Back to Journals » Clinical Epidemiology » Volume 12
Time to Cure and Predictors of Recovery Among Children Aged 6–59 Months with Severe Acute Malnutrition Admitted in Jimma University Medical Center, Southwest Ethiopia: A Retrospective Cohort Study
Authors Hussen Kabthymer R , Gizaw G , Belachew T
Received 16 June 2020
Accepted for publication 8 September 2020
Published 22 October 2020 Volume 2020:12 Pages 1149—1159
DOI https://doi.org/10.2147/CLEP.S265107
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eyal Cohen
Robel Hussen Kabthymer,1 Getu Gizaw,2 Tefera Belachew2
1Human Nutrition Unit, School of Public Health, Dilla University, Dilla, Ethiopia; 2Department of Population and Family Health, College of Health Sciences, Jimma University, Jimma, Ethiopia
Correspondence: Robel Hussen Kabthymer Tel +251913575702
Email [email protected]
Purpose: Treatment at a stabilization center is an important intervention to avert the huge burden of mortality for children with complicated severe acute malnutrition (SAM). Despite the improvement in hospital coverage and the development of standardized WHO treatment guidelines, recent reviews indicated a wide range in recovery rate (34– 88%) due to several context-specific factors. This study aimed to estimate time to recovery and to determine predictors of time to recovery among children aged 6– 59 months with severe acute malnutrition.
Patients and Methods: An institution-based retrospective cohort study design was used among 375 children aged 6– 59 months admitted to Jimma University Medical Center, Jimma, Ethiopia from September 2015 to September 2017. All eligible children were enrolled and assessed using a pretested questionnaire. Kaplan–Meir estimates and survival curves were used to compare the time to recovery using log rank test among different characteristics. Cox proportional hazard model was used to identify significant predictors of time to recovery. A p-value less than 0.05 was declared statistically significant.
Results: The rate of recovery was 4.06 per 100 person days. Median time of recovery for our cohort of SAM children’s was 19 days (95% CI: 17.95– 20.05). Independent predictors of time to recovery were play stimulation (AHR=1.93, 95% CI: 1.23– 3.03), vaccination status (AHR=2.26, 95% CI: 1.12– 4.57), tuberculosis (AHR= 0.48, 95% CI: 0.27– 0.87), malaria (AHR=0.34,95% CI:0.13– 0.88), use of amoxicillin (AHR=1.54, 95% CI: 0.008– 2.34), deworming (AHR=1.8, 95% CI: 1.18– 2.73), and shock (AHR=0.18, 95% CI: 0.05– 0.59).
Conclusion: The findings of this study showed that the average length of stay on treatment and median time for recovery are within the sphere standard. Psychosocial stimulation, appropriate provision of routine medication and management of medical co-morbidity are needed to promote fast recovery.
Keywords: retrospective cohort, predictors, recovery, severe acute malnutrition, Jimma
Introduction
In acute malnutrition, the level of most of macronutrients accessible to our body cells is not enough to maintain its normal function. Insufficient diet, abnormally reduced nutrient absorption or longstanding inflammatory condition that raise the need for nutrients while accelerating wasting would give rise to macronutrient deficiency.1
Acute malnutrition at early stages of life poses a greater risk of mortality or morbidity from common childhood illnesses like pneumonia and diarrheal disease. It also dictates future adulthood health by compromising physical and intellectual growth.2
Depending on level of wasting and the presence of edema acute malnutrition is categorized into severe acute malnutrition (SAM) and moderate acute malnutrition (MAM). It is severe acute malnutrition if the wasting is severe (weight for height < 70% NCHS median or a low MUAC i.e. <110 mm) or if there is edema. Acute malnutrition is defined as moderate acute malnutrition if the wasting is less severe (weight for height between 80% NCHS median and 70% NCHS median); edematous cases were always classified as severe. In many health facilities the death rate from severe malnutrition was over 20% currently, which is unacceptable.3
Severely malnourished children are highly vulnerable to serious infections such as pneumonia, diarrhea, skin infection, overgrowth of bacteria of the gut, and others. Adherence to the management protocol while treating severely malnourished children results in improved recovery. The treatment integrates both nutritional deficiency and medical co-morbidities.4
Cure rate, mortality rate, average length of stay and weight gain are the key parameters to assess the effectiveness of the management. Thus tolerable levels of death, cure rate and default rate should be less than 10%, above 75% and below 15%, respectively.5 On the other hand, many low income countries did not achieve the above parameters.6
