median length of stay in the hospital was short (4 days [interquartile range =3, 5]). Time to recover from severe pneumonia was significantly influenced by being underweight, age, antibiotics administered first, and antibiotic change. Measures such as providing nutritious meals to children and ensuring that underweight children are properly managed should be bolstered.
Keywords: time to recovery, severe pneumonia, under 5 children, NEMMCSH
Background
Pneumonia is a form of acute respiratory infection that affects the lungs.1 Severe pneumonia is diagnosed when children with acute onset of cough and or difficult breathing present with any danger signs such as central cyanosis, inability to breastfeed or drink, or vomiting everything, convulsions, lethargy or unconsciousness, grunting and head nodding.2 Streptococcus pneumonia and Haemophilus influenzae type b (Hib) are the most common bacterial causes of pneumonia in children.1 Severe pneumonia is the single largest infectious cause of death in children worldwide.1 Despite the fact that pneumonia affects children worldwide, the risk and repercussions of the disease are more prevalent in poor and middle income nations, where the bulk of deaths and cases occur.3 The majority of the victims are children under the age of two.4 It is also a common reason for children to be admitted to hospitals, especially in communities with little resources.5 Hospitalization is also lengthier in developing countries than in developed countries. Aside from case fatality, the length of hospitalization is a commonly accepted indicator of pneumonia severity and service efficiency.6,7
Severe pneumonia is still the greatest infectious cause of morbidity and mortality in children under the age of five around the world.8 In 2015, 22 million cases of severe pneumonia resulted in the death of 920,136 children under the age of 5 years old.1 Killing approximately 2,400 children a day, severe pneumonia accounted for approximately 16% (880,000) of the 5.6 million under-five deaths in 2016.9 Almost all severe pneumonia deaths (98%) occur in developing countries10 with Africa and Southeast Asia accounting for more than three-quarters of all pneumonia deaths. In 2015, WHO Africa region had the highest burden of severe pneumonia mortality (0•5 million).3
Head nodding, presence of edematous protein-energy malnutrition, severe wasting, hypoxemia at presentation,7 younger age, radiographic pneumonia, presence of any danger signs,11 lack of exclusive breastfeeding and proper vaccination, and overcrowding6,12 were predictors for prolonged recovery time.
Clinical services’ major aim is to identify severe disease episodes in children, provide appropriate treatment, and ensure that they leave the health facility healthy and without complications.13 A common question asked by admitted patients is, “When will I be better?”.14
Increased hospitalization length places a tremendous burden on the hospitalized child, his or her family, and health services especially in areas with poor resources and without health insurance.6,15 In low-income nations, the projected average daily cost of care for a hospitalized under-five child with severe pneumonia is greater than the average daily wage. A hospital stay accounts for 46.8% of a household’s total expense for a single episode of severe pneumonia.16
Direct medical costs for severe pneumonia in a hospital inpatient environment range between 26.6 and 115.8% of monthly household income. Non-medical costs (transportation and food) during the hospitalization time of a kid with severe pneumonia accounts for 9.0% and 31.0% of monthly household income in Kenya and Guinea, respectively.17
According to Bozzani et al, a hospital’s senior clinical staff spent 38% of their average time prescribing and providing treatment regimens for children admitted with severe pneumonia, and 26% on ward rounds.18
For an average episode, a hospital stay includes a 5.5% chance of an adverse drug reaction, a 17.6% chance of infection, and a 3.1% chance of ulcer. Each additional night in the hospital raises the chance of bad drug reactions by 0.5%, infections by 1.6%, and ulcers by 0.5.19 Hospital acquired malnutrition is also linked to a longer stay in the hospital.20
It takes time away from work for parents and increases the chance of contracting diseases that are costly to treat.21,22 It can also lead to “discharge against medical advice” (DAMA) which can lead to readmission, complications, and even death.23
The Ethiopian federal ministry of health (FMoH) has been working to reduce under-five mortality by introducing PCV and Hib vaccines, implementing strategies such as integrated community case management (ICCM) and integrated management of newborn and child illnesses (IMNCI), and training health extension workers (HEWs).24,25 The Government plans to reduce the under-five mortality rate to 31 and 14 per 1000 live births in 2025 and 2035, respectively, as part of the national goal of the Health Sector Transformation Plan (HSTP), which was launched in 2015.26
