Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Time to Immunologic Recovery and Its Determinant Factors Among Adult HIV Patients Who Initiated Antiretroviral Treatment at Hiwot Fana Specialized University Hospital, Harar, Ethiopia
Authors Demeke Bayou F , Nigussie Amare S
Received 26 August 2021
Accepted for publication 22 November 2021
Published 1 December 2021 Volume 2021:13 Pages 1009—1014
DOI https://doi.org/10.2147/HIV.S336167
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Fekade Demeke Bayou,1 Shambel Nigussie Amare2
1Department of Epidemiology, College of Medicine and Health Sciences, Jigjiga University, Jigjiga, Ethiopia; 2Department of Clinical Pharmacy, School of pharmacy, College of Health and Medical Science, Haramaya University, Harar, Ethiopia
Correspondence: Fekade Demeke Bayou Email [email protected]
Objective: To determine the time to immunologic recovery and its determinant factors among adult HIV patients who initiated antiretroviral treatment at Hiwot Fana Specialized University Hospital from February, 2018 to January, 2020.
Methods: A facility-based retrospective cohort study was conducted among 301 adult HIV patients who initiated antiretroviral treatment from February, 2018 to January, 2020. Five trained nurses collected the data using data abstraction checklists. The collected data were entered into the computer using EpiData version 3.1 and then exported to Statistical Package for Social Sciences (SPSS) version 25. The median survival time to immunologic recovery was described using Kaplan–Meier (KM) estimator. Cox proportional hazards regression model was used to identify the potential determinant factors of the time to immunologic recovery. An adjusted hazard ratio (AHR) with its 95% confidence interval (CI) and p-values < 0.05 were used to determine the strength and significance of associations between variables.
Results: In this study, the overall median time required to reach normal CD4 count was 11 months [95% CI = (9.50, 12.51)]. Baseline functional status, ambulatory [AHR=1.383, 95% CI (1.05, 1.83)], bedridden [AHR=1.712 (1.11, 2.64)], first-line treatment classes (TDF/3TC/DTG) [AHR= 1.63, 95% CI (1.21, 2.18)], and baseline CD4 count > 350 cells/mm3 [AHR=1.65, 95% CI (1.11, 2.45)] were significantly associated with the time to immunologic recovery.
Conclusion: The median time to immunologic recovery was relatively shorter. Baseline functional status (ambulatory and bedridden), baseline CD4 count, and first-line treatment class were significant predictors of time to immunologic recovery. HIV patients with working functional status should be given the necessary attention. Utilization of dolutegravir-based regimens should be encouraged to attain a normal CD4 count earlier.
Keywords: time to immunologic recovery, CD4 count, Harer, Ethiopia
Introduction
Although treatment options have expanded significantly, human immunodeficiency virus (HIV) remains an important global public health issue.1 According to the 2020 World Health Organization (WHO) report, there were 38 million people living with HIV, 1.7 million newly infected people, and about 0.7 million people died of acquired immune deficiency syndrome (AIDS) related Illnesses.2 In Ethiopia, the number of new HIV infections diagnosed each year increased by 36% among all ages and doubled among adults.3 In 2019, there were more than 670,000 PLHIV and 11,546 annual deaths due to HIV/AIDS.4 To avert this problem; the government of Ethiopia adopted and are implementing the global 90-90-90 HIV prevention strategies to eliminate HIV/AIDS epidemic by 2030. As a result, ART coverage has increased by 90% among all ages and tripled among pregnant women within six years. Consequently, AIDS-related deaths declined by 77% and 79% among all ages and children respectively.3,5
Effective use of ART can suppress HIV viral replication, slow down the disease progression, improve immunity, and delay mortality.6 Early initiation and long-term adherence to ART are critical for achieving optimal response and reducing the risk of transmission to uninfected partners.7 Response to ART can be assessed by clinical evaluation, immunologic recovery (CD4 count), or retroviral load.8 Compared to clinical or immunological monitoring, virological monitoring provides an early and more accurate indication of the clinical outcomes. However, due to high costs and technical demands of the test, clinical and immunological assessments are usually recommended to start and monitor the efficacy of therapy.9 CD4-cell counts provide an overall picture of the immune status of PLHIV and are often used to determine health status and when to initiate and discontinue the use of medications to treat opportunistic infections.10
One of the aims of ART is to shorten the length of time to reach normal CD4 count and to help patients retain their former better immunity level as early as possible. Evidence from a previous study indicated that baseline CD4 count, baseline age, and sex were potential determinants of the time to immunologic recovery.11 For proper management of the treatment, measuring the length of time required to reach normal range CD4 count from ART initiation and identifying determinants of the time to immunologic recovery, are of paramount importance. Understanding the time to immunologic recovery also helps to determine the severity of immunosuppression and is a guide for decisions on how prophylaxis for opportunistic infections should be given. However, evidence on these issues is scare in Ethiopia, particularly in the study setting (HFSUH). Therefore, this study was aimed at determining the time to immunologic recovery and its determinant factors among adult HIV patients who initiated ART at Hiwot Fana Specialized University Hospital, Eastern Ethiopia
Methods and Materials
Study Setting, Design, and Period
This study was conducted at Hiwot Fana Specialized University Hospital, located in Harari region. Harari region is one of the ten regional states found in the eastern part of Ethiopia at a distance of 526 km from Addis Ababa. The estimated total population of the region is 183,344. In the region, there are three public hospitals, two private hospitals, four urban and three rural health centers, and 33 private clinics. A facility-based retrospective cohort study was conducted from June 15 to July 15, 2021.