Globally acute malnutrition affects nearly 52 million children under the age of five in 2015. Africa and Asia share more than 90% of this effect. Severe forms of acute malnutrition (SAM) is associated with the death of one million children under the age of five each year. Globally, it is estimated that 25–35 million children under the age of five are severely malnourished, with its prevalence ranging from 5 to 30% in 2015, more than two thirds of all acutely malnourished children under the age of five lived in Asia and more than one quarter lived in Africa. In Africa, 14.1 million children less than five years old were wasted, of which 4.3 million are severely wasted. Sub-Saharan Africa (SSA) accounts for 13.2 million children next to south Asia (27.8 million) of acute malnutrition burden.5 In sub-Saharan Africa alone, there are 137 million children under the age of five, of which 12.3 million were wasted.7
In Ethiopia, the level of stunting, underweight and wasting was 38.4, 23.6 and 9.9%, respectively. Among 9.9% of wasted children, 2.9% are severely wasted. In Oromia Region the prevalence of stunting, underweight and wasting was 36.5, 22.5 and 10.6 respectively.8 A study done in Jimma Zone on nutritional status of children revealed that 14.4% were underweight, while 33.9% were stunted and 19.2% were wasted.9
As part of the effort to improve the standard of inpatient care for severely malnourished children and decrease case fatality rates, standardized guidelines have been prepared by WHO10 and Ethiopia3 for treating SAM patients. Using these guidelines, case fatality rate has been reduced considerably.11 At stabilization centers, treatment for complicated SAM children is an important target intervention to significantly reduce the huge burden of mortality. More than 15% of SAM children need admission to therapeutic feeding units. Recent reviews indicated that the recovery rates for inpatient treatment of severe acute malnutrition using the WHO protocol ranged from 33.6% to 88.4%.12–14 A study done in Jimma University Medical Center nutritional rehabilitation unit on severe acute malnutrition showed a recovery rate of 77.8%.15
Although there are studies on recovery of admitted children with SAM and their predictors16–18 the majority were limited to children below the age of 6 months, had smaller sample size and unable to clearly show the effects of some factors like season and psychosocial stimulation. As the factors vary according to context, this study aimed to estimate time to recovery and the effect of psychosocial stimulation on time to recovery from SAM after adjusting for their contextual factors.
Patients and Methods
An institution-based, retrospective cohort study was conducted in Jimma University Medical Center (JUMC). This University is one of the teaching and tertiary medical centers in Ethiopia located in Oromia Region, Jimma Zone; Jimma Town. Jimma Town is located at about 346 km, southwest of Addis Ababa. JUMC offers services for nearly 9,000 inpatient and 80,000 outpatient attendants in a year within the catchment area covering a 250 km radius.
Severe acute malnutrition is defined as weight for height < 70% NCHS median or a low MUAC ie, <110 mm or if there is bilateral pitting edema.
Recovered (Cured)
When the child reaches ≥ 85% of median WFH or WFH Z score ≥ - 2 on more than one occasion or no edema for 10 days.
SAM cases whose card has incomplete data on outcome variable and SAM cases with documented other causes of edema were excluded. This study included all eligible 375 SAM cases. Data were collected from SAM registries and medical records of children. Six graduated diploma nurses were recruited to collect the data. Length of stay and average weight gain were calculated from the available secondary information from cards.
We included all cases of children with SAM treated in Jimma University Medical Center nutritional rehabilitation unit (NRU) between September 2015 and September 2017 and we excluded those SAM cases whose card has incomplete data on outcome variable, those SAM cases who were readmitted within the study period and those SAM cases with documented other causes of edema. Schematic presentation of sampling procedure is shown in Figure 1.
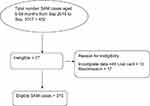 |
Figure 1 Schematic presentation of sampling procedure. |
Data Collection Method
A structured and pretested data collection tool (format) was used to abstract the data from medical records. Data were abstracted from SAM registries and cards of children retrieved from card room using medical record number. Six graduated diploma nurses were recruited to collect the data from the patient medical record and SAM treatment registry. Before the actual data collection, the data collection tool was pretested and necessary amendments were made. One supervisor with principal investigator followed the data collection closely. Length of stay and average weight gain were calculated from the available secondary information from cards. Anthropometric data of patient and other data were taken from medical cards and SAM registry.
