Back to Journals » Infection and Drug Resistance » Volume 15
Tigecycline Suppresses the Virulence Factors of Multidrug-Resistant Acinetobacter baumannii Allowing Human Neutrophils to Act
Authors Sato Y , Hatayama N, Ubagai T , Tansho-Nagakawa S, Ono Y , Yoshino Y
Received 1 April 2022
Accepted for publication 16 June 2022
Published 28 June 2022 Volume 2022:15 Pages 3357—3368
DOI https://doi.org/10.2147/IDR.S368890
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Yoshinori Sato,1 Nami Hatayama,1 Tsuneyuki Ubagai,1 Shigeru Tansho-Nagakawa,1 Yasuo Ono,1,2 Yusuke Yoshino1
1Department of Microbiology and Immunology, Teikyo University School of Medicine, Itabashi-ku, Tokyo, 173-8605, Japan; 2Teikyo Heisei University, Faculty of Health and Medical Science, Toshima-ku, Tokyo, 170-8445, Japan
Correspondence: Yoshinori Sato, Department of Microbiology and Immunology, Teikyo University School of Medicine, 2-11-1 Kaga, Itabashi-ku, Tokyo, 173-8605, Japan, Tel +81-3-3964-1211, Fax +81-3-5375-5284, Email [email protected]
Purpose: To determine the ability of human neutrophils to kill multidrug-resistant Acinetobacter baumannii (MDRAB) in the presence of tigecycline (TGC).
Methods: Clinical isolates of MDRAB were cultured with human neutrophils and H2O2 in the presence of TGC. The numbers of viable bacteria, catalase activity, gene expression at the K locus of the MDRAB, reactive oxygen species (ROS) production, and granule exocytosis in human neutrophils were determined.
Results: There was a time-dependent increase in the numbers of MDRAB after co-culturing with human neutrophils, whereas there was a significant decrease in the MDRAB numbers when co-cultured with both, human neutrophils and TGC for 6 h. The presence or absence of TGC did not affect total ROS production or the expression of CD11b, CD15, and CD63 on human neutrophils occurred when co-cultured with MDRAB. TGC significantly suppressed catalase activity and gene expression at the K locus of MDRAB, and significantly reduced the thickness of the capsule. Additionally, the bacterial viability of TGC-treated MDRAB cultured with H2O2 was lower than that without H2O2 after 6 h of culture.
Conclusion: TGC significantly suppressed the expression of catalase and the capsule in MDRAB without adverse effects on neutrophil function, allowing human neutrophils to kill MDRAB. TGC is an effective antibiotic for treating MDRAB infections.
Keywords: multidrug-resistant Acinetobacter baumannii, MDRAB, catalase, capsule, tigecycline, TGC, human neutrophils
Introduction
Acinetobacter baumannii is an important opportunistic pathogen associated with nosocomial infections, such as central line-associated bloodstream infections (CLABSI), catheter-associated urinary tract infections (CAUTI), surgical site infections (SSI), and ventilator-associated pneumonia (VAP).1–3 Additionally, A. baumannii is among the six nosocomial pathogens that have acquired multidrug resistance and virulence, namely, Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, A. baumannii, Pseudomonas aeruginosa, and Enterobacter spp. (ESKAPE).4,5 Although A. baumannii has been described as a pathogen with low-virulence,6 recent studies have shown that it exhibits several forms of pathogenicity, such as biofilm formation, adherence, invasion of lung epithelial cells, host cell death, and iron acquisition.7,8 Therefore, A. baumannii, and particularly, multidrug-resistant A. baumannii (MDRAB) has gained importance as a human pathogen in hospitals.
Some bacterial pathogens can circumvent destruction by neutrophils, which play a crucial role in eliminating bacteria from the host.9 A. baumannii also has some virulence factors (eg catalase production10 and expression of a capsule8,11) to evade neutrophil functions (eg killing, phagocytosis, and neutrophil extracellular traps (NETs)).12 Notably, because MDRAB clinical isolates are known to survive the oxidative stress within the phagolysosome of macrophages,10 it is conceivable that MDRAB may escape the antimicrobial effects of the reactive oxygen species (ROS) produced by neutrophils. Additionally, A. baumannii masks its surface antigens by expressing a polysaccharide capsule8,9 and evades killing by human neutrophils in vitro.13 Loss of capsule severely decreases the virulence of A. baumannii both, in vitro and in vivo.11 Conversely, changes in capsule structure increase the virulence of A. baumannii by preventing host complement-mediated opsonophagocytosis.14–16 Therefore, the capsule of A. baumannii is a virulence factor that plays a crucial role in preventing neutrophil-mediated cytotoxicity.
