Back to Journals » Breast Cancer: Targets and Therapy » Volume 12
Time to Adjuvant Chemotherapy and Its Predictors Among Women with Breast Cancer at the University of Gondar Compressive Specialized Hospital: A Retrospective Follow-Up Study
Authors Zeleke Alem A , Gebeye Zeleke E, Akalu TY
Received 4 May 2020
Accepted for publication 7 August 2020
Published 17 September 2020 Volume 2020:12 Pages 97—108
DOI https://doi.org/10.2147/BCTT.S260341
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Adugnaw Zeleke Alem, Ejigu Gebeye Zeleke, Temesgen Yihunie Akalu
Department of Epidemiology and Biostatistics, Institute of Public Health, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
Correspondence: Adugnaw Zeleke Alem Email [email protected]
Background: Early adjuvant chemotherapy improves the outcomes of breast cancer patients by increasing the benefit provided by the cytotoxic systemic therapies. Despite these, the recommended time to adjuvant chemotherapy and its predictors is very limited. Therefore, this study was determining the time to adjuvant chemotherapy and its predictors among women with breast cancer at the University of Gondar Comprehensive Specialized Hospital.
Methods: An institution-based retrospective follow-up study was conducted at the University of Gondar Compressive Specialized Hospital from January 2015 to February 2019 among all women with breast cancer. Stata version 14 was used for data analysis. A stratified Cox regression model was fitted to identify the potential predictors. The adjusted hazard ratio (AHR) with a 95% confidence interval (CI) was reported to show the strength of the association. Cox–Snell residual test was used to check the goodness of fit.
Results: In this study, the median time to adjuvant chemotherapy was 67 days with an interquartile range of 34– 102 days. More than three-fourth (79.9%) of patients received chemotherapy after 30 days. Of the total, 96.6% of patients with co-morbidity received adjuvant chemotherapy after 30 days. Regarding surgical complications, 97.0% of the patients with a surgical complications were received adjuvant chemotherapy after 30 days. Older patients (AHR= 0.34, 95% CI: 0.16,0.71), presence of co-morbidity (AHR= 0.43, 95% CI: 0.29, 0.62), positive surgical margin (AHR= 0.40, 95% CI: 0.25, 0.64), and presence of surgical complication (AHR= 0.55, 95% CI: 0.34, 0.88) were significantly associated with delayed time to adjuvant chemotherapy.
Conclusion: In this study, time to adjuvant chemotherapy among women was longer. Age, co-morbidity, surgical complications, and margin status were significant predictors of time to adjuvant chemotherapy. Close follow-up is important for women with surgical complications, co-morbidities, elder patients, and patients with a positive margin.
Keywords: adjuvant chemotherapy, breast cancer, survival analysis, Gondar
Background
Globally, breast cancer (BC) is the most common malignant tumor in women and causes about 1.7 million new cases (11.9% of total cancer cases) and over half a million deaths annually. In developing countries, the incidence of BC has gradually increased.1–4 According to 2016 global cancer statistics, it is the most common type of cancer and the second leading cause of death in women.5
In Ethiopia, cancer contributes about 5.8% of the total deaths. Breast cancer is the leading cause of cancer and contributes to 30.2% of cancer cases.1 In Tikur Anbessa Specialized Hospital a total of 16,622 new cases of cancer were registered between 1997 and 2012. Of these, about 20.8% of new cases were BC patients with an annual incidence of 216 cases.6
Adjuvant chemotherapy (AC) is commonly used to improve outcomes of patients with breast cancer particularly to patients with large primary tumors, estrogen receptor (ER) negative, high-grade tumors, and with the invasion of lymph nodes.3,7,8 Adjuvant chemotherapy is routinely recommended to 60–80% of breast cancer patients after surgery9 and decreases 30–40% risk of death.10
The time between surgery and the first adjuvant chemotherapy would appear to have an impact on overall survival (OS) and disease-free survival (DFS) in patients with BC.3,7,8,11–18 However, the selected cutoff points for the definition of early and delayed start of adjuvant chemotherapy were differed. For example, studies showed that a three-month or longer delay between surgery and adjuvant chemotherapy can decrease the survival of patients with BC.7,14–16,18 On the other hand, an interval between surgery and the start of chemotherapy >60 days was associated with poor outcomes of breast cancer.11,17 Moreover, a recent meta-analysis showed that a 4-week increase in TAC was associated with an increase of 4–8% of death among BC patients.19 Another systematic review and meta-analysis indicated that a 4-week increase in time to initiation of AC led to a significant decrease of 15% of overall survival of breast cancer patients.9 Although the optimal time to adjuvant chemotherapy administration for patients with breast cancer is not precisely defined, early initiation of AC improves the survival of BC patients through increasing the benefit provided by cytotoxic systemic therapies by preventing accelerated growth of micro-metastases, increased tumor angiogenesis, or development of primary resistance has been hypothesized.3,7,20
According to different pieces of literature lack of financial issues, lack of knowledge of breast cancer, late presentation or trying to access affordable medical care,1,21 age,21–24 residence,14,21,25,26 body mass index,23,27 marital status,14,24 year of diagnosis,22,25 type of surgery,14,24,26 stage of cancer,14,22,24 co-morbidities,14,21–23,27 number of excisions,21,23 tumor grade,23 surgical complication,21 and surgical margin status21 were predictors of TAC.