Variables/Characteristics at Admission
Variable taken as admission variable if recorded or diagnosed within the first 48 hours of admission.
Play Stimulation
A play session was held by a trained nurse at decorated play stimulation room with different toys and playing aids based on the age of the child for at least for 30–45 min/day. A child will be considered as getting play stimulation if he attended a minimum of three play sessions; otherwise, it is not considered as having play stimuli.
Special Medication
Special medication is a general term used to describe use of treatments like Resomal fluid, blood transfusion, IV fluid and NG tube as per the national guideline.
To assure data quality, the data collection tool was pretested on 20 patient cards treated in Jimma University Medical Center. After pre-test necessary amendments to the tool were made for the final data collection. Then the data collection tool was corrected and data collectors were made aware of the changes made. Two days of intensive training was given for six data collector diploma nurses on how to extract the data from the patient registry. The data that are collected daily were checked by the supervisor and principal investigator for completeness and consistencies. To check the existence of selection bias. We compared the age and sex of the excluded (48 cases) and included (375 cases). Using independent t-test for mean age and chi square test for sex. But there is no statistically significant difference (p-value < 0.05).
Data were entered into Epidata version 3.02 to avoid clerical errors. Variables like anemia were crosschecked by taking recorded measures of hemoglobin from the chart against the physician’s diagnosis to improve data quality. In the case of the play stimulation variable, a list of children indexed by their medical record number who received play stimulation therapy was taken from play stimulation clinics record in the NRU.
Data Processing and Analysis
Data were coded and entered to Epidata software version 3.02. Then data were exported to SPSS version 24 for cleaning, checking and analysis. Age, weight, height and edema were further exported to ENA-SMART software to calculate WFH% and HAZ score from admission and discharge measurements. Descriptive statistics using frequency, percent and measure of central tendency was done. A survival curve was also used to compare time to cure by play stimulation status. The outcome variable was dichotomized as cured and censored for survival analysis. Factors related to time to recovery were analyzed using multivariable Cox proportional hazard model. Proportional hazard assumption of Cox proportional hazard model was checked by plotting log-minus-log survival plot against time for different variables. To control confounding effect of variables, multivariable Cox proportional hazard model was used. Variables with a p-value less than 0.25 in the bivariate analysis were selected and included in the multivariable analysis. Associations with a p-value less than 0.05 were declared as statistically significant associations.
Results
Socio Demographic and Care-Related Characteristics
A cohort of 375 SAM children were followed retrospectively for a median time of 17 days with an inter quartile range of 10 days. Of these children, 191 (50.9%) were females while the rest are males. The mean age (in months) of the study subjects was 26.8 months with a standard deviation of 14.6 months with more than half (53%) aged between 12 and 36 months. Most (255, 68%) were from rural areas. Nearly one third 107 (28.5%) of the children were admitted in the winter (June to August) season. Nearly two-thirds of the children 238 (63.5%) were fully vaccinated for their age (Table 1).
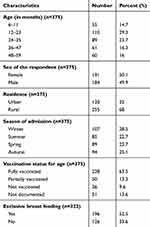 |
Table 1 Socio Demographic and Care-Related Characteristics of SAM Children Admitted in JUMC, Nutritional Rehabilitation Unit from September 2015 to September 2017 |
Anthropometry and Complication-Related Characteristics
More than half (65.9%) of the children had edematous SAM and 224 (59.7%) passed the appetite test. Over two thirds (66.6%) of the children were stunted while nearly half (48.3%) had a WFH% median below 70% (Table 2).
 |
Table 2 Baseline Nutritional Status of SAM Children Admitted in JUMC, Nutritional Rehabilitation Unit from September 2015 to September 2017 |
A total of 356 (94.9%) had at least one type of complication at admission. As shown in Table 3 diarrhea and anemia were the most common comorbidities among the admitted SAM children. About 268 (71.4 %) and 159 (42.4%) had diarrhea and anemia, respectively, as a major comorbidity. These cases were followed by pneumonia (29.6%), dehydration (14.7%), tuberculosis (14.4%), malaria (5.6%) and HIV (3.5%), respectively. Overall, 25 (6.7%) of them had septic or hypo-volumic shock at admission. And about 304 (81.1%) were conscious at admission.