Colistin (CST) and TGC are last-resort antibiotics used against several multidrug-resistant bacteria,17 although there have been reports regarding resistance to these antibiotics worldwide.18,19 TGC belongs to the glycylcycline class of semi-synthetic antimicrobial agents, which were developed to treat polymicrobial infections caused by multidrug-resistant gram-positive and -negative bacteria.20 TGC acts as a bacteriostatic agent against such multidrug-resistant bacteria; for instance, it suppresses bacterial growth and Shiga toxin expression in Shiga toxin-producing Escherichia coli (STEC).21 Our previous study showed that biofilm formation by MDRAB decreases in the presence of TGC in a dose-dependent manner.22 Additionally, Navidifar et al reported that the expression of biofilm-related genes was significantly decreased in the clinical isolates of A. baumannii exposed to TGC.23 These results suggest that TGC is an effective antibiotic for suppressing bacterial growth and restricting the expression of genes associated to virulence factors.
These studies indicate that A. baumannii has several virulence factors that evade immune responses, such as neutrophil killing. Although TGC is an effective antibiotic for suppressing bacterial growth and inhibiting the expression of genes associated with virulence factors, its effects on neutrophil killing to eliminate A. baumannii remain unknown. In this study, we focused on the bacteriostatic agent TGC and analyzed the ability of human neutrophils to kill MDRAB in the presence of TGC.
Materials and Methods
Bacterial Strains and Growth Conditions
A. baumannii R1, R2, and R3 were isolated from Teikyo University Hospital during an outbreak in 2010.10 Bacteria were isolated on CHROMagar Acinetobacter (CHROMagar, Paris, France) and incubated for 24 h at 37 °C. The R1 strain was isolated from the sputum of a patient with interstitial pneumonia. The R2 strain was isolated from the urine sample of a patient with malignant lymphoma and pneumonia, whereas the R3 strain was isolated from the blood of a patient with sepsis and myelodysplastic syndrome. The isolates were streaked onto blood agar plates and cultivated for 24 h to obtain monoclonal colonies that were identified as A. baumannii by sequencing the partial RNA polymerase β-subunit (rpoB) gene.24 The isolates were confirmed to be non-clonal using pulsed-field gel electrophoresis (data not shown). After identification, the isolates were stored in glycerol stock at −80 °C in the Department of Microbiology & Immunology, Teikyo University School of Medicine. Antimicrobial susceptibility testing was performed on the three A. baumannii strains based on the minimum inhibitory concentrations (MICs) of imipenem (IPM), amikacin (AMK), and ciprofloxacin (CPFX). The MICs of IPM, AMK, and CPFX for the three strains were >8 μg/mL, >32 μg/mL, and >2 μg/mL, respectively. Thus, the isolated strains were identified as MDRAB. The MIC of TGC for the MDRAB strains R1 and R2 was 0.5 μg/mL, whereas that for strain R3 was 1 μg/mL.10 The MDRAB clinical isolates, A. baumannii strain ATCC 19606, and Pseudomonas aeruginosa strain PAO-1 were cultured on Luria-Bertani (LB) agar plates (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) for 16 h at 37 °C. Thereafter, the bacteria were suspended in Roswell Park Memorial Institute-1640 with L-glutamine and sodium (RPMI-1640) (Sigma–Aldrich, St. Louis, MO, USA) supplemented with 2% fetal bovine serum (FBS) (Gibco, grand island, NY, USA) at a concentration of 2×108 CFU/mL. The cell concentration was determined by measuring the optical density (OD) at 595 nm. Bacterial suspensions thus obtained were used for performing all subsequent assays.
Determination of MICs
The MICs of TGC for MDRAB strains in this study were measured in RPMI-1640 supplemented with 2% heat-inactivated FBS (Gibco, NY, USA), according to the CLSI methodology.