Population
All HIV positive adults who initiated ART treatment in the hospital from February, 2018 - January, 2020 were the source population. All selected HIV positive adults who initiated ART treatment in HFSUH from 2018–2020 were included in the study. Patients who had incomplete information on major study variables (like CD4 count, date of ART initiation and recovery) were excluded.
Sample Size and Sampling Technique
In this study, 301 adult HIV patients who initiated antiretroviral treatment from February, 2018 - January, 2020 participated. The medical record numbers of adult HIV positive cases who initiated ART from February, 2018 - January, 2020 were obtained from the registration books. After getting the medical record numbers of HIV cases, the charts of the respective medical record numbers were evaluated for completeness and eligibility based on the inclusion criteria.
Data Collection Methods and Procedure
The data were extracted from the patients’ charts using pretested data abstraction format (see S1Text), which was constructed after reviewing different literature. It was prepared in English language and included variables like sociodemographic characteristics, clinically related factors and, drug-related variables. Five B.Sc. level nurses collected the data after getting two days’ training on the data collection tool and processes. Two master’s holder lecturers experienced in public health research supervised the data collection processes. The collected data were examined for completeness and consistency during data abstraction on a daily basis.
Study Variables
The outcome variable was the time to immunologic recovery (time to reach normal CD4 count). Variables including: sex of the participants, age of the participants, comorbidity status, baseline functional status, baseline WHO clinical stage, first treatment class, baseline CD4 count, and adverse effect of treatments were independent variables.
Operational Definitions
Survival Time
It was defined as the starting date of ART initiation to the time of reaching the minimum value of the normal range CD4 count (500 cells/ mm3) determined for each participant.
Time to Immunologic Recovery
It was determined by calculating the differences in the number of months it took from date of initiation of treatment until a patient attained/reached normal range CD4 count.
Censored
Participants who never attained the normal range CD4 count for different reasons over the follow-up period (right censored), lost to follow-up, or died before reaching immunologic recovery during the study.
Method of Data Analysis
The collected data were entered into the computer using EpiData version 3.1 and then exported to Statistical Package for Social Sciences (SPSS) version 25. The data were checked, coded, cleaned, and edited before analyses. Patients’ characteristics were described using frequency and percentage. The median survival time until patients reached the normal range CD4 counts was also described using Kaplan-Meier (KM) estimator. Log rank test was used to compare the survival experience of different categories of covariates. Cox proportional hazards regression model was used to identify the potential determinant factors associated with the time to immunologic recovery among HIV patients. The Schoenfeld residual test was used to check the proportional hazard assumption. An adjusted hazard ratio (AHR) with its 95% confidence interval (CI) and p-values < 0.05 were used to determine the strength and significance of associations between predictor variables and time to immunologic recovery.
Ethical Consideration
Ethical clearance letter was obtained from Haramaya University, College of Health and Medical Sciences, Institutional Health Research Ethics Review Committee (IHRERC) under reference number of IHRERC/088/2021. Haramaya University, College of Medical and Health Sciences IHRERC allowed the review of patients’ medical records with justifiable reason (for research purposes) regardless of patients’ consent. The head administrator of the hospital provided consent on behalf of the patients. In addition, a formal permission letter to conduct the data collection was obtained from the head administrator of the hospital. Moreover, the names and unique identification numbers of patients were kept confidential and anonymous in compliance with the Declaration of Helsinki. Basic COVID-19 safety measures (wearing a mask, keeping social distance and using alcohol-based hand rubs) were properly applied throughout the study to protect the data collectors as well as the supervisors from the deadly pandemic.
