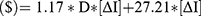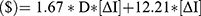Back to Journals » Clinical Ophthalmology » Volume 16
Time and Distance Cost of Longer Acting Anti-VEGF Therapies for Macular Degeneration: Contributions to Drug Cost Comparisons
Authors Meer EA , Oh DH, Brodie FL
Received 4 August 2022
Accepted for publication 26 October 2022
Published 22 December 2022 Volume 2022:16 Pages 4273—4279
DOI https://doi.org/10.2147/OPTH.S384995
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Elana A Meer,1 Dennis H Oh,2,3 Frank L Brodie1,4
1Department of Ophthalmology, University of California, San Francisco, San Francisco, CA, USA; 2Dermatology Service, San Francisco Veterans Affairs Medical Center, San Francisco, CA, USA; 3Department of Dermatology, University of California, San Francisco, CA, USA; 4Department of Ophthalmology, San Francisco Veterans Affairs Medical Center, San Francisco, CA, USA
Correspondence: Frank L Brodie, University of California, San Francisco, Wayne and Gladys Valley Center for Vision, 490 Illinois Street, San Francisco, CA, 94158, Email [email protected]
Purpose: To evaluate the holistic cost of longer acting anti-VEGF therapy for macular degeneration when considering the associated costs of travel to the retina clinic.
Design: Theoretical evaluation of cost using publicly available pricing data and reimbursements at the Veterans Affairs (VA) Medical Center.
Patients and Methods: Setting: VA Medical Center. Study population: Patients with age related macular degeneration. Main outcome measures: Three-year cost of therapy when considering medication as well as travel costs and time spent in transit.
Results: Based on cost data derived purely from wholesale acquisition cost and projected injection frequency over the first three years of treatment, faricimab is less expensive than ranibizumab and aflibercept by $37,709 and $6359, respectively. Aflibercept is less expensive ranibizumab by $31,350 over the first 3 years of treatment. When considering even small distances traveled by patients, these cost differences grow, amplified at even larger distances: at 25 miles, ranibizumab becomes $38,814 and $32,133 more expensive than faricimab and aflibercept, respectively. Aflibercept becomes $6681 more expensive than faricimab. At 100 miles, ranibizumab becomes $41,502 and $34,038 more expensive than faricimab and aflibercept, respectively. Aflibercept becomes $7464 more expensive than faricimab.
Conclusion: Longer acting anti-VEGF therapies may differ not only in their wholesale acquisition cost, but also in the frequency of per label injections and associated clinic visits. Taking into account distance and time cost of travel may contribute to a more holistic view of cost differences among these therapies.
Keywords: distance cost, travel cost, longer acting therapy, macular degeneration, anti-VEGF injections
Introduction
With the introduction of longer acting therapies for macular degeneration, cost-benefit analysis considerations become even more essential in considering their role in clinical practice. Studies comparing intravitreal, anti-VEGF therapy ranibizumab (Lucentis, Genentech-Roche, South San Francisco, California, monospecific molecule anti-VEGF), faricimab (Vabysmo, Roche, Basil, Switzerland, bispecific molecule anti-ANG2 and anti-VEGF) and aflibercept (Eylea, Regeneron, Eastview, New York) monotherapies for treating neovascular, age-related macular degeneration (NVAMD) have demonstrated similar visual outcome and adverse event profiles while varying in frequency of injections required to maintain these excellent outcomes.1–10,28
Comprehensive societal cost-benefit analyses comparing such intravitreal therapies have demonstrated considerable financial return on investment to patients and insurers; all have been considered cost-effective using societal and direct ophthalmic medical cost perspectives and average cost-utility ratios with bevacizumab being the most cost-effective.1,28 Such analyses have included a range of cost perspectives, comparing ophthalmic direct medical costs (eg 2018 average, national, medicare fee schedule), non-ophthalmic costs (eg depression, injury, nursing home costs), direct nonmedical costs (eg caregiver costs, activities of daily living, etc), and indirect medical (productivity) costs across bevacizumab, ranibizumab, and aflibercept.1,11,12,28 Brown et al found that over an 11 year period, substituting bevacizumab for ranibizumab and aflibercept with neovascular AMD therapy in 2018 could have theoretically saved $1.343 billion in direct ophthalmic medical expenditures. This analysis emphasizes the importance of considering cost savings for emerging therapies,13 however further work is needed to characterize cost savings that may be unique to specific health systems, such as transportation costs.