Preparation of Neutrophils
Neutrophils were isolated from the peripheral blood of healthy volunteers, as described previously.25 Briefly, heparinized human whole blood (25 mL) was mixed with 7 mL of 6% dextran solution (Wako, Osaka, Japan) and 10 mL of Hank’s balanced salt solution (HBSS)(-) (Gibco), and allowed to stand for 30 min at 25 °C until stratification had occurred. Leukocyte-rich plasma was collected and centrifuged at 500 × g in lymphocyte separation medium 1077 (Promo Cell, Heidelberg, Germany) for 30 min. A hypotonic (0.2%) saline solution was added to lyse the erythrocytes, and osmolality was restored by adding hypertonic (1.6%) saline. Neutrophils were adjusted to a final concentration of 1×107 cells/mL in HBSS. Neutrophil purity was determined using the Celltac MEK 6450 hematology analyzer (Nihon Kohden, Tokyo, Japan). Cell viability ≥95% was confirmed before each assay via trypan blue exclusion staining.
Neutrophil and H2O2 Killing Assays
Isolated human neutrophils were co-cultured with MDRAB at a multiplicity of infection (MOI) of 50 bacteria per cell in the absence or presence of 8 μg/mL TGC at 37 °C under 5% CO2 for 2, 4, and 6 h. All assays were performed at a volume of 200 μL. The H2O2 killing assay was performed according to a previous study.10 Bacterial cell suspensions (5 × 107 CFU/mL) were cultured with 0.078% H2O2 (Nacalai Tesque, Kyoto, Japan) in the absence or presence of 8 μg/mL TGC at 37 °C under 5% CO2 for 2, 4, and 6 h. Thereafter, the bacterial suspensions were serially diluted and inoculated on LB agar to determine the bacterial counts.
ROS Production
Total ROS production in human neutrophils, which were co-cultured with MDRAB, was determined in the absence or presence of 8 μg/mL TGC using a luminol-dependent chemiluminescence (CL) assay.26,27 Human neutrophils (5 × 105 cells) were preincubated at 37 °C for 10 min in 1 mL RPMI-1640 supplemented with 2% FBS and luminol solution. After incubation, bacteria were added to the neutrophil suspension (MOI = 50) and ROS production was monitored using a six-channel Biolumat LB 9505 (Berthold Co., Bad Wildbad, Germany) at 37 °C for 120 min.
Flow Cytometry Analysis
Exocytosis of the primary/secondary granules within the human neutrophils was analyzed by monitoring the cell surface proteins CD11b, CD15, and CD63.28 Briefly, human neutrophils were co-cultured with MDRAB in the absence or presence of 8 μg/mL TGC and harvested after 4 h. The harvested cells were washed with phosphate buffered saline (PBS) (Nacalai Tesque) and enumerated. To analyze the expression of the cell surface proteins, human neutrophils were stained with specific monoclonal antibodies (mAbs) against the cell surface markers for 30 min at 4 °C. The mAbs included phycoerythrin (PE)-labeled anti-CD11b, allophycocyanin (APC)-labeled anti-CD63, and APC-Cy-7-labeled CD15 (BioLegend, San Diego, CA, USA). The cells were washed twice with staining buffer (BD Biosciences, San Jose, CA, USA) and subsequently fixed with the BD Cytofix™ Fixation Buffer (BD Biosciences). The stained cells were analyzed using a FACSCant II flow cytometer that was equipped with the FACS Diva software (BD Biosciences). All flow cytometry data were analyzed using the FlowJo v10.8.0 software (BD Biosciences).
Catalase Activity
Catalase activity in MDRAB was measured using the Catalase Colorimetric Activity Kit (Arbor Assays, MI, USA) according to the manufacturer’s protocol. Briefly, the bacteria were cultured on LB agar plates (Becton, Dickinson and Company, MD, USA) for 16 h at 37 °C. Thereafter, they were suspended in RPMI-1640 supplemented with 2% FBS and adjusted to a concentration of 5×107 CFU/mL by measuring the OD at 595 nm. The bacterial cell suspensions were then used for the catalase activity assay.