Despite the programs and initiatives indicated previously, there are still numerous instances and deaths caused by childhood pneumonia. In Ethiopia, an estimated 3,370,000 children contract pneumonia each year. Pneumonia is a leading killer of under-five children27, killing over 40,000 children each year and accounting for 20% of all deaths.24 The country is among the top five countries with the greatest pneumonia deaths.3
Despite the aforementioned initiatives and methods, little is known regarding recovery time (prolonged hospitalization) from severe pneumonia and its determinants in Ethiopia and the research area. Understanding the indicators of time to recovery in children with pneumonia can help parents understand how long their child will be in the hospital. This will enable them to mobilize resources for the hospital care of the child and re-adjust to any disruption that the admission may cause. This may reduce the incidence of DAMA, thereby ensuring adequate management and reducing risk of death. Recovery time reflects quality of the available health services in the health institution. Therefore, the aim of this study was to determine time to recovery and its predictors among children 2–59 months of age with severe pneumonia admitted to pediatric ward of Nigist Eleni Mohammed Memorial Comprehensive Specialized Hospital (NEMMCSH) using proportional Cox model.
Methods
Study Area
The research was carried out at NEMMCSH, which is located in Hosaena town, Hadiya Zone, Southern Ethiopia. Hosaena is 230 kilometers from Ethiopia’s capital, Addis Ababa, and 194 kilometers from Hawassa, the regional capital. NEMMCSH is a referral and teaching hospital that serves the people of Hadiya Zone and the neighboring areas with inpatient and outpatient services.
Study Design and Period
Facility based retrospective cohort study was conducted from January 2017 to December 2020.
Population
Source Population
All children with severe pneumonia aged 2 to 59 months who were admitted to pediatric ward of NEMMCSH.
Study Population
All children with severe pneumonia aged 2 to 59 months who were admitted to pediatric ward of NEMMCSH from January 2017 to December 2020.
Inclusion and Exclusion Criteria
Inclusion Criteria
All children with severe pneumonia aged 2 to 59 months who were managed in pediatric ward of NEMMCSH from January 2017 to December 2020.
Exclusion Criteria
Incomplete charts for major variables (medical record number, date of admission, date of discharge, discharge status and age).
Sample Size Determination
The sample size was determined using Stata software Version 15. A hazard ratio of covariates was predetermined to obtain the maximum sample size. A hazard ratio of 0.79 for wasting when other covariates held constant was found to be a covariate of interest that maximized the sample size. Other parameters were standard deviation (1.005), probability of success (recovery) observed (0.95), 1% margin of error, and 90% power.27 The total sample size required was 280 with the number of events required to be observed in the study, E=266.
The formula for manual calculation of the sample size was as follows.28
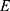 = the number of events required to be observed
= the number of events required to be observed
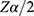 = standard normal percentile of confident coefficient
= standard normal percentile of confident coefficient
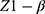 = standard normal percentile for the power to be achieved
= standard normal percentile for the power to be achieved
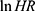 = the natural logarithm of the hazard ratio.
= the natural logarithm of the hazard ratio.
Then, the total sample size needed in order to achieve the calculated number of events was calculated using the following formula.
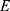 = the number of events to be observed (calculated previously)
= the number of events to be observed (calculated previously)
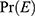 = the probability that the event of interest (which is recovery in this context) will occur.
= the probability that the event of interest (which is recovery in this context) will occur.
Sampling Technique
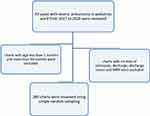
A simple random sampling technique was used. First, pediatric ward registration book was reviewed for children admitted to pediatric ward of NEMMCSH with severe pneumonia from January 2017 to December 2020. Then, those records of children aged less than two months, more than 5 years, and with no information for age were excluded. From the remaining cases, those with missing value for the medical record, date of admission, date of discharge and discharge status were excluded. The MRN of all the rest of the records of the children with severe pneumonia was registered. A total of 280 records of children with severe pneumonia were reviewed using simple random sampling techniques (Figure 1).