As we consider longer acting therapies, reduction in the number of visits generates additional savings beyond clinic visit and material costs classically considered in formal cost-benefit analyses. Due to the fact that intravitreal therapy for AMD requires care by a retina specialist, frequently care is not available in more rural communities and patients need to travel to access care. The health care system operated by Veterans Affairs (VA) is a unique system, which bears the cost of patient travel. Therefore, it is particularly ideal to use as a case study to consider the effect of distance and time cost savings of travel that may be achieved with longer acting therapies. In this study, we estimate the effect of transportation time and distance on the costs of anti-VEGF therapy for macular degeneration.
Methods
A theoretical cost-saving analysis was performed for the use of faricimab, ranibizumab, and aflibercept over the first three-year time period of initial medication usage. Institutional Review Board approval was not required as we used publicly available data derived from the VA website14 and pharmaceutical company list prices. The research adhered to the Declaration of Helsinki, and no state or federal regulations were violated.
Publicly available wholesale acquisition prices for the medications were extracted. Only FDA approved anti-VEGF therapies for AMD at the time of writing were analyzed, including faricimab, ranibizumab, and aflibercept. Frequency of injections in the first three years of therapy for each medication was determined by drug label. The time period of the first three years was utilized as this represents the time period that the VA health system uses to evaluate new drugs, and a longer time period was thought to only further expand differences and minimize the effect of the loading period. Ranibizumab 0.5 mg (10 mg/mL) is a monthly injection per label (12 injections per year based on standard prescribing and pivotal study data).15,16 The label instructions for aflibercept (40 mg/mL) is for 8 injections for the first year, and 6.5 injections per year for year 2 and 3.17 The label for faricimab (120 mg/mL) allows for a flexible dosing interval and therefore we derived average frequency from pivotal study data: 45% of patients received faricimab injections every 16 weeks, 33% every 12 weeks, and 22% every 8 weeks, the weighted average interval was considered to be 12.92 weeks with 4.02 injections per year and 6.79 injections in the first year.10 The wholesale acquisition prices (ie the public pricing before any negotiated discounts not necessarily the actual proprietary costs paid by the payer) included $2190 for faricimab 5 mg, $1950 for ranibizumab 0.5 mg, and $1850 for aflibercept.
Transportation costs were derived from publicly available VA data.14,18 VA reimburses qualified patients at $0.585 per mile.14,19 An additional consideration, although not reimbursed, is the time spent for travel and clinic appointments for both the patient and, if applicable, a caregiver. This cost is an important consideration as patients with macular degeneration are often elderly and accompanied by family members or caregivers, potentially doubling the time cost of travel. As many as one-third of patients with AMD require caregiving services due to their AMD,20 and over 20% of patients report that caregivers take time away from work and personal activities to provide transportation to appointments, with a substantial time and financial burden.21 In studies investigating the cost of patient and caregiver time, self-reported wages may be employed to estimate opportunity cost.22–26 In the absence of self-reported wages, we use the federal minimum wage of $15 per hour as a proxy.22 Although professional caregivers may have rates exceeding federal minimum wage rates, we use federal minimum wage to conservatively estimate the cost savings to the patient. The VA system does provide a formula to calculate the estimated time of travel per given distances, which allowed us to estimate this time cost of travel within the VA system.14 For distances less than 30 miles, transportation time (minutes) was calculated as 3.42 + 1.70*distance (miles).14 For distances greater than or equal to 30 miles, it was assumed an average freeway speed of 60 miles/hour for additional mileage transportation time (minutes) calculated as 3.42 + 1.70 * 30 + 1*(distance – 30).