Result
Characteristics of the Study Participants
A total of 301 cases were followed-up retrospectively to determine the time to immunologic recovery and its determinant factors. The majority (67.4%) of the participants were female and the median age was 39.0 years (interquartile range = 18 years). Out of the total participants, 182 (60.5%) of them lived in rural areas. About 112 (37.2%) had baseline WHO Clinical Stage III, 54 (17.9%) had comorbidities, and 49.5% had working functional status. The majority of the participants (41.9%) were taking TDF/3TC/DTG as the first treatment regimen and 116 (38.5%) had history of changing the first-line ART regimens (Table 1).
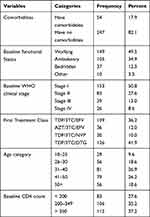 |
Table 1 Characteristics of HIV Patients at Hiwot Fana Specialized University Hospital, February, 2018 - January, 2020 (n=301) |
Time to Immunologic Recovery
The overall median time to attain immunologic recovery (normal CD4 count or > 500 cells/mm3) was 11 months, ranging from 1 to 42 months. Participants aged between 18 and 25 years had the shortest duration (7 months) to reach normal CD4 count while those above 50 years old had the longest duration (13 months) (Table 2).
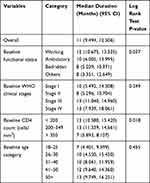 |
Table 2 Median Time (in Months) to Immunologic Recovery by Different Factors Among HIV Patients at Hiwot Fana Specialized University Hospital, February, 2018- January, 2020 |
Determinants of Time to Immunologic Recovery
In this study the baseline functional status, ambulatory: [AHR=1.383, 95% CI (1.05, 1.83)], bedridden: [AHR=1.712 (1.11, 2.64)], first line treatment classes (TDF/3TC/DTG): [AHR= 1.63, 95% CI (1.21, 2.18)] and baseline CD4 count > 350 cells/mm3: [AHR=1.65, 95% CI (1.11, 2.45)] were statistically significant determinants of the time to immunologic recovery. Accordingly, bedridden HIV patients were nearly two times more likely to recover faster (reach their normal CD4 count) as compared to those with working functional status. On the other hand, patients who were on TDF/3TC/DTG treatment were 1.6 times more likely to reach their normal CD4 count within the shortest time compared to those on TDF/3TC/EFV treatment. Similarly, patients with baseline CD4 count between 200 and 349 cells/mm3 were 1.7 times more likely to recover faster than those with CD4 count < 200 cells/mm3 (Table 3).
 |
Table 3 Determinant Factors of Time to Reach Normal Range CD4 Count Among Adult HIV Patients Who Initiated ART in Hiwot Fana Specialized University Hospital February, 2018 - January, 2020 |
Discussion
In this study, a total of 301 HIV patients were followed-up retrospectively to determine the time to immunologic recovery and its determinant factors. Accordingly, the overall median time required to reach normal CD4 count was 11 months (95% CI = 9.50, 12.51). Baseline functional status, ambulatory: [AHR=1.383, 95% CI (1.05, 1.83)], bedridden: [AHR=1.712 (1.11, 2.64)], first-line treatment classes (TDF/3TC/DTG): [AHR= 1.63, 95% CI (1.21, 2.18)], and baseline CD4 count > 350 cells/mm3: [AHR=1.65, 95% CI (1.11, 2.45)] were significantly associated with the time to immunologic recovery (time to reach normal CD4 count).
This study found ambulatory and bedridden HIV patients were more likely to recover faster as compared to those with working functional status. Although this finding is not in line with findings from other similar studies,12,13 the possible justification might be that bedridden patients are more likely to have good adherence to ART treatments as they are under close follow-up by health care givers. On the other hand, patients who were on TDF/3TC/DTG treatment were more likely to reach their normal CD4 count within the shortest time than those on TDF/3TC/EFV treatment. Even though there is no similar study which has investigated this association so far, there is strong evidence which revealed better safety and viral load suppression effect of DTG based ART regimens.14–17 The drug safety again enhances adherence to the treatments, while effective viral load suppression may yield better improvement of the immune system. As a result, patients on this treatment regimen may return to their normal range CD4 count within a shorter time. Higher baseline CD4 count was associated with a shorter time to immunologic recovery. This finding is supported by other similar studies.11,18 The possible explanation for this association might be; as patients with higher baseline CD4 count are closer to the normal range CD4 count, it may take less time to get immunologic recovery as compared to those with low baseline CD4 count. This study has the following limitation, since the study was based on secondary data, information related to some relevant variables was not available; hence, their effect on the time to immunologic recovery was not determined.
Conclusion and Recommendation
The median time required to attain normal range CD4 count was relatively shorter. Baseline functional status (ambulatory and bedridden), baseline CD4 count, and first-line treatment class (TDF/3TC/DTG) were significant predictors of the time to immunologic recovery. HIV patients with working functional status should be given the necessary attention in spite of good performance of their day-to day-activities. Utilization of DTG (dolutegravir)-based regimens should be encouraged to achieve and retain normal range CD4 count early.