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (RT-PCR)
To analyze the expression of the K locus genes (wzc, gnaA, and galU), the total RNA from A. baumannii, which was cultured in RPMI-1640 with 2% FBS in the absence or presence of TGC at 37 °C under 5% CO2 for 2 h, was extracted using an RNeasy Protect Bacteria Mini Kit (Qiagen, Tokyo, Japan). The harvested RNA samples were quantified using a NanoDrop spectrophotometer (Thermo Fisher Scientific, MA, USA). Total RNA was reverse-transcribed into complementary DNA (cDNA) using the PrimeScript™ 1st strand cDNA Synthesis Kit (Takara Bio, Shiga, Japan). To analyze the mRNA levels of all genes, cDNA was amplified using PowerUp SYBR Green Master Mix (Thermo Fisher Scientific) with consensus primers to detect the rpoB, wzc, gnaA, and galU genes.10,37–39 The primer sequences are listed in Table S1. rpoB was used as an internal control to quantify the wzc, gnaA, and galU genes. Real-time PCR was performed as follows: 40 denaturation cycles at 95 °C for 15s, annealing at 60 °C for 30s, and extension at 72 °C for 1 min. The amplified PCR products were quantitatively monitored using the StepOne Real-Time PCR System (Applied Biosystems, CA, USA). Fold changes in the expression level of each gene were calculated by the 2−ΔΔCt method. The expression of each gene was evaluated relative to the control sample (TGC-untreated MDRAB), which was assigned a value of one arbitrary unit.
Microscopy
Human neutrophils co-cultured with the MDRAB strain R1 were stained with Gram–Hucker’s stain solution (Muto Pure Chemicals, Ltd., Tokyo, Japan) and imaged using a BX53 biological microscope (Olympus, Tokyo, Japan). Bacterial capsules were visualized via the wet-film India ink method. Images were acquired using an all-in-one fluorescence microscope BZ-X800 (KEYENCE, Osaka, Japan).
Statistics
For the neutrophil killing, ROS production, and catalase activity assays and flow cytometry analysis quantitative results were compiled from two independent experiments (n = 6) and presented as mean ± standard error of the mean (SEM). Results for the K locus gene expression were compiled from two independent experiments (n = 4) and are presented as mean ± SEM. Comparisons of numerical data were performed using the Student’s t-test. For all analyses, a 2-tailed probability of <5% (ie, *P < 0.05) was considered significant.
Results
Human Neutrophils Effectively Kill MDRAB in the Presence of TGC
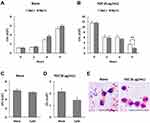
We determined the ability of human neutrophils to kill MDRAB cells in the presence of TGC, which is a bacteriostatic antibiotic. First, we determined the MIC of TGC for the MDRAB strains R1, R2, and R3 in RPMI-1640 medium containing 2% FBS. The MIC of TGC for MDRAB strains R1 and R2 was 2 μg/mL, whereas that for strain R3 was 16 μg/mL (Table 1). Next, a representative MDRAB strain R1, was co-cultured with human neutrophils and 8 μg/mL TGC in RPMI-1640 with 2% FBS, and the MDRAB numbers were analyzed. Figure 1A shows a time-dependent increase in the number of MDRAB strain R1 cells co-cultured with human neutrophils. Additionally, co-culturing with or without human neutrophils did not affect the growth of MDRAB strain R1, indicating that human neutrophils killed very few MDRAB. However, there was a significant decrease in the number of MDRAB strain R1 cells after co-culturing with human neutrophils and 8 μg/mL TGC for 6 h (Figure 1B), suggesting that human neutrophils killed TGC-treated MDRAB. To determine whether human neutrophils phagocytosed MDRAB in the presence of TGC, we analyzed the numbers of MDRAB strain R1 cells after 6 h of co-culturing with cytochalasin D-treated human neutrophils in the presence and absence of 8 μg/mL TGC. There was no difference in the number of MDRAB strain R1 cells co-cultured with mock-treated and cytochalasin D-treated human neutrophils (Figure 1C). Similarly, we observed a non-significant difference in the numbers of MDRAB strain R1 cells after co-culturing with mock-treated and cytochalasin D-treated human neutrophils in the presence of 8 μg/mL TGC (Figure 1D). Gram staining showed that human neutrophils did not phagocytose MDRAB strain R1 in the presence or absence of 8 μg/mL TGC (Figure 1E). These results suggest that human neutrophils kill TGC-treated MDRAB via a mechanism other than phagocytosis.