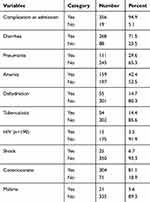 |
Table 3 Medical Complications and Clinical Features of SAM Children Admitted in JUMC, Nutritional Rehabilitation Unit from September 2015 to September 2017 |
Medication and Nutritional Therapy-Related Characteristics
Regarding medication and nutritional therapy, 299 (79.4%) of them had taken folic acid and 270 (72%) of them took amoxicillin. While 158 (42.1%), 126 (33.6%), 129 (34.4%) and 161 (42.9) took vitamin A, iron, deworming and special medication, respectively. A total of 296 (78.9%) had taken IV antibiotic and 68 (18.1%) had taken IV fluid therapy. A few, 30 (8%), had undergone a blood transfusion and 130 (34.4%) used an NG tube. Almost all, 364 (97.1%), were taking formula 75 nutritional therapy while 293 (78.1%), 237 (63.2%) received formula 100 and plumpy nut therapy, respectively. Seventy four (19.7%) of the children also received play stimulation.
Brief Overview of Standard of Care
Regarding Routine Medications
On the day of admission (day 1), vitamin A was given to all children except those with edema or those who had received vitamin A in the past 6 months. On the day of admission, one single dose of folic acid (5 mg) were given to children with clinical signs of anemia.
Regarding Antibiotics
Antibiotics were given to every severely malnourished patient, even if they did not have clinical signs of systemic infection.
Survival Pattern and Time to Recovery
Regarding the outcome of the treatment of the cohort of children with SAM, 274 (73.1%, 95% CI: 68.5–77.4) recovered while 46 (12.35, 95% CI: 9.06–15.6), 10 (2.7%, 95% CI: 1.05–4.35) and 45 (12%, 95% CI: 8.71–15.28) died, transferred out and defaulted, respectively (Figure 2).
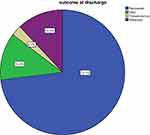 |
Figure 2 Treatment outcome of admitted SAM children. |
The overall follow up time the for 375 SAM children was 6,748 days with cumulative incidence of recovery 0.0406 recovery per person day (40.6 recovery/1000 person days) among admitted children.
Table 4 and Figure 3 illustrate median time of recovery from SAM among severely malnourished children treated at our nutritional rehabilitation unit. The figure showed that the median length of stay for the all SAM children in this study was 17 days ±10 days of interquartile range. The mean length of stay for the entire cohort is 18 days with a minimum of 1 day and maximum of 56 days. Life Table analysis showing the cumulative probability of nutritional recovery was 99, 78, 30, 8, and 1% at 5, 10, 15, 20, 30 and 50 days, respectively. This shows that the probability of recovery decreases as the length of stay increases and vice versa.
 |
Table 4 Actuarial Life Table Analysis Showing Survival of SAM Children Admitted in JUMC from 2015 to 2017, Jimma, Southwest Ethiopia |
 |
Figure 3 Depicted survival graph for length of stay (days) of entire cohort of children admitted with SAM. |
Predictors of Time to Recovery
Bivariate analysis was performed for the variables such as socio-demographic characteristics, anthropometry, type of malnutrition, complication at admission, clinical features, type of medications and nutritional therapy using bivariate Cox regression. Statistical significance was observed in children with play stimulation, categories of age, season of admission, exclusive breast feeding, vaccination status, type of SAM, edema, complication at admission, TB, HIV, malaria, shock, appetite, complication after admission, folic acid, amoxicillin, ampicillin, deworming, gentamicin, special medication, blood transfusion, IV antibiotic, IV fluid, NG tube use and plumpy nut, F-100 (p< 0.25).
Multivariable Analysis
Multivariable Cox regression analysis was performed for variables identified in the bivariate Cox regression having a p-value < 0.25 to adjust for confounders using multivariable Cox regression method.
Vaccination status (fully vaccinated), play stimulation, TB, malaria, amoxicillin, deworming and shock were found to be independent predictors of nutritional recovery time. The likelihood of early recovery in fully vaccinated children was 2.26 (AHR=2.26, 95% CI: 1.12–4.57) times those who were not vaccinated. Similarly, children who received play stimulation therapy were 1.93 (AHR=1.93, 95% CI: 1.23–3.03) times likely to recover fast. Likewise, SAM children with TB were 52% (AHR= 0.48, 95% CI: 0.27–0.87) more likely to have a delayed recovery time compared to children without TB. Also, children with malaria 65.9% (AHR=0.34, 95% CI: 0.13–0.88) have more probability of delayed recovery than their counterparts. In addition, children who took amoxicillin had a 1.54 (AHR=1.54, 95% CI: 0.008–2.34) times more likelihood of fast recovery than their counterparts. Children with SAM who took deworming medicine were 1.8 (AHR=1.8, 95% CI: 1.18–2.73) times more likely to have early recovery than children without deworming. The likelihood of delayed recovery was 82% (AHR=0.18, 95% CI: 0.05–0.59) more in those who developed shock relative to those who did not develop shock (Table 5).