Despite there are several studies in developed nations, still there is a limited information on time to adjuvant chemotherapy and its predictors in developing countries including Ethiopia. Moreover, previous studies done on TAC and its predictors were focused on the categorical outcome that does not show the exact time to AC, but this study is able to determine time to adjuvant chemotherapy.
Methods
Patients and Data Collection
An institution-based retrospective follow-up study was conducted among all women with breast cancer and who underwent surgery and treated at the University of Gondar Compressive Specialized Hospital between January 1, 2015, and February 1, 2019. The Hospital is located in the North Gondar administrative zone, Amhara National Regional State, which is 180 km far from Bahir Dar (the capital city of the region) and 727 Km from Addis Ababa, which is the capital city of Ethiopia. In Ethiopia next to Tikur Anbessa Specialized Referral Hospital, the University of Gondar Compressive Specialized Hospital is the second oldest Hospital by starting cancer treatment and follow-up service. The Hospital was started cancer treatment and follow-up service since January 2015. It has outpatient and in-patient departments to provide the service.
Medical records with incomplete data on date of surgery or date of the first dose of adjuvant chemotherapy and patients treated with neoadjuvant chemotherapy before adjuvant chemotherapy were excluded from this study (Figure 1).
 |
Figure 1 Flowchart of medical record abstraction procedure. |
Three BSC degree public health officers collected the data and one medical doctor and principal investigator supervised the overall process. Initially, breast cancer patients were identified from other types of cancer patients using medical records/cancer registers. Then, data extraction was made using an English version extraction checklist from all eligible women with breast cancer. Adjuvant chemotherapy was defined as systemic cancer-directed chemotherapy given after surgery and before any recurrence.
Variables Definitions
The response variable was time to adjuvant chemotherapy which is defined as a time in days between surgery to the first dose of chemotherapy. Independent variables included in this study were: type of surgery (breast conserving surgery (BCS) and mastectomy), age at diagnosis, body mass index (BMI; <18.5 kg/m2 “underweight”, 18.5–25 kg/m2 “normal”, >25 kg/m2 “overweight”), American Joint Committee on Cancer stage at diagnosis (TMN) criteria28 was used to classify stage of cancer as I, II, III and stages IA and IB were categorized as stage I, stages IIA and IIB was collapsed as stage II, and stages IIIA, IIIB and IIIC were collapsed as stage III, number of lymph nodes involved (0, 1–3, >3); co-morbidity was defined as the presence of one of the conditions (congestive heart failure, respiratory diseases, acute myocardial infarction, liver disease, diabetes, renal disease, HIV/AIDS or another type of cancer) was denoted as “yes‟, while the absence of these conditions was denoted as “no‟, which was collected using the Charlson co-morbidity index,29,30 residence, marital status, histological grade, year of diagnosis, margin status, number of excision was defined as the number of ipsilateral excisional procedures performed on separate days before initiation of adjuvant chemotherapy (1 and 2), and surgical complication was defined as the presence of one of the seroma, hematoma, lymphedema or surgical wound infection was recorded as “yes” and absence of these conditions was recoded as “no”.