Results
Based on medication labeling and pivotal study data (as listed above), the anticipated pricing per year of each drug per patient for the first three years of use are calculated in Table 1, with total cost over the first three years of treatment $32,491 for faricimab, $70,200 for ranibizumab 0.5 mg, and $38,850 for aflibercept (Table 1). Faricimab is less expensive than ranibizumab and aflibercept by $37,709 and $6,359, respectively.
 |
Table 1 Cost of Anti-VEGF Therapies for the First Three Years of Therapy Based on Wholesale Acquisition Cost and Frequency of per Label Injections |
Using the VA hospital travel cost data, and per label injection visits over the first three years, we can derive additional travel costs for each visit per drug as shown in Table 2. The VA system also considers the time cost of travel in their calculations (formulas presented in the methods) up to 30 miles. If we consider a $15 minimum wage (the federal minimum wage as of 2022),27 then the time-cost of visits (opportunity cost of patient or caregiver) may be described as in Table 3.
 |
Table 2 Travel Cost Calculation Based on Distance Traveled to Receive Care During the First Three Years of Treatment |
 |
Table 3 Time Cost of Travel Calculation Based on Distance Traveled to Receive Care During the First Three Years of Treatment |
When considering time cost of travel and the distance cost of travel, even for a distance of 25 miles, the price differentials for the first three years of treatment grow (Table 4). Whereas, the total cost of ranibizumab for the first three-year period of treatment was $37,709 more expensive than that of faricimab, at a distance of 25 miles away from care, it becomes $38,814 more expensive. Similarly, whereas the cost of ranibizumab for the first three-year period of treatment was $31,350 more than that of aflibercept, at a distance of 25 miles away from care, it becomes $32,133 more expensive. Finally, while the cost of aflibercept for the first three-year period of treatment was only $6,359 more expensive than that of faricimab, at a distance of 25 miles away from care, it becomes $6,681 more expensive (Table 4). When considering larger distances, these cost differences grow further: at 100 miles, ranibizumab becomes $41,502.2 more expensive than faricimab and $34,038.2 more expensive than aflibercept, and aflibercept becomes $7,464 more expensive than faricimab over the first three years of treatment (Table 4).
 |
Table 4 Total Cost Data and Cost Effectiveness Data Including Drug, Distance and Time Cost Data* |
Discussion
In this brief report, we estimate the effect of the time and distance cost of travel on the holistic cost of anti-VEGF therapy. In particular, we utilize the Veterans Affairs hospital system to calculate the distance and time cost of travel to exemplify the effect it may have on cost-benefit analysis calculations for anti-VEGF therapies. We demonstrate that when taking into account distance and time cost of travel, longer duration therapies for macular degeneration may have even greater cost benefits than previously thought due to a reduction in frequency of injections and clinic visits. Over the first three years of treatment, faricimab is less expensive than aflibercept, both of which are considerably less expensive than ranibizumab. When considering the distance and time costs of travel, these differences become amplified, with newer medications such as faricimab and aflibercept becoming substantially more cost effective due to the reduction in visit and injection frequency requirements.
Based on these data the relationship between injection frequency, distance from clinic and incremental cost of the drug with equivalent holistic value can be generalized to the below equations with D being the patient distance from clinic and  being the difference in injection frequency.
being the difference in injection frequency.
For each fewer injection per year, the increased value ($), segmented by patient distance from clinic, is equivalent to:
Studies that have demonstrated an overall financial benefit to use intravitreal bevacizumab, ranibizumab, and aflibercept monotherapies when compared to no treatment, however, did not focus on comparing across therapies, taking into account the differences in injection frequency on distance and time costs.28
While cost comparisons across faricimab, aflibercept, and ranibizumab are limited, this data is in line with past studies that have suggested the net societal gain of aflibercept is greater than that of ranibizumab.1 Similarly, individual treatment costs for bevacizumab, ranibizumab, and aflibercept have demonstrated the greatest medical savings for bevacizumab, followed by aflibercept, and ranibizumab.29 However, few studies have included faricimab in cost comparisons or accounted for the effects of travel costs.