 |
Table 5 Multivariate Cox Proportional Hazard Regression Model for Predictors of Time to Recovery from SAM Among Children Admitted in Jimma University Medical Center, Jimma, Southwest Ethiopia |
Discussion
This study revealed important information about nutritional recovery time and its predictors on children with SAM managed in a nutritional rehabilitation unit. Generally we have understood from the life table analysis as the length of stay increases the likelihood of recovery decreases. The overall median length of stay of the entire cohort was 17 days ± 9 days and median nutritional recovery time for the cohort of SAM children was observed to be 19 days both are within the accepted national minimum standards of average length of stay for inpatient treatment.3 This result was in line with other studies conducted in northern Ethiopia and other developing countries.19 However, in contrast to studies conducted in southern Ethiopia,11,16 this study found a shorter recovery time. This may be due to differences in implementation of SAM management guideline,3 staffing and setting. Looking at the type of SAM, edematous malnutrition accounted for more than half 247 (65.9%). This was in line with studies done in Ethiopia and other African countries.11,15,20 The high prevalence of edematous SAM may be due to frequent intake of carbohydrate and low intake of protein rich foods.20 Diarrhea is the most common co-morbidity seen in 268 (71.5%) children which is consistent with reports from studies in Kenya (70.3%),21 in Sekota (44.6%),13 and in Mekelle (63.4%).17
Regarding treatment outcome, the minimum acceptable standard of sphere project is >75% recovery, <15% default rate, <10% death rate.5 In this study the recovery rate was 73.1% which is in line with the minimum standard. This result is greater than the reports from Gondar University Referral Hospital,22 Mekelle,17 and Bahirdar.23 In contrast to a study done in Southern Ethiopian therapeutic feeding centers they reported that the nutritional recovery rate was 3.61 per 100 person day observations. Rate of recovery in this study was 4.06 per 100 person days (40.6 recovery/1000 person days). This difference can be attributed to the existence of high level professionals like pediatricians and pediatric residents in this study setup.
Our findings indicated that children who were provided with a combination of psychosocial stimulation and therapeutic feeding tended to gain weight at a faster rate of 1.93 times more likely to recover than those who received only therapeutic feeding. Even if play stimulation was included in Ethiopian SAM management guideline, it was not common to see the service in health institutions except in Jimma University Medical Center. The introduction of play stimulation therapy to the existing SAM management has a rewarding effect via reducing hospital stay. This shortened hospital stay has a direct implication in terms of cost and quality of SAM management. Although further studies are required to substantiate, the findings call for arguments to include this very important, but neglected component of treating children with SAM. In addition to dietary and medical therapy, play stimulation therapy needs to be given in all other health facilities in the country.
Among the socio-demographic characteristics, vaccination status is an independent predictor of nutritional recovery time. In this study, children who are not fully vaccinated for age took 2.26 longer recovery time compared to their fully vaccinated for age counterparts. Similarly, a study conducted in Felegehiwot Referral Hospital reported that fully vaccinated SAM children had 4.12 times better recovery rate than their unvaccinated counterparts.23 This can be explained by the role of vaccination in preventing several contagious diseases.
Findings from play therapy in Africa24 indicated that play stimulation increased speed of recovery. Children who received play therapy were discharged as cured before the end of the 4th and the 5th week from admission compared to the control groups who did not receive play stimulation and who were not discharged before the end of 6th week. Besides another study conducted in Bangladesh reported that severely malnourished children who received play stimulation improved weight for age Z-score than their counterparts.25 Results from a randomized placebo controlled trial conducted in Southern Malawi on SAM children showed that nutritional recovery was 1.38 times greater for those who took amoxicillin than the placebo group and it also indicated nutritional recovery time was shorter for those who took amoxicillin than the placebo group.26 Similarly, this study found that amoxicillin speeded up recovery. Children who took amoxicillin were found to have 54% (AHR =1.54, 95% CI: 1.008–2.34) increased speed of recovery than those children who did not take it. Recent updates of WHO10 and the national guideline for SAM management3 recommend provision of a broad spectrum of antibiotics like amoxicillin for all severely malnourished children regardless of signs of infection and complications. Deworming was also found to be a predictor of a shorter recovery time. Severely malnourished children who took albendazole/mebendazole were 1.8 times more likely to have a shorter recovery time as compared to those children who did not (AHR=1.8, 95% CI: 1.18–2.73). This may be due to the high prevalence of intestinal parasite in severely malnourished children which results in reduced appetite and nausea in children who do not get deworming.