 |
Table 1 MDRAB Clinical Isolates Used in the Present Study MIC of TGC for MDRAB Was Measured in RPMI-1640 Supplemented with 2% FBS |
Human Neutrophils Co-Cultured with MDRAB Produced Low Levels of ROS and the Presence of TGC Did Not Alter the Total ROS Production
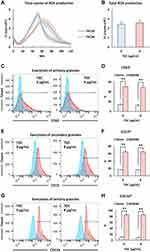
ROS play a crucial role in the rapid killing of bacteria.29 Therefore, we confirmed the ROS levels in human neutrophils co-cultured with the representative MDRAB strain R1, A. baumannii strain ATCC 19606, and P. aeruginosa strain PAO-1. ROS production in human neutrophils co-cultured with the P. aeruginosa strain PAO-1 peaked (451 ± 31.9×105 cpm) at 23.4 ± 1.43 min. ROS production in human neutrophils co-cultured with A. baumannii strains ATCC 19606 and R1 reached a maximum a value of 7.28 ± 0.35×105 cpm and 6.88 ± 0.63×105 cpm at 60.0 ± 1.90 min and 51.4 ± 0.78 min, respectively (Tables 2 and 3, and Figure 2A). Additionally, the total ROS production in human neutrophils co-cultured with P. aeruginosa strain PAO-1 was 104 ± 4.84×107 counts, whereas that of human neutrophils co-cultured with A. baumannii strains ATCC 19606 and R1 was 2.97 ± 0.19×107 and 2.44 ± 0.28 × 107, respectively (Tables 1 and 2). These results indicated that A. baumannii induces low levels of ROS production in human neutrophils in vitro. However, since our previous results indicated that human neutrophils could effectively kill MDRAB in the presence of TGC (Figure 1), we investigated ROS production in human neutrophils after co-culturing with the representative MDRAB strain R1, in the presence of TGC. ROS production in human neutrophils co-cultured with MDRAB strain R1 and 8 μg/mL TGC reached a maximum value of 5.58 ± 0.56×105 cpm at 62.2 ± 3.67 min (Table 3 and Figure 2A). The total ROS production in human neutrophils co-cultured with MDRAB strain R1 and 8 μg/mL TGC was 2.60 ± 0.33×107 counts (Table 3 and Figure 2B). These results indicated the total ROS production in human neutrophils co-cultured with MDRAB did not differ significantly in the absence or presence of TGC (Figure 2A).
 |
Table 2 Total ROS Production in Human Neutrophils Co-Cultured with Acinetobacter baumannii Strain ATCC 19606 and P. aeruginosa Strain PAO-1 |
 |
Table 3 Total ROS Production in Human Neutrophils Co-Cultured with MDRAB Strain R1 in the Presence or Absence of TGC |
Granule Exocytosis in Human Neutrophils Co-Cultured with MDRAB Was Not Altered in the Presence of TGC
Granule proteins released by human neutrophils have anti-microbial effects.30 Because the absence and presence of TGC did not cause a significant change in the total ROS production in human neutrophils co-cultured with MDRAB, we focused on the expression of granule proteins released by human neutrophils. The readiness of human neutrophil granules to be exocytosed leads to changes in neutrophil cell surface protein composition, in addition to affecting the release of granule matrix proteins.28 Thus, the expression of cell-surface proteins on human neutrophils can be used as a marker for granule exocytosis.28 To clarify whether TGC affects granule exocytosis in human neutrophils, we determined the expression of CD11b, CD15, and CD63 proteins in human neutrophils, which were co-cultured with the representative MDRAB strain R1 in the presence of TGC. We observed that the expression of CD11b, CD15, and CD63 proteins on human neutrophils, which were co-cultured with MDRAB strain R1 for 4 h, did not differ significantly depending on the presence or absence of 8 μg/mL TGC (Figure 2C–H). These results indicated that TGC does not affect granule exocytosis in human neutrophils.