Data Collection Tool and Procedure
Data collection tool was developed after detailed review of literature and observing under-five patient charts and pediatric ward registration log book to establish the trend of registration, physical examination, and history taking.
Data were collected using pre-tested and structured questionnaire. The questionnaire had socio-demographic and clinical sub-sections.
Child’s age in months (continuous), sex, and place of residence (categorical) were under socio-demographic variables.
On the clinical subsection the variable outcome status (categorized as discharged, dead, absconded, transferred and unknown) was obtained which was later used to determine the occurrence of event. Other variables included were type of first written chief complaint (categorical), delay to come to hospital (in days), body temperature at admission (continuous), level of consciousness (categorized as conscious or unconscious), exclusive breast feeding (categorical), vaccination status (categorical), child’s HIV status (categorical), presence of other comorbidity (categorical), type of comorbidity (categorical), antibiotic administered (categorical), anemia and other danger signs. A variable length of stay (LOS in days) was derived by subtracting the date of admission from the date of discharge.
The data for date of admission, date of discharge, outcome status, child age, sex and classified diagnosis were extracted from the pediatric ward registration log book. The remaining data were retrieved from individual patient charts.
Data Quality Assurance
Data collectors and supervisors were trained on content of data collection tool, data collection procedures, and risks of poor data quality. Four pediatrics nurses collected the data while under supervision of two public health professionals. Data entry and cleaning were done in Epi data. Continuous variables were checked for outliers by box plot.
Study Variables
Dependent Variable
Time to recovery from severe pneumonia (number of days).
Independent Variables
Socio-demographic factors: Sex, Age, and Place of residence
Clinical factors: type of first written chief complaint, delay to come to hospital (in days), body temperature at admission, level of consciousness, exclusive breast feeding, vaccination status, child’s HIV status, antibiotic administered, anemia, stunting, wasting, underweight, inability to feed/drink, vomiting everything, head nodding, grunting.
Data Analysis
Descriptive Statistics
Data were entered into Epi-data for windows and analyzed using Stata version 15. Percentages and frequencies were used to summarize categorical variables. The results were presented by tables, texts and graphs based on the nature of variable. Distribution of the continuous variables was checked by box plot.
Mean with standard deviation and median with interquartile range were used to summarize normally and non-normally distributed continuous variables, respectively. But in the case of survival time, median was calculated because mean cannot provide accurate information because of censoring.
Kaplan Meier survival curve was used to describe proportion of hospital stays over time after initiation of inpatient treatment and to compare groups. The log rank test was used to test the null hypothesis that there was no difference in the distribution of survival times.
Bivariate analysis was performed using Cox proportional hazards regression to identify which variables had association with the time to recovery from severe pneumonia. All covariates which had an association with the outcome variables at p-value of 0.25 or less were entered into the multivariable model. The independent effects of covariates on hazard of recovery time were analyzed using multivariable Cox proportional hazard model. Adjusted hazard ratios with 95% Confidence Interval (CI) were estimated and p-value less than 0.05 was used to declare presence of significant association between recovery time and covariates. The proportional hazard assumption was checked on Stata by a global test using estat phtest. The global test tests the null hypothesis that the effect of covariate is the same over time. Multicollinearity was checked using Variance Inflation Factor (VIF).29
Operational Definition (Variable Definition)
Event: recovery from severe pneumonia.
Discharged (recovered): those who left the hospital with clinical improvement confirmed by a physician.
Recovery time: the time from admission to when the child is discharged from the hospital due to improvement in symptoms calculated by number of days (hospital stay).
Censored: a child with severe pneumonia who had been admitted to the ward and who had died, self-discharged, absconded, transferred or with unknown outcome status.
Median time of recovery: is the time when 50% of the children had recovered.
Absconded: those who left the hospital by themselves (without being discharged by a physician).