Distance considerations are particularly important, especially in the VA system which accounts for cost distance of travel in reimbursement calculations. While data is limited on VEGF therapy, a recent study found most veterans receive ophthalmic care between 20–30 miles from their home.30 Furthermore, many veterans are rural, with significant travel times affecting access to care, with a significant impact on prevalence of vision loss.31 By our basic calculations, at these average range of distances from care,30 the cost-effectiveness of anti-VEGF therapies magnifies simple price calculations. At a distance of 20–30 miles, faricimab, may save around $38,814 and $6,681 over the first three-year period of treatment when compared to ranibizumab and aflibercept, respectively. Similarly, ranibizumab increases to $32,133 more than aflibercept over the first three year treatment period after taking into account distance and time cost of travel for the relatively short distance of 20–30 miles. When considering greater distances of 50–100 miles for veterans living in more rural locations, the cost differences grow. These calculations demonstrate the importance of considering the distance and time cost of a reduction in visits required for anti-VEGF therapy for macular degeneration. In addition, these likely underestimate the time cost of individuals as patients often present to clinic accompanied by family members or caregivers. Multiple studies have demonstrated the importance of considering caregiver time, and the opportunity cost as well as valuation of lost productivity in caregivers.22,23,25,32–34 Therefore, in many cases actual time cost may be significantly greater than the projections presented here, further increasing the differences in cost between the newer therapies with fewer visits and the older therapies (eg ranibizumab). While the addition of time and distance costs might seem small compared to the total cost of therapies per year, it is important to note that these are costs borne by the patient and caregiver, making even relatively small savings essential to consider.
We acknowledge several limitations. The first and main limitation is that this study involves estimations based on nominal costs and listed assumptions but does not utilize cost and claims data. These calculations do not incorporate a comprehensive cost benefit analysis as has been done before.1 Instead, we aim to demonstrate the importance of incorporating time and distance costs of travel by using a simple model. We hope that by demonstrating this effect, we can drive future research to incorporate more comprehensive travel cost calculations in big data cost-effectiveness analyses. Second, this analysis was based on the assumption of time and distance cost data from a veterans affairs hospital in California.14 While this served as a helpful base for calculations, given the unique sponsorship of travel costs by the VA system, the specific time and distance cost calculations may not apply across all health systems and all states. Third, we use minimum wage as a blunt instrument to measure time value, however it is an imperfect way to measure the value of patient, family member, and caregiver time in the absence of self-reported wages for each patient and caregiver.22,23,25,32–35 In addition, as mentioned above, we present the time cost of travel in the results based on only the patient traveling to clinic visits, however, in reality, they would likely be accompanied by a family member or caregiver, significantly increasing the time cost of each clinic visit. There are many additional associated costs with clinic visits and injections frequency not considered in this analysis that would further amplify the cost benefit to the longer acting therapies. Furthermore, pharmaceutical companies offer numerous incentives and rebates to health systems and doctors, lowering the net cost of their drugs – these are not accounted for here and could well affect the ultimate analysis. In addition, this paper represents an objective estimation of travel costs according to travel reimbursement standards, however, these standards may become obsolete when fuel prices become prohibitive as occurring in instances of world instability. Finally, this study utilizes approved anti-VEGF therapies and therapeutic regimens for AMD, with the goal of focusing on the FDA on label regimens to allow for a direct like-for-like comparison. Future studies are needed to explore the effect of time and distance cost on different treatment arms that have been described as alternative regimens in the literature, such as in the EXCITE trial.36
Overall, we demonstrate that the distance and time cost of travel may have considerable effects on the holistic cost of therapies which differ not only in their wholesale acquisition cost, but also in the frequency of injections. More specifically, we found that when considering time and distance costs of travel, faricimab is less expensive than ranibizumab and aflibercept by $ 37,709 and $6,359, respectively over the first three years of therapy. We hope this work drives further considerations surrounding incorporating travel costs into cost-benefit analyses for longer acting therapies.