The hazard of death due to TB was nearly three times higher than children with no TB (AHR= 2.88, 95% CI= 1.72, 4.65) as reported by Kebede.13 In this study; however, it was found that among children who had tuberculosis, nutritional recovery time was delayed by 51.7%. This can be explained by diminished immunity of SAM children which predisposes them to tuberculosis resulting in disease and inflammatory response. This in turn worsens the nutritional state thus delaying the recovery time. This may highlight the vicious cycle of malnutrition and disease; however the mechanism underlying the association between tuberculosis and malnutrition remains unclear.27
Malaria is among the common febrile illness which affects children. Its effect is highly devastating in severely malnourished children. Even if there is a shortage of studies showing the impact of malaria on recovery rate and recovery time a study done in Sekota Hospital13 showed that malaria increases the death rate and shortens the time to death (AHR= 2.13, 95% CI: 1.12–7.15). However, this study revealed that those severely malnourished children with malaria had a 65.9% delayed recovery than their non malarious counterparts. This could be due to the febrile nature of the disease which puts the child in a catabolic state which in turn may worsen the child’s nutritional state, maybe via reduced appetite. The above-mentioned possible reasons thus could lengthen the time needed to recover.
A study done in therapeutic feeding centers in Gedeo Zone showed that development of shock significantly increased the hazard of death by 3.8 times more.28 But the finding of this study shows that children who developed shock had 82% longer nutritional recovery time than those who did not develop shock. The possible explanation may be because, in children who developed shock there is a decreased perfusion to vital organs that may lead to organ damage, thus prolonging the nutritional recovery time.
A study done in Woldia Hospital indicated that severely malnourished children having HIV/AIDS co-morbidity were 90% less likely to be cured as compared to those without HIV/AIDS co-morbidity. HIV infection was a predominant factor that compromised recovery rate and increased mortality rate.29 There are also other studies that support the finding that being co-morbid with HIV reduces the likelihood of recovery.6,10 However, in this study, HIV was not a significant predictor of nutritional recovery time (p >0.05). This may be due to a shift in practice from routine provider-initiated HIV counseling and testing (PIHCT).
SAM children who did not get vaccination for their age have a longer hospital stay so; implementation of the extended immunization program should be strengthened. The results pose arguments for integration of play stimulation therapy in the management of severe acute malnutrition at scale level to prevent childhood mortality thereby achieving sustainable development goals. Provision and use of routine medications like amoxicillin and deworming, management of complications like shock, TB, and malaria should also stick to the national guideline for management of SAM. All the aforementioned results showed reduced hospital stay thus, implying reduction in the cost needed for treatment and burden of health institutions; which will in turn ensure quality of care.
The longitudinal nature of the study design, giving an insight for researchers who wish to use a prospective design and addressing the effect of variables like season of admission, vaccination status, and play stimulation were some of the strengths of this study. Despite the bulk of evidence regarding severe acute malnutrition, the novel aspect of this study is that it has shown the effect of variables like play stimulation and season of admission on time to recovery.
However, difficulty ascertaining the reliability of recorded data, potential bias due to excluded records and unknown status of defaulters, and failing to address variables like educational status, household wealth index, socioeconomic status, maternal nutritional status, and child’s feeding practice that might have an affect on recovery, were some of the limitations of the study.
Conclusion
Based on the findings of this study, average length of stay for the entire cohort and median time of recovery was within the sphere standard. The probability of recovery decreases with increased hospital stay. Play stimulation, vaccination, amoxicillin and deworming were independent predictors of short nutritional recovery, while the presence of malaria, TB and shock were found to be independent predictors of delayed nutritional recovery time. Availing of routine medication and management of medical co morbidity as per the national SAM management guideline promotes fast recovery.