The event was defined as women with breast cancer who take either cyclophosphamide, 5-fluorouracil, Adriamycin, or combination of cyclophosphamide and Adriamycin during follow-up period. Breast cancer patients who did not take any form of adjuvant chemotherapy at the end of the follow-up period and those who died lost to follow-up, and transfer to another care unit during the study before taking adjuvant chemotherapy were defined as censored.
Statistical Analysis
Data was entered into Epi-data version 3.1 software and transferred to Stata version 14 for statistical analysis. Descriptive statistics were performed and presented using tables, graphs, and texts. Time to adjuvant chemotherapy was calculated by subtracting the date of surgery from the date of the event occurred (adjuvant chemotherapy started). Time to adjuvant chemotherapy was measured and coded as 1 for event and 0 for censored.
Kaplan–Meier survival curve was used to estimate the overall survival and comparison among groups was made by the Log-rank test. Variables with p-value <0.2 in the bi-variable analysis were included in the multivariable analysis. The adjusted hazard ratio (AHR) with a 95% confidence interval (CI) was reported to show the strength of association. The Cox-proportional hazard model assumption was checked by graphical methods and Schoenfeld residual tests. However, in the Schoenfeld residual test number of excision violated the proportional hazards assumption. Then, the model was stratified by the number of excisions. Hence, stratified Cox regression with interaction and without interaction was compared by log-likelihood test and parsimonious model was selected. Cox–Snell residual test was used to check the goodness of fit. Finally, the Stratified Cox regression model without interaction was fitted to identify the predictors of adjuvant chemotherapy. In this study, a hazard ratio (HR) >1 for a particular group indicates that chemotherapy was initiated earlier in that group compared with the reference, whereas a HR <1 indicates that chemotherapy was delayed in that group compared with the reference category.
Results
Background Characteristics of the Study Participants
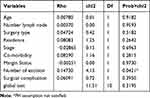
In this study, a total of 202 BC patients were included in the final analysis. The mean age of participants at the time of diagnosis was 48.17 years with SD ± 15.02 years. Of all, 147 (72.77%) of patients were married and 163 (82.3%) were orthodox by religion. More than two-thirds, 141 (69.8%) of the women were at clinical stage III breast cancer at the time of diagnosis. The majority, 181 (89.60%) of patients had mastectomy surgery. Fifty-nine (29.21%) of the women with breast cancer were living with additional co-morbidity. Of these, 44.1% of them had cardiovascular diseases which followed by respiratory diseases (27.1%). Thirty-three (16.3%) of the study participants had a surgical complication. Of these, around two-thirds (63.5%) of them had surgical wound infections (Table 1).
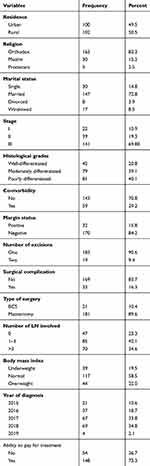 |
Table 1 Characteristics of Women with Breast Cancer at the University of Gondar Compressive Specialized Hospital |
Time to Adjuvant Chemotherapy and Comparison Survival Functions Among Different Categories of Breast Cancer Patients
Kaplan–Meier survival function was used to show the existence of survival difference between categorical variables. Accordingly, the KM curve plots for BC patients with co-morbidity were higher than patients without co-morbidity. These showed that BC patients with and without co-morbidity had different survival experience (Figure 2). Breast cancer patients with and without surgical complications had different survival experiences (Figure 3).
 |
Figure 2 Kaplan–Meier survival curves for women with BC among co-morbidity categories. |
 |
Figure 3 Kaplan–Meier survival curves compare TAC in women with BC among surgical complication categories. |
The median time to adjuvant chemotherapy among BC patients was 67 days (95% CI 55.76, and interquartile range of 34–102). A Log-rank test was performed to test the equality of survival among the groups. In this study, the survival time among categories of co-morbidity, surgical complication, surgical margin status, age, type of surgery, and lymph node involvement were significantly different. Of the total, 96.6% of patients with co-morbidity received adjuvant chemotherapy after 30 days. Regarding surgical complications, 97.0% of patients with surgical complications were received adjuvant chemotherapy after 30 days (Table 2).