Results
Socio-Demographic Characteristics of Study Participants
Males accounted for 152 (54.29%) of the 280 participants, with a male to female ratio of 1.18:1. The median age of the children was 9 months (IQR=5.5 months), with 60% of the children aged 2 to 11 months and 36.43% aged 12 to 35 months (Table 1).
 |
Table 1 Frequency and Percentage Distribution of Socio-Demographic Characteristics of Children Aged 2–59 Months with Severe Pneumonia Admitted from January 2017 to December 2020 at NEMMCSH |
Cough was present in about two-thirds of the children (67%). Seventy-two percent (72%) of them visited the hospital within three days after becoming ill. After seven days, twelve (4.29%) of the patients had presented to the hospital. More than two-thirds (70.36%) of the children were exclusively breastfed, and no information was recorded in the charts of 58 (20.71%) of the children. Nearly half (47.14%) of the children were fully vaccinated, while 54 (19.29%) were not. Among the 280 children, 243 (86.79%) had chest indrawing and 208 (72.9%) required oxygen support at the time of admission. Nearly a quarter of the youngsters (19.29%) were underweight, and 35.71% were stunted. Crystalline penicillin was given to 63.85% of the children at the time of admission. Antibiotics were changed for 17.50% of the children (Table 2).
 |
Table 2 Frequency and Percentage Distribution of Clinical Characteristics of the Children Aged 2–59 Months with Severe Pneumonia Admitted from January 2017 to December 2020 at NEMMCSH |
Incidence of Recovery and Treatment Outcome
Out of 280 children, 260 (92.86%) of them were discharged with improvement, only 3 patients were found to have died and 10 (3.57%) children had left the hospital without medical advice (Figure 2).
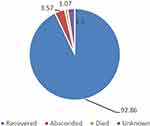 |
Figure 2 Outcome of children aged 2–59 months with severe pneumonia admitted from January 2017 to December 2020 at NEMMCSH. |
The total person days contributed by the study participants were 1076 days. The overall incidence density of recovery obtained was 24.16 per 100 person-days. The incidence density of recovery within five days, ten days, and fifteen days was 33.97 per 100 PD, 23.2 per 100 PD, and 21.1 per 100 PD respectively. The incidence density of recovery from severe pneumonia among males was 24.7 per 100 PD and that of females was 23.54 per 100 PD (Table 3).
 |
Table 3 Incidence Density of Recovery Among Strata of Categorical Variables of Children Aged 2–59 Months with Severe Pneumonia Admitted from January 2017 to December 2020 at NEMMCSH |
Median Time to Recovery from Severe Pneumonia (Survival Estimation)
According to the Kaplan Meier survival estimation the overall median recovery time was four days (95% CI = 3.33–4.68) (Figure 3).
 |
Figure 3 Cumulative survival distribution of children aged 2–59 months with severe pneumonia admitted from 2017 to 2020 at NEMMCSH. |
Predictors of Time to Recovery from Severe Pneumonia
In a bivariate Cox regression analysis, predictors such as age, antibiotics given first, vaccination, exclusive breast feeding, weight for height, delay to come to hospital, inability to feed or drink, needing oxygen support, antibiotic change, and weight for age were associated with time to recovery.
After fitting the multivariable Cox regression, the variables age, antibiotics given first, antibiotic change, and weight for age were found to be significant predictors of time to recovery from severe pneumonia in children aged from 2–59 months.
The proportional hazard assumption was satisfied for the significantly associated variables. When compared to children aged 2–11 months, those aged 12–35 months were twice as likely to recover from severe pneumonia (AHR: 2.0, 95% CI=1.30–3.30). Children who were treated with ampicillin and gentamicin were 65% less likely to recover than children who were treated with C. penicillin (AHR: 0.35, 95% CI: 0.13–0.80). Underweight children were 44% less likely to recover from severe pneumonia than children with normal weight for age (AHR = 0.56, 95% CI = 0.38–0.80). Those children for whom antibiotic changed had 66% lower hazard of recovery as compared to children for whom antibiotics were not changed (AHR = 0.34, 95% CI = 0.21–0.53) (Table 4).
 |
Table 4 Adjusted Hazard Ratio, 95% Confidence Interval and p-value of Variables That were Included in the Multivariable Cox Regression Analysis |
Discussion
The aim of this study was to find out the incidence of recovery, median time to recovery from severe pneumonia and its predictors among children aged 2–59 months who were admitted to pediatric ward of NEMMCSH from January 2017 to December 2020.