Abbreviations
AIDS, acquired immune deficiency syndrome; AHR, adjusted hazard ratio; CI, confidence interval; F 75, formula milk 75; F 100, formula milk 100; HIV, human immune deficiency virus; JUMC, Jimma University Medical Center; MRN, medical registration number; MUAC, mid upper arm circumference; NRU, nutritional rehabilitation unit; OR, odds ratio; RUTF, ready to use therapeutic food; SAM, severe acute malnutrition; SPSS, Statistical Package for Social Sciences; TFC, therapeutic feeding center; TFU, therapeutic feeding unit; TB, tuberculosis; UNAIDS, United Nations on HIV/AIDS; UNICEF, United Nations International Children’s Emergency Fund; WFH/A, weight for height/age; WHO, World Health Organization.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon request.
Ethics Statement
Ethical approval and clearance was obtained from Jimma University, Institute of Health Institutional Review Board with approval number of IHRPGC/516/207. Written permission letters were also obtained from concerned bodies of Jimma University Medical Center to access the medical records. Confidentiality of the information was assured and privacy of the study subjects was maintained.
Acknowledgments
We would like to acknowledge Jimma University Medical Center who allowed us to undertake this study. Lastly we are extremely grateful to the participants involved in the study, data collectors, and the rest of the research team members.
Author Contributions
All authors made a significant contribution to the work reported, that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Jimma University funded the expenses for data collection.
Disclosure
The authors report no conflicts of interest in this work. This paper was presented at the Conference named ‘Research for Sustainable Development’ as a poster presentation. The poster’s abstract was published in the Proceedings of Dilla University 8th annual conference. Also the abstract was submitted to the 8th Africa Nutritional Epidemiology conference but not presented. Still the abstract was published in the Conference Proceedings available at www.ansnet.org.
References
1. Pelletier DL, Frongillo EA. Changes in child survival are strongly associated with changes in malnutrition in developing countries. J Nutr. 2003;133(1):107–119. doi:10.1093/jn/133.1.107
2. Victora CG, Adair L, Fall C, et al. Maternal and child under nutrition: consequences for adult health and human capital. Lancet. 2008;371(9609):340–357. doi:10.1016/S0140-6736(07)61692-4
3. Federal Ministry of Health. Protocol for the Management of Severe Acute Malnutrition. Addis Ababa: Ethiopia; 2007:1–120. Available from: https:/files.ennonline.net/attachments/897/ethiopia-sam-guideline-march-2007.pdf.
4. Hobbs B, Bush A. A 10 point plan for tackling acute malnutrition in under fives. Acute malnutrition an everyday’s emergency. 2015. Available from: https:/www.popline.org/node/579688.
5. The Sphere Project Humanitarian Charter and Minimum Standards in Humanitarian Response. Minimum Standards in Food Security and Nutrition: Management of Acute Malnutrition and Micronutrient Deficiencies. 2011:168–169. Available from: http://www.spherehandbook.org/en/management-of-acute-malnutrition-and-micronutrient-deficiencies-standard-2-severe-acute-malnutrition/.
6. Bhutta Z, Das J, Rizvi A, et al. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Matern Child Nutr. 2013;2:1–15.
7. UNICEF/WHO/World Bank Group Joint Child Malnutrition Estimates. Levels and trends in child malnutrition. 2017. Available from: https://www.who.int/nutgrowthdb/estimates.
8. CSA, ICF. Ethiopia Demographic and Health Survey 2017: Key Indicators Report. Addis Ababa, Ethiopia, and Rockville, Maryland, USA; 2017.
9. Beyene TT. Predictors of nutritional status of children visiting health facilities in Jimma Zone, South West Ethiopia. Int J Adv Nurs Sci Pract. 2012;1(1):1–13.
10. WHO. Guideline: Updates on the Management of Severe-Acute Malnutrition in Infants and Children. Geneva: World Health Organization. 2013:1–13. Available from: http://www.who.int/elena/titles/full_recommendations/sam_management/en/.
11. Teferi E, Lera M, Sita S, Bogale Z, Datiko DG, Yassin MA. Treatment outcome of children with severe acute malnutrition admitted to therapeutic feeding centers in Southern Region of Ethiopia. Ethiop J Health Dev. 2010;24(3):234–238.
12. Saaka M, Osman S, Amponsem A, et al. Treatment outcome of severe acute malnutrition cases at the Tamale Teaching Hospital. J Nutr Metab. 2015;2015:1–8. doi:10.1155/2015/641784
13. Kebede S. Survival status and predictors of mortality among children aged 0–59 months with severe acute malnutrition admitted to stabilization center at Sekota Hospital Waghemra Zone. J Nutr Disord Ther. 2015;5(2):160–171.