 |
Table 2 Survival Time and Log Rank Test for Different Categorical Variables of Breast Cancer Patients at University of Gondar Compressive Specialized Hospital from Jan 2015 to Feb 2019 |
Assessing the Proportional Hazard Assumption
A Cox PH model assumes that the hazard ratio comparing any two specifications of independent variables is constant over time. Equivalently, this suggests that the hazard for one category is proportional to the hazard for any other category, where the proportionality constant is independent of time.
To assess the PH assumption plot of -ln [- ln(survival probability) versus ln of survival time was done for co-morbidity and number of excision. As it is observed from the graph of co-morbidity, the hazards did not cross and which means that the proportional hazard assumption was satisfied (Figure 4). On the other hand, the graphs cross each other between categories of a number of excisions. This indicated that the proportional hazard assumption was not satisfied (Figure 5). Even if the hazard functions do not cross, it is possible that the PH assumption is not satisfied. The goodness of fit (GOF) approach provides a single test statistic for each variable being assessed. Therefore, the global test of the proportional-hazards assumption based on the Schoenfeld residuals was done. A significant p-value suggests that the PH assumption is not met. Based on GOF, all variables except a number of excisions (P=0.04) satisfies the proportional hazard assumption (Table 3).
 |
Figure 4 Proportional hazard plot by co-morbidity categories among women with BC. |
 |
Figure 5 Proportional hazard plot by number of excision among BC patients. |
Model Selection
After the proportional hazard assumption was checked the most parsimonious model was chosen using the Log-likelihood ratio test. Since the number of excisions violated the proportional hazards assumption (p=0.04), the models were stratified according to the number of excision variable. Hence, stratified Cox regression model with and without interaction was compared by the log-likelihood test and the parsimonious model was selected. Based on the comparison technique used the Stratified Cox regression model without interaction was found to be the appropriate model (p=0.052 at a degree of freedom=1). Interpretations and conclusions were thus being based on the stratified Cox model with no interaction assumption.
In multivariable model age, co-morbidity, margin status, and surgical complications were found to be significant predictors for TAC among BC patients at a 5% level of significance. The hazard of starting adjuvant chemotherapy was decreased by 66% (AHR= 0.34, 95% CI: 0.16–0.71) among age >70 BC patients than the age<40 BC patients. The hazard of starting adjuvant chemotherapy was decreased by 57% (AHR= 0.43, 95% CI: 0.29, 0.62) among BC patients with co-morbidity than BC patients without co-morbidity. The hazard of starting adjuvant chemotherapy was decreased by 45% (AHR= 0.55, 95% CI: 0.34, 0.88) among BC patients with surgical complications than BC patients without surgical complications. The hazard of delay of adjuvant chemotherapy was increased by 60% (AHR= 0.40, 95% CI: 0.25, 0.64) among women with a positive surgical margin than those women with a negative surgical margin (Table 4).
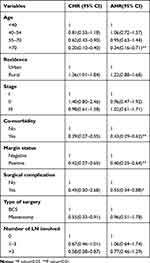 |
Table 4 Bi-Variable and Multivariable Stratified Cox Regression Analysis of Women with Breast Cancer Patients at University of Gondar Compressive Specialized Hospital from Jan 2015 to Feb 2019 |
Goodness of Fit
To check the goodness of fit, the Cox–Snell residuals (together with their Nelson-Aalen cumulative hazard function) were obtained from fitting stratified Cox model. The figure shows that the plot of the Nelson–Aalen cumulative hazard function against Cox–Snell follows the 45° line closely. Hence, the graph showed that Cox–Snell residual was satisfied with the overall model fitness (Figure 6).