TGC Suppressed Catalase Production and Capsule Expression in MDRAB
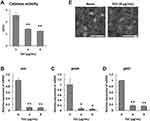
We next elucidated the effect of TGC on the virulence of the representative MDRAB strain R1 by measuring catalase activity and monitoring the expression of genes associated to the capsule. Catalase activity was significantly decreased in MDRAB strain R1 cells treated with 4 and 8 μg/mL TGC for 2 h, compared to that in untreated cells (Figure 3A). This suggested that TGC-treated MDRAB may not be efficient at neutralizing the ROS produced by human neutrophils. Furthermore, we analyzed the expression of genes associated to the capsule in A. baumannii, which allows the bacteria to mask its surface antigens. Expression of the K locus genes (wzc, gnaA, and galU) in MDRAB strain R1 was significantly suppressed after 2 h of treatment with 4 and 8 μg/mL TGC (Figure 3B–D). Additionally, there was a significant decrease in the thickness of the bacterial capsule after 4 h of treatment with 8 μg/mL TGC (capsule width in the presence and absence of TGC was 0.46 ± 0.02 and 0.36 ± 0.02 μm, respectively; **P < 0.01, n=4) (Figure 3E), and TGC-treated bacteria were rounded. These results indicate that both catalase activity and MDRAB capsule expression were effectively suppressed by TGC treatment.
TGC-Treated MDRAB are Killed by H2O2
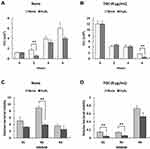
Next, we further analyzed the killing effect of H2O2, a bactericidal molecule produced by human neutrophils, on MDRAB in the presence of TGC. First, we examined the concentration of H2O2 for MDRAB treatment in RPMI-1640 with 2% FBS, and observed that a concentration of ≤ 0.078% H2O2 was not effective in killing MDRAB after 18 h of culturing. Thus, we determined the bacterial count for the representative MDRAB strain R1 when cultured with 0.078% H2O2 and 8 μg/mL TGC. As shown in Figure 4A, there was a significant decrease in the bacterial count for the MDRAB strain R1 after 2 h of culturing in the presence of 0.078% H2O2 than that in the absence of H2O2. This gradually increased in a time-dependent manner, suggesting that MDRAB decomposed the H2O2. After 6 h of incubation, the numbers of MDRAB strain R1 cells decreased significantly when cultured with 0.078% H2O2 and 8 μg/mL TGC, compared to that without H2O2 (Figure 4B). Furthermore, we calculated the viability of the MDRAB strains R1, R2, and R3 (which have been reported to show the same levels of catalase gene expression and activity10) when cultured with H2O2 in the absence and presence of TGC. There was an increase in the viability of MDRAB strains R1, R2, and R3 after 6 h of incubation with 0.078% H2O2 compared to that when the culture started (Figure 4C). In the presence of 8 μg/mL TGC, MDRAB strains R1, R2, and R3 cultured with 0.078% H2O2 and 8 μg/mL TGC showed significantly lower viability than the strains cultivated without H2O2 for 6 h (Figure 4D). These results indicated that TGC-treated MDRAB cells were more susceptible to cytotoxic substances.
Discussion
A. baumannii has recently emerged as a major nosocomial pathogen,1–3 and the increase in outbreaks of MDRAB worldwide has become a cause of concern.4,5 TGC acts as a bacteriostatic agent against MDRAB and suppresses bacterial growth and the expression of virulence factors.22,23 In the present study, we observed that human neutrophils effectively killed the representative MDRAB strain R1 in the presence of TGC. However, human neutrophils did not phagocytose A. baumannii. Notably, co-culturing with cytochalasin D-treated human neutrophils resulted in decreased numbers of MDRAB cells compared to that after incubation with mock-treated human neutrophils. A previous study reported that cytochalasin D impaired matrix metallopeptidase 9 (MMP-9) secretion in neutrophils, but initiated the release of cathepsin G, other granular bactericides, and proinflammatory agents.31 Consequently, it is plausible that the bactericidal molecules produced by human neutrophils kill TGC-treated MDRAB. Importantly, we observed that the markers for granule exocytosis were upregulated on human neutrophils after co-culture with MDRAB. Moreover, there was a significant decrease in the numbers of MDRAB cells after 6 h of co-culturing with human neutrophils and TGC. Furthermore, TGC-treated MDRAB strains R1, R2, and R3 exhibited increased susceptibility to H2O2. These results suggest that TGC treatment increases the susceptibility of MDRAB to the cytotoxic substances produced by human neutrophils.