Abbreviations
AIDS, acquired immunodeficiency syndrome; AHR, adjusted hazard ratio; ART, antiretroviral therapy; CD4, cluster of differentiation 4; CI, confidence interval; DTG, Dolutegravir; FDC, fixed dose combination; HFSUH, Hiwot Fana Specialized University Hospital; PLHIV, People Living with Human Immunodeficiency Virus; WHO, World Health Organization.
Acknowledgment
We would like to thank Haramaya University, College of Health and Medical sciences and School of Pharmacy for the assistance to conduct this study.
Author Contributions
All authors made a significant contribution throughout the work, i.e, in the conception of the study, study design, execution, acquisition of the data, analysis and interpretation of the result, in drafting the manuscript, revising the article, gave final approval of the version to be published, have agreed on the journal to which the article has been submitted and agreed to be accountable for all aspects of the work.
Funding
There was no funding source for this study.
Disclosure
The authors declare that they have no competing interests.
References
1. UNAIDS. Ending AIDS progress towards the 90- 90-90 targets. Geneva: Global AIDS update; 2017.
2. World Health Organization. Latest HIV estimates and updates on HIV policies uptake. Geneva; 2020.
3. Girum T, Wasie A, Worku A. Trend of HIV/AIDS for the last 26 years and predicting achievement of the 90-90-90 HIV prevention targets by 2020 in Ethiopia: a time series analysis. BMC Infect Dis. 2018;18:320. doi:10.1186/s12879-018-3214-6
4. The Ethiopian Public Health Institute. HIV related estimats and projections in Ethiopia for the year-2019. Adis Ababa: EPHI; 2020.
5. The Ethiopian Public Health Institute. HIV related estimates and projections for Ethiopia. Adis Ababa; 2017.
6. Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trails Group 320 Study Team. N Engl J Med. 1997;337:725–733. doi:10.1056/NEJM199709113371101
7. Sax PE, Meyers JL, Mugavero M, et al. Adherence to antiretroviral treatment and correlation with risk of hospitalization among commercially insured HIV patients in the United States. PLoS One. 2012;7:e31591.
8. Aldous JL, Haubrich RH. Defining treatment failure in resource-rich settings. Curr Opin HIV AIDS. 2009;4(6):459–466. doi:10.1097/COH.0b013e328331dea5
9. World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach.
10. World Healths Organization. WHO guidelines on the use of CD4, viral load and early infant diagnosis (EID) tests for initiation and monitoring of ART. Geneva: WHO; 2015.
11. Gezie LD, Gelaye KA, Worku AG, et al. Time to immunologic recovery and determinant factors among adults who initiated ART in Felege Hiwot Referral Hospital, northwest Ethiopia. BMC Res Notes. 2017;10:1–7.
12. Lenjiso GA, Endale BS, Bacha YD. Clinical and immunological failure among HIV-positive adults taking first-line antiretroviral therapy in Dire Dawa, eastern Ethiopia. BMC Public Health. 2019;19(1):1–10. doi:10.1186/s12889-019-7078-5
13. Nigusie T, Asfaw D, Belete B. Determinants of change in CD4 count and relationship with survival among children with HIV in Ethiopia. AIDS Care. 2020;33:1237–1241.
14. Rossetti B, Baldin G, Sterrantino G, et al. Efficacy and safety of dolutegravir-based regimens in advanced HIV-infected naive patients: results from a multicenter cohort study. Antiviral Res. 2019;169:104552. doi:10.1016/j.antiviral.2019.104552
15. Mehari EA, Muche EA, Gonete KA. Virological suppression and its associated factors of Dolutegravir based Regimen in a resource-limited setting: an observational retrospective study in Ethiopia. HIV/AIDS. 2021;13:709.
16. Ndashimye E, Arts EJ. Dolutegravir response in antiretroviral therapy naïve and experienced patients with M184V/I: impact in low-and middle-income settings. Int J Infect Dis. 2021;105:298–303. doi:10.1016/j.ijid.2021.03.018
17. Rutherford GW, Horvath H. Dolutegravir plus two nucleoside reverse transcriptase inhibitors versus efavirenz plus two nucleoside reverse transcriptase inhibitors as initial antiretroviral therapy for people with HIV: a systematic review. PLoS One. 2016;11(10):e0162775. doi:10.1371/journal.pone.0162775
18. Limmade Y, Fransisca L, Rodriguez-Fernandez R, et al. HIV treatment outcomes following antiretroviral therapy initiation and monitoring: a workplace program in Papua, Indonesia. PLoS One. 2019;14(2):e0212432. doi:10.1371/journal.pone.0212432
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