 |
Figure 6 Goodness of fit for the stratified Cox model of BC patients. |
Discussion
In this study, the median time to start adjuvant chemotherapy was 67 days (95% CI 55.76). Age, co-morbidity, surgical complication, and margin status were predictors of time to adjuvant chemotherapy among BC patients. This finding is higher than studies conducted in Egypt (35 days),21 United States (46 days),7 Turkey (21 days),13 New Zealand (49 days),25 Italy (38 days),12 and Canada (44 days).31 The possible explanations for this difference could be an increase in cancer incidence with disproportionately smaller increases in resources for cancer treatment and differences in healthcare. Even though the burden of cancer cases is increasing in Ethiopia, the health systems in the country have traditionally concentrated on the control of communicable diseases. The country has few cancer specialists and treatment centers. This makes it difficult for a great majority of the population to access cancer treatment services, which results in a long waiting time to get the service.6 Another possible explanation could be more than one-fourth (27%) of breast cancer patients in this study could not pay for chemotherapy because of the expensiveness of the treatment since the service was not exempted.
However, this study had a shorter duration of commencement of adjuvant chemotherapy compared with a study conducted in Brazil (137 days).32 This could be due to the majority of breast cancer women were diagnosed with advanced-stage (stage III) in this study, but in Brazil, most of the women were stage I. In fact, stage I BC patients were more likely to have delays in adjuvant chemotherapy administration.7 Even though the optimal timing of chemotherapy initiation is controversial, findings from systematic and meta-analysis recommended that adjuvant chemotherapy should preferably start within 30 days.9,19 Integrating the multidisciplinary team primarily in the process of diagnosis and treatment of breast cancer patients would reduce the time to initiating adjuvant chemotherapy. This evidence was supported by asystematic review which showed that a multidisciplinary team caring for the patient optimizes the work and improves the outpatient and Hospital management.33
Advanced age (>70 years) was a significant predictor for delayed adjuvant chemotherapy. This is in line with previous studies conducted from the National Comprehensive Cancer Network databases showing that older patients are less likely to receive adjuvant chemotherapy.14,22,23 Besides, another study conducted in China showed older age has resulted in delayed initiation of adjuvant chemotherapy on breast cancer survival.24 This finding is also in agreement with a study conducted in Egypt.21 This could be due to advanced age may be related to a higher prevalence of postoperative complications and co-morbidities. Another plausible explanation may be due to financial issues. This study result revealed that only 47.0% of elderly (>70 years) patients could pay for treatment, but more than three-fourth (78.4%) of patients <40 years could pay for treatment and limited mobility of elderly patients.
In this study, co-morbidity was a significant predictor associated with delayed TAC. This finding is in line with retrospective studies conducted in the United States22,23 and Egypt.21 This could be due to patients with co-morbidities are challenged for decision-making regarding the use and selection of adjuvant chemotherapy34 and the financial burden from health care costs and fear of burden of pill burden from both conditions that make the treatment duration is longer among patients with additional co-morbidities. Moreover, BC patients with co-morbidities are more likely to need an increased time to recover from surgery.
The presence of surgical complications was another predictor associated with a delayed time of adjuvant chemotherapy. This finding is comparable with a retrospective study conducted in Egypt.21 This could be chemotherapy could affect the blood-forming cells of bone marrow, which decreases immunity by minimizing white blood cell counts.
Women with positive surgical margin was another predictor significantly associated with delayed time to adjuvant chemotherapy. This finding is supported by a retrospective study conducted in Egypt.21 This could be breast cancer patients with positive surgical margins were more likely to undergoes surgery to remove any remaining cancerous cells.
This study may give insight for future researchers and policymakers on TAC and its predictors among breast cancer patients to avoid unnecessary delay. Assessment of predictors of delay of adjuvant chemotherapy is important; they may indicate areas to be targeted for interventions aimed at improving outcomes for women with breast cancer. The limitation of this study was breast subtype, educational status, and wealth index which were significant in other studies were not assessed due to the retrospective nature of the data. Besides, this study does not address the effect of timing of initiation of adjuvant chemotherapy over survival outcomes in breast cancer that is important for clinicians and public health experts.
Conclusion
Time to adjuvant chemotherapy was longer. Advanced age, presence of co-morbidity, presence of surgical complications, and positive margin status were predictors of delayed time to adjuvant chemotherapy. It is better to emphasize surgery to reduce surgical complications and to avoid remaining cancerous cells’ positive margins. It is better to give greater attention to breast cancer patients with co-morbidity and older patients to reduce the delay of adjuvant chemotherapy.