To ascertain the mechanism underlying the increased susceptibility of MDRAB to cytotoxic substances after TGC treatment, we analyzed catalase activity and the expression of capsule-associated genes in TGC-treated MDRAB, which are resistant to the bactericidal functions of neutrophils.8,11 Catalase activity and the mRNA expression of wzc, gnaA, and galU were significantly suppressed in MDRAB in the presence of TGC after 2 h of culture, indicating that TGC exerted immediate effects on the virulence factors of MDRAB. Moreover, the ability of TGC to suppress the expression of the K locus genes in MDRAB caused a significant reduction in the thickness of the capsules after 4 h of culturing with the antibiotic. However, there was a significant reduction in the number of TGC-treated MDRAB after 6 h of incubation with human neutrophils and H2O2. These results suggest that TGC does not immediately affect bacterial surface antigens or the structure of the enveloping polysaccharide capsule. Instead, TGC treatment gradually alters the capsule structure and facilitates killing by human neutrophils in a time-dependent manner.
Although TGC-treated MDRAB strains showed susceptibility to H2O2, the numbers of TGC-treated MDRAB strain R3 did not significantly decrease in the presence of H2O2. As shown in Table 1, the MIC of TGC for MDRAB strain R3 was 16 mg/L, although the strain shares its virulence characteristics with MDRAB strains R1 and R2.10 These results suggest that TGC treatment increased the susceptibility of MDRAB to the cytotoxic substances produced by human neutrophils; however, this increase depends on the MIC of TGC for MDRAB.
A. baumannii is an emerging pathogen among the elderly and immunocompromised hosts in community hospitals and nursing homes.32 Although neutrophils play a crucial role in eliminating MDRAB,33 we previously showed that MDRAB does not induce neutrophil activation and functional maturation in the lungs of an aged mouse model.34 Therefore, bactericidal agents rather than bacteriostatic agents may be more effective in eliminating MDRAB in elderly hosts. However, there have been reports of multidrug resistance against last-resort antibiotics, such as CST and TGC.19,35 Additionally, either monotherapy or combination therapy should be considered to treat MDRAB infection because there are differences in the effectiveness of bactericidal activity in vitro and in vivo.36 Further studies are required to clarify the effectiveness of antimicrobial agents in the treatment and management of bacterial infections.
Conclusion
MDRAB causes nosocomial infections, especially VAP, and is associated with a high mortality rate in patients that are critically ill.4 However, the antibiotic TGC can exert bacteriostatic effects against the pathogen when therapeutic concentrations of the drug are reached in the body fluids and tissues, such as the lungs, skin, liver, heart, bone, and kidneys.20 Moreover, TGC suppresses the virulence factors of MDRAB, and ensures that human neutrophils can effectively kill MDRAB and subsequently eliminate the bacteria from the host. Thus, TGC is considered an effective antibiotic for the treatment of MDRAB, which has various virulence factors.
Ethical Approval
The study was approved by the Ethics Committee of the Teikyo University School of Medicine, Japan (TUIC-COI 21-1242), and the experimental procedures were performed according to the Declaration of Helsinki. Written informed consent was obtained from all patients before blood sampling.
Acknowledgments
We thank our colleagues from the Department of Microbiology and Immunology, Teikyo University School of Medicine, for their constructive discussions regarding this study. We thank the Honyaku Center, Inc. (www.honyakuctr.com) for editing the draft of this manuscript. This research was supported by JSPS KAKENHI (Grant Numbers 21K08516 and 20K08827).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–951. doi:10.1038/nrmicro1789
2. Antunes LC, Visca P, Towner KJ. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis. 2014;71:292–301. doi:10.1111/2049-632X.12125
3. Rice LB. Federal funding for the study of anti-microbial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis. 2008;197:1079–1081. doi:10.1086/533452
4. Ayoub Moubareck C, Hammoudi Halat D. Insights into Acinetobacter baumannii: a review of microbiological, virulence, and resistance traits in a threatening nosocomial pathogen. Antibiotics. 2020;9:119. doi:10.3390/antibiotics9030119
5. Mulani MS, Kamble EE, Kumkar SN, et al. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Front Microbiol. 2019;10:539. doi:10.3389/fmicb.2019.00539
6. Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi:10.1128/CMR.00058-07
7. Uppalapati SR, Sett A, Pathania R. The outer membrane proteins OmpA, CarO, and OprD of Acinetobacter baumannii confer a two-pronged defense in facilitating its success as a potent human pathogen. Front Microbiol. 2020;11:589234. doi:