Funding
No funding/support was received for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Brown GC, Brown MM, Rapuano SB, Boyer D. A cost-benefit analysis of VEGF-inhibitor therapy for neovascular age-related macular degeneration in the United States. Am J Ophthalmol. 2021;223:405–429. doi:10.1016/J.AJO.2020.07.010
2. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi:10.1056/NEJMOA054481/SUPPL_FILE/NEJM_ROSENFELD_SA1.PDF
3. Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57–65.e5. doi:10.1016/J.OPHTHA.2008.10.018
4. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi:10.1016/J.OPHTHA.2012.09.006
5. Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six–week results of the VIEW studies. Ophthalmology. 2014;121(1):193–201. doi:10.1016/J.OPHTHA.2013.08.011
6. Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. doi:10.1016/J.OPHTHA.2012.03.053
7. Maguire M, Martin D, Ying G, et al. Five-year outcomes with anti–vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular. Ophthalmology. 2016;123(8):1751–1761. doi:10.1016/j.ophtha.2016.03.045
8. Khanani AM, Heier J, Ruiz CQ, et al. Faricimab in neovascular age-related macular degeneration: 1-year efficacy, safety, and durability in the phase 3 TENAYA and LUCERNE trials. Invest Ophthalmol Vis Sci. 2021;62(8):428.
9. Sharma A, Kumar N, Kuppermann BD, Bandello F, Loewenstein A. Faricimab: expanding horizon beyond VEGF. Eye. 2019. doi:10.1038/s41433-019-0670-1
10. Heier JS, Khanani AM, Quezada Ruiz C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399(10326):729–740.
11. Javitt JC, Zhou Z, Willke RJ. Association between vision loss and higher medical care costs in Medicare beneficiaries costs are greater for those with progressive vision loss. Ophthalmology. 2007;114(2):238–245.e1. doi:10.1016/J.OPHTHA.2006.07.054
12. Gillies MC, Campain A, Barthelmes D, et al. Long-term outcomes of treatment of neovascular age-related macular degeneration: data from an observational study. Ophthalmology. 2015;122(9):1837–1845. doi:10.1016/J.OPHTHA.2015.05.010
13. Smith AF, Brown GC. Understanding cost effectiveness: a detailed review. Br J Ophthalmol. 2000;84(7):794–798. doi:10.1136/BJO.84.7.794
14. HERC. Estimating travel costs. Veterans affairs hospital. Available from: https://www.herc.research.va.gov/include/page.asp?id=travel-costs.
15. Busbee BG, Ho AC, Brown DM, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120(5):1046–1056. doi:10.1016/J.OPHTHA.2012.10.014
16. Li E, Donati S, Lindsley KB, Krzystolik MG, Virgili G. Treatment regimens for administration of anti-vascular endothelial growth factor agents for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2020;2020(5). doi:10.1002/14651858.CD012208.PUB2/MEDIA/CDSR/CD012208/IMAGE_N/NCD012208-CMP-004.03.SVG
17. Ohr M, Kaiser PK. Aflibercept in wet age-related macular degeneration: a perspective review. Ther Adv Chronic Dis. 2012;3(4):153. doi:10.1177/2040622312446007
18. Weinstein MC, Russell LB, Gold MR, Siegel JE. Cost-Effectiveness in Health and Medicine. Oxford university press; 2016.
19. IRS issues standard mileage rates for 2022 | internal revenue service. Available from: https://www.irs.gov/newsroom/irs-issues-standard-mileage-rates-for-2022.