14. Berti A, Bregani ER, Manenti F, Pizzi C. Outcome of severely malnourished children treated according to UNICEF 2004 guidelines: a one-year experience in a Zone hospital in rural Ethiopia. Trans R Soc Trop Med Hyg. 2008;102(9):939–944. doi:10.1016/j.trstmh.2008.05.013
15. Habtamu J, Alemseged W, Fissehaye A. Survival status and predictors of mortality in severely malnourished children admitted to Jimma University Specialized Hospital from 2010 to 2012, Jimma, Ethiopia: a retrospective longitudinal study. BMC Pediatr. 2015;15(76):1–13.
16. Gebremichael DY. Predictors of nutritional recovery time and survival status among children with severe acute malnutrition who have been managed in therapeutic feeding centers, Southern Ethiopia: retrospective cohort study. BMC Public Health. 2015;15(1):1–11. doi:10.1186/s12889-015-2593-5
17. Melaku G, Afework MB, Mache T. Treatment outcomes and associated risk factors of severely malnourished under five children admitted to therapeutic feeding centers of Mekelle City, Northern Ethiopia. Open J. 2015;1(4):1–9.
18. Banbeta A, Seyoum D, Belachew T, Birlie B, Getachew Y. Modeling time-to-cure from severe acute malnutrition: application of various parametric frailty models. Arch Public Health. 2015;73(6):1–8.
19. Maggie P. Critical appraisal of the management of severe acute malnutrition in Malawi: a case of two hospitals in Zomba. 2011:19–27. Available from: http://ummafrapp.de/skandal/haart/annex%209.pdf.
20. Munthali T, Jacobs C, Sitali L, Dambe R, Michelo C. Mortality and morbidity patterns in under-five children with severe acute malnutrition (SAM) in Zambia: a five-year retrospective review of hospital-based records (2009–2013). Arch Public Health. 2015;73(1). doi:10.1186/s13690-015-0072-1
21. Talbert A, Thuo N, Karisa J, et al. Diarrhoea complicating severe acute malnutrition in Kenyan children: A prospective descriptive study of risk factors and outcome. PLoS One. 2012;7(6):e38321. doi:10.1371/journal.pone.0038321
22. Abeje AT, Gudayu TW, Malefia YD, Befftu BB. Analysis of hospital records on treatment outcome of severe acute malnutrition: the case of Gondar University Tertiary Hospital. Pediatr Therapeut. 2017;6:283. doi:10.4172/2161-0665.1000283
23. Desyibelew HD, Fekadu A, Woldie H. Recovery rate and associated factors of children age 6 to 59 months admitted with severe acute malnutrition at inpatient unit of Bahir dar Felege Hiwot referral hospital therapeutic feeding unit, northwest Ethiopia. PLoS One. 2017;12(2):e0171020. doi:10.1371/journal.pone.0171020
24. Emotional stimulation in the context of emergency food interventions, Play Therapy Africa: the society for protection and therapeutic aid in Africa; final report, Addis Ababa 2009. Available from: http://www.ennonline.net/emotionalstimulation.
25. Nahar B, Hamadani JD, Ahmed T, et al. Effects of psychosocial stimulation on growth and development of severely malnourished children in a nutrition unit in Bangladesh. Eur J Clin Nutr. 2009;63(6):725–731. doi:10.1038/ejcn.2008.44
26. Food and Nutrition Technical Assistance III Project (FANTA). Studying the Use of Antibiotics for Treating Severe Acute Malnutrition. Washington, DC; 2015. Available from: https://www.fantaproject.org/…/FANTA-Impact-CMAM-Antibiotics-May2015_0.pdf.
27. Jaganath D, Mupere E. Childhood tuberculosis and malnutrition. J Infect Dis. 2012;206(12):1809–1815. doi:10.1093/infdis/jis608
28. Girum T, Kote M, Tariku B, Bekele H. Survival status and predictors of mortality among severely acute malnourished children <5 years of age admitted to stabilization centers in gedeo zone: a retrospective cohort study. Ther Clin Risk Manag. 2017;13:101–110.
29. Chane T, Oljira L, Atomesa GE, Agedew E. Treatment outcome and associated factors among under-five children with severe acute malnutrition admitted to therapeutic feeding unit in Woldia Hospital, North Ethiopia. J Nutr Food Sci. 2015;4(329).
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