Abbreviations
AC, adjuvant chemotherapy; BMI, body mass index; BC, breast cancer; BCS, breast-conserving surgery; NCCN, National Comprehensive Cancer Network; TAC, time to adjuvant chemotherapy.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Consideration
Ethical clearance and approval to conduct the research was obtained from the ethical review committee of the Institute of Public Health, College of Medicine and Health Science, University of Gondar. As the study was conducted through a review of medical records, the individual patient was not subject to harm and the official letter of co-operation to the University of Gondar Compressive Specialized Hospital was taken from the Institute of Public health. Permission was taken from the oncology unit head manager. To keep the confidentiality, name and other identifiers of patients and health care professionals were not recorded on the data extraction format.
Acknowledgments
We want to thank the University of Gondar Comprehensive Specialized Hospital for their cooperation and permitting data access. Our honest gratitude also goes to data collectors and chart room workers.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Abate S, Assefa ZY, Tigeneh W. Trends of breast cancer in Ethiopia. Int J Cancer Res Mol Mech. 2016;2:2–6.
2. Bizualem S, Yalemtsehay M, Daniel S, Endegena A, Wondwossen EADWL. Cancer Science & Therapy Biological and Clinicopathological Characteristics of Breast Cancer at. Breast Cancer. 2017;9(12):755–760.
3. Zhan Q, Fu J, Fu F, Zhang J, Wang C. Survival and time to initiation of adjuvant chemotherapy among breast cancer patients: a systematic review and meta-analysis. Oncotarget. 2018;9(2):2739–2751.
4. Fkih M’hamed I, Privat M, Ponelle F, Penault-Llorca F, Kenani A, Bignon YJ. Identification of miR-10b, miR-26a, miR-146a and miR-153 as potential triple-negative breast cancer biomarkers. Cell Oncol. 2015;38(6):433–442. doi:10.1007/s13402-015-0239-3
5. Siegel RL, Miller KDJA. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30.
6. Mahlet K, Kunuz A, Tekelech M, et al. National cancer control plan of Ethiopia. In: the situation of cancer in ethiopia. J Cancer Prevent. 2015:13–15.
7. Christina A, Daphne Y, Lichtensztajn DY, Giordano SH. SHdI of ACAPWBC. Delayed Initiation of Adjuvant chemotherapy among patients with breast cancer. JAMA Oncol. 2016;2(3):322–329. doi:10.1001/jamaoncol.2015.3856
8. Damila C, Leadndro L, Patricia XAD. Adjuvant treatment delay in breast cancer patients. Revista da Associação Médica Brasileira. 2015;61(5):411–416.
9. Yu K, Huang S, Zhang J, Liu G, Shao Z. Association between delayed initiation of adjuvant CMF or anthracycline-based chemotherapy and survival in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2013;13(1). doi:10.1186/1471-2407-13-240
10. Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomised trials. The Lancet. 2012;379:432–444.
11. Gagliato DDM, Gonzalez-angulo AM, Lei X, Theriault RL, Giordano SH. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J clin oncol. 2014;32:8.
12. Farolfi A, Scarpi E, Rocca A, et al. Time to initiation of adjuvant chemotherapy in patients with rapidly proliferating early breast cancer. Eur J Cancer. 2015;51(14):1874–1881. doi:http://dx.doi.10.1016/j.ejca.2015.07.003
13. Alkis N, Durnali AG, Arslan UY, Kocer M. Optimal timing of adjuvant treatment in patients with early breast cancer. Med Oncol. 2011;28:1255–1259.
14. Hershman DL, Wang X, McBride R, Jacobson JS, Grann VR, Neugut AI. Delay of adjuvant chemotherapy initiation following breast cancer surgery among elderly women. Breast Cancer Res Treat. 2006;99(3):313–321. doi:10.1007/s10549-006-9206-z
15. Nurgalieva ZZ, Franzini L, Morgan RO, Vernon SW, Liu CC, Du XL. Impact of timing of adjuvant chemotherapy initiation and completion after surgery on racial disparities in survival among women with breast cancer. Med Oncol. 2013;30:1–9.