20. Schmier JK, Halpern MT, Covert D, Delgado J, Sharma S. Impact of visual impairment on use of caregiving by individuals with age-related macular degeneration. Retina. 2006;26(9):1056–1062. doi:10.1097/01.IAE.0000254890.48272.5A
21. Prenner JL, Halperin LS, Rycroft C, Hogue S, Williams Liu Z, Seibert R. Disease burden in the treatment of age-related macular degeneration: findings from a time-and-motion study. Am J Ophthalmol. 2015;160(4):725–731.e1. doi:10.1016/J.AJO.2015.06.023
22. Chari AV, Engberg J, Ray KN, Mehrotra A. The opportunity costs of informal elder-care in the United States: new estimates from the American time use survey. Health Serv Res. 2015;50(3):871. doi:10.1111/1475-6773.12238
23. Russell LB. Completing costs: patients’ time. Med Care. 2009;47(7 Suppl 1):S89–S93. doi:10.1097/MLR.0B013E31819BC077
24. Ray KN, Chari AV, Engberg J, Bertolet M, Mehrotra A. Opportunity costs of ambulatory medical care in the United States. Am J Manag Care. 2015;21(8):567.
25. Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276(15):1253–1258. doi:10.1001/JAMA.1996.03540150055031
26. Becker GS. A theory of the allocation of time. Econ J. 1965;75(299):493–517. doi:10.2307/2228949
27. State Minimum Wage Laws | U.S. Department of Labor. Bureau of labor statistics; 2022. Available from: https://www.dol.gov/agencies/whd/minimum-wage/state#ca.
28. Brown GC, Brown MM, Rapuano S, Boyer D. Cost-utility analysis of VEGF inhibitors for treating neovascular age-related macular degeneration. Am J Ophthalmol. 2020;218:225–241. doi:10.1016/J.AJO.2020.05.029
29. Cost–benefit analysis of VEGF inhibitors for neovascular AMD | practiceUpdate. Practice update. Available from: https://www.practiceupdate.com/content/cost-benefit-analysis-of-VEGF-inhibitors-for-neovascular-amd/103842.
30. Pettey WBP, Wagner TH, Rosen AK, Beilstein-Wedel E, Shwartz M, Vanneman ME. Comparing driving miles for department of veterans affairs-delivered versus department of veterans affairs-purchased cataract surgery. Med Care. 2021;59:S307–S313. doi:10.1097/MLR.0000000000001491
31. McDaniel JT, Albright DL, Wallace JP, Jenkins WD. Vision loss in older veterans is greater in rural than urban areas. Eye Rep. 2020;6(1). doi:10.16964/ER.V6I1.98
32. Gelfand A, Sou J, Sawatzky R, et al. Valuation of lost productivity in caregivers: a validation study. Front Psychol. 2021;12:3785. doi:10.3389/FPSYG.2021.727871/BIBTEX
33. Kanti Biswas R, Roy R, Maksane N, Bhavsar M, Sanyal A. Treatment burden and quality of life of patients with neovascular age-related macular degeneration (nAMD) and their caregivers—a review. Int J Ophthalmol Vis Sci. 2021;6(3):164–171. doi:10.11648/j.ijovs.20210603.13
34. Mulligan K, Seabury SA, Dugel PU, Blim JF, Goldman DP, Humayun MS. Economic value of anti–vascular endothelial growth factor treatment for patients with wet age-related macular degeneration in the United States. JAMA Ophthalmol. 2020;138(1):40–47. doi:10.1001/JAMAOPHTHALMOL.2019.4557
35. Hanemoto T, Hikichi Y, Kikuchi N, Kozawa T. The impact of different anti-vascular endothelial growth factor treatment regimens on reducing burden for caregivers and patients with wet age-related macular degeneration in a single-center real-world Japanese setting. PLoS One. 2017;12:12. doi:10.1371/JOURNAL.PONE.0189035
36. Schmidt-Erfurth U, Eldem B, Guymer R, et al. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology. 2011;118(5):831–839. doi:10.1016/J.OPHTHA.2010.09.004
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.