The overall incidence density of recovery was 24.16 per 100 person-days. This finding is higher than the incidence density of recovery in University of Gondar Comprehensive Specialized Hospital,30 Debre Markos Referral Hospital.31 The difference in the quality of services and time when the studies were conducted may explain the discrepancy among studies. The two studies were conducted earlier than this study, over time, there could have been differences in treatment protocol, service quality, and in availability of medical equipment. Also the difference in comorbidity status among children could explain the variation in the finding.
The median time to recover from severe pneumonia is four days (IQR=2 days). Being a tertiary hospital in a developing African country, this could be termed as shorter duration of hospitalization (fast progress). The United States Centers for Diseases Control and Prevention estimated that the average length of hospital stay for treatment of pneumonia in children is 5 days.32 The CDC’s higher estimation of length of hospital stay could be because they estimated for all children under the age of 15 years. Studies conducted in University of Gondar Comprehensive Specialized Hospital, Northern Ethiopia (3 days)30 and Debre Markos Referral Hospital (4 days)31 found nearly similar median time to recover. But the median time to recovery in this study was higher than the finding in Nepal (2 days),11 and lower than findings reported by two studies conducted in Tanzania (6 and 10 days).33,34 This difference could be due to the time difference among studies and the method (model) of analysis used; as both the Tanzanian studies used the logistic regression which does not consider censoring of study subjects.
At the multivariable level of analysis, the variables antibiotic given first, antibiotic change, weight for age, and age were significant predictors of time to recovery.
Older children were 2 times more likely to recover from severe pneumonia as compared to children of young age. This finding is consistent with the findings in University of Gondar Comprehensive Specialized Hospital, Northern Ethiopia30 and Nepal.11
It took a longer time to recover from severe pneumonia for underweight children than children with normal weight for age, this finding is in agreement with the finding in University of Gondar Comprehensive Specialized Hospital, Northern Ethiopia,30 Gambia by Kuti et al, and Nepal by Sudha Basnet et al.11,32 The weakened immune system and increased susceptibility, of malnourished children, to severe infection and complications could be a possible explanation for a prolonged time to recovery among underweight children.35 Children who were treated with ampicillin and gentamicin as first line drugs had long time to recovery. To our knowledge, this is the first study to identify ampicillin and gentamicin as an independent predictor of the time to recovery from severe pneumonia. This could be due to the fact that ampicillin and gentamicin are administered for very severe pneumonia (severe pneumonia with heart failure as a danger sign) and severe pneumonia plus malnutrition which need longer time to recover. Also, the possibility of severe drug reactions in children treated with ampicillin and gentamicin could explain the longer duration of recovery.36,37
Those children for whom antibiotic was changed stayed in hospital longer in order to recover as compared to children for whom antibiotics were not changed. Antibiotics are changed when there is treatment failure on first line drugs. The extra time taken by second line drugs to act could explain the longer time to recovery.
Conclusion
The median length of hospital stay was short (4 days). Underweight (weight for age), age, antibiotics given first, and antibiotic change were significant predictors of time to recovery from severe pneumonia. However, exclusive breast feeding, vaccination, sex, place of residence, and delay to come to hospital were not significantly associated with the recovery time. Measures like feeding children nutritious meals and careful management of underweight children should be strengthened. Special attention should be given to children with the identified predictors while treating them. Further prospective studies are recommended to incorporate laboratory results and other clinical parameters that are not included in this study.
Ethical Consideration
Ethical clearance was obtained from the ethical review committee of Wachemo University, College of medicine and Health Sciences. A formal letter was submitted to Wachemo University, Nigist Elleni Memorial Comprehensive specialized hospital and permission was assured. Parental informed consent to review their medical records was not required by the review committee because parents gave permission for data to be used for research purposes while they were at hospital attending to their children.
All information collected from patient cards was kept strictly confidential and names of patients were not included in the checklist. The study was in compliance with the Declaration of Helsinki.