16. Gelmon KA, Speers C, Barnett J, Olivotto I. Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J clin oncol. 2006;24:245–257.
17. Wang B, Huang J, Hung W, Lin C. Impact on survival on interval between surgery and adjuvant chemotherapy in completely resected stage IB-IIIA lung cancer. PloS one. 2016;4:1–10.
18. Yon S, Susan J, Faina MS. The effect of delays in treatment for breast cancer metastasis on survival. Br cancer res treatment. 2011;130:953–964.
19. Raphael MJ, Biagi JJ, Kong W, Mates M, Booth CM, Mackillop WJ. The relationship between time to initiation of adjuvant chemotherapy and survival in breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2016;160(1):17–28. doi:10.1007/s10549-016-3960-3
20. Jung SY, Sereika SM, Linkov F, Brufsky A, Weissfeld JL, Rosenzweig M. The effect of delays in treatment for breast cancer metastasis on survival. Breast Cancer Res Treat. 2011;130(3):953–964. doi:10.1007/s10549-011-1662-4
21. Mosalam NA, Mohamed H, El A, Saad AS, Mohamed AW. Journal of Oncology Translational Research the impact of adjuvant chemotherapy initiation time on the outcome of breast cancer. Breast Cancer. 2017;3(1):1–8.
22. Fedewa SA, Ward EM, Stewart AK, Edge SB. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: A National Cohort Study 2004–2006. J Clin Oncol. 2015;28(27):4135–4141.
23. Vandergrift JL, Niland JC, Theriault RL, et al. Time to adjuvant chemotherapy for breast cancer in national time to adjuvant chemotherapy for breast cancer in National Comprehensive Cancer Network Institutions. J National Cancer Inst. 2012;105:115.
24. Yu K, Fan L, Qiu L, Ling H, Jiang Y, Shao Z. Influence of delayed initiation of adjuvant chemotherapy on breast cancer survival is subtype-dependent. Oncotarget. 2017;8(28):46549–46556.
25. Seneviratne S, Campbell I, Scott N, Kuper-hommel M, Round G, Lawrenson R. Ethnic differences in timely adjuvant chemotherapy and radiation therapy for breast cancer in New Zealand: a cohort study. BMC cancer. 2014;14:1–9.
26. He X, Ye F, Zhao B, et al. Risk factors for delay of adjuvant chemotherapy in non-metastatic breast cancer patients: a systematic review and meta-analysis involving 186982 patients. PloS one. 2017;1–12.
27. Alderman AK, Collins ED, Schott A, Hughes ME. The impact of breast reconstruction on the delivery of chemotherapy. PloS one. 2010;1791–1800.
28. Edge SB. American Joint Committee on Cancer, American Cancer Society.AJCC CANCER STAGING MANUAL: From the AJCC Cancer Staging Manual.
29. Kartal M, Tezcan S, Canda T. Diagnosis, treatment characteristics, and survival of women with breast cancer aged 65 and above: a hospital-based retrospective study. BMC Womens Health. 2013;13(1):1–7. doi:10.1186/1472-6874-13-34
30. Maskarinec G, Pagano I, Lurie G, Bantum E, Gotay CC, Issell BF. Factors affecting survival among women with breast cancer in Hawaii. J Women’s Heal. 2011;20(2):231–237.
31. Plotogea A, Chiarelli AM, Mirea L, et al. Factors associated with wait times across the breast cancer treatment pathway in Ontario. Plos One. 2013;1–9.
32. Alessandra F, Janaina V, Refael M, et al. Análise do tempo decorrido entre diagnóstico e tratamento do câncer de mama. Anal Time Interval. 2018;28(4):6–11.
33. Taplin BSH, Weaver S, Salas E, et al. Reviewing cancer care team effectiveness. Journal of Oncology Practice. 2015;11(3):239–246. doi:10.1200/JOP.2014.003350
34. Agarwala V, Choudhary NGS, Gupta S. A risk-benefit assessment approach to selection of adjuvant chemotherapy in elderly patients with early breast cancer: a mini review. Indian J Med Paediatr Oncol. 2017;38(4):526–534. doi:10.4103/ijmpo.ijmpo_96_17
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.