Acknowledgments
We would like to thank Wachemo University for giving the opportunity to conduct the study.
We are grateful to the pediatric ward nurse and card room staff for their cooperation in the process of the data collection.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
WCU.
Disclosure
The authors reported no conflicts of interest for this work.
References
1. WHO. Pneumonia Fact Sheet. WHO; 2016.
2. Southern Cross Medical Library. Pneumonia - Causes, Symptoms, Diagnosis, Treatment. Southern Cross Medical Library; 2015.
3. McAllister DA, Liu L, Shi T, et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Global Health. 2019;7(1):e47–e57. doi:10.1016/S2214-109X(18)30408-X
4. UNICEF. UNICEF data: monitoring the situation of children and women; 2016. New York. Available from: http://data.unicef.org/child-protection/child-marriage.html.
5. Williams BG, Gouws E, Boschi-Pinto C, et al. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002;2(1):25–32. doi:10.1016/S1473-3099(01)00170-0
6. Tiewsoh K, Lodha R, Pandey RM, et al. Factors determining the outcome of children hospitalized with severe pneumonia. BMC Pediatr. 2009;9(1):15. doi:10.1186/1471-2431-9-15
7. Kuti BP, Adegoke SA, Oyelami OA, et al. Predictors of prolonged hospitalisation in childhood pneumonia in a rural health centre. South African J Child Health. 2014;8(1):11–15. doi:10.7196/sajch.663
8. UNICEF. UNICEF Data: Monitoring the Situation of Children and Women. UNICEF; 2018.
9. UNICEF. UNICEF data: monitoring the situation of children and women; 2018. Available from: http://data.unicef.org/topic/child-health/pneumonia.
10. Song P, Theodoratou E, Li X, et al. Causes of death in children younger than five years in China in 2015: an updated analysis. J Glob Health. 2016;6(2). doi:10.7189/jogh.06.020802.
11. Basnet S, Sharma A, Mathisen M, et al. Predictors of duration and treatment failure of severe pneumonia in hospitalized young Nepalese children. PLoS One. 2015;10(3):e0122052. doi:10.1371/journal.pone.0122052
12. Ansari ME, Kumar A, Aggarwal KC, Meena KR, Kamal M. Outcome predictors of severe and very severe pneumonia in children between 2 and 59 months of age admitted in a tertiary care hospital. Indian J Child Health. 2016;4(1):39–43. doi:10.32677/IJCH.2017.v04.i01.011
13. World Health Organization. Acute Respiratory Infections in Children: Case Management in Small Hospitals in Developing Countries, a Manual for Doctors and Other Senior Health Workers. World Health Organization; 1990.
14. Wootton DG, Dickinson L, Pertinez H, et al. A longitudinal modelling study estimates acute symptoms of community acquired pneumonia recover to baseline by 10 days. Eur Respir J. 2017;49(6):1602170. doi:10.1183/13993003.02170-2016
15. Mulholland EK, Smith L, Carneiro I, Becher H, Lehmann D. Equity and child-survival strategies. Bull World Health Organ. 2008;86:399–407. doi:10.2471/BLT.07.044545
16. Madsen HO, Hanehøj M, Das AR, et al. Costing of severe pneumonia in hospitalized infants and children aged 2–36 months, at a secondary and tertiary level hospital of a not‐for‐profit organization. Trop Med Int Health. 2009;14(10):1315–1322. doi:10.1111/j.1365-3156.2009.02374.x
17. Zhang S, Sammon PM, King I, et al. Cost of management of severe pneumonia in young children: systematic analysis. J Glob Health. 2016;6(1). doi:10.7189/jogh.06.010408.
18. Bozzani FM, Arnold M, Colbourn T, et al. Measurement and valuation of health providers’ time for the management of childhood pneumonia in rural Malawi: an empirical study. BMC Health Serv Res. 2016;16(1):314. doi:10.1186/s12913-016-1573-5
19. Hauck K, Zhao X. How dangerous is a day in hospital? A model of adverse events and length of stay for medical inpatients. Med Care. 2011;49:1068–1075. doi:10.1097/MLR.0b013e31822efb09
20. Quadros D-R, Kamenwa R, Akech S, et al. Hospital-acquired malnutrition in children at a tertiary care hospital. South African J Clin Nutr. 2018;31(1):8–13. doi:10.1080/16070658.2017.1322825
21. Chola L, Robberstad B. Estimating average inpatient and outpatient costs and childhood pneumonia and diarrhoea treatment costs in an urban health centre in Zambia. Cost Effect Resource Alloc. 2009;7(1):16. doi:10.1186/1478-7547-7-16
22. Ayieko P, Akumu AO, Griffiths UK, et al. The economic burden of inpatient paediatric care in Kenya: household and provider costs for treatment of pneumonia, malaria and meningitis. Cost Effect Resource Alloc. 2009;7(1):3. doi:10.1186/1478-7547-7-3
23. Eke G, Opara P. Discharge against medical advice amongst patients admitted into the Paediatric wards of the University of Port Harcourt Teaching Hospital. Niger J Paediatr. 2013;40(1):40–44.
24. UNICEF. Survival and health; 2014. Available from: www.unicef.org/ethiopia.
25. Central Statistical Agency (CSA) [Ethiopia] and ICF. Ethiopia Demographic and Health Survey 2016. Addis Ababa, Ethiopia, and Rockville, Maryland, USA: CSA and ICF; 2016.
26. The Federal Democratic Republic of Ethiopia Ministry of Health. Health Sector Transformation Plan. Ethiopia: The Federal Democratic Republic of Ethiopia Ministry of Health; 2015:70.
27. Malla T, Poudyal P, Malla K. Modifiable demographic factors that differentiate bronchiolitis from pneumonia in Nepalese children less than two years–a hospital based study. Kathmandu Univ Med J. 2014;12(3):175–180. doi:10.3126/kumj.v12i3.13710
28. Pintilie M. Advance Statistical Methods for Clinical trials: power and sample size for time to event analysis. 2013.
29. Neter J, Kutner MH, Nachtshem CJ, Wasserman W. Applied Linear Statistical Models. Chicago: Irwin; 1996.
30. Assfaw T, Yenew C, Alemu K, et al. Time-to-recovery from severe pneumonia and its determinants among children under-five admitted to University of Gondar Comprehensive Specialized Hospital in Ethiopia: a retrospective follow-up study; 2015–2020. Pediatr Health Med Therap. 2021;12:189. doi:10.2147/PHMT.S305383
31. Mengist B, Tesfa M, Kassie B, Patman S. Time to recovery and predictors of severe community-acquired pneumonia among pediatric patients in Debre Markos referral hospital, North West Ethiopia: a retrospective follow-up study. PLoS One. 2020;15(9):e0239655. doi:10.1371/journal.pone.0239655
32. Kuti BP, Adegoke SA, Oyelami OA, Ota MO. Predictors of prolonged hospitalisation in childhood pneumonia in a rural health centre. South African J Child Health. 2014;8(1):11–15.
33. Caggiano S, Ullmann N, De Vitis E, et al. Factors that negatively affect the prognosis of pediatric community-acquired pneumonia in district hospital in Tanzania. Int J Mol Sci. 2017;18(3):623.
34. Forsberg P. Pneumonia Among Hospitalized Children Aged 1–9 Years: A Prospective and Retrospective Study at a Referral Hospital in Northern Tanzania. Sahlgrenska Academy, Gothenburg University; 2012.
35. Blossner M, De Onis M, Prüss-üstün A. Malnutrition: Quantifying the Health Impact at National and Local Levels. World Health Organization; 2005.
36. World Health Organization. Revised WHO Classification and Treatment of Childhood Pneumonia at Health Facilities: Evidence Summaries. Geneva: World Health Organization; 2014.
37. Asghar R, Banajeh S, Egas J, et al. Chloramphenicol versus ampicillin plus gentamicin for community acquired very severe pneumonia among children aged 2–59 months in low resource settings: multicentre randomised controlled trial (SPEAR study). BMJ. 2008;336(7635):80–84. doi:10.1136/bmj.39421.435949.BE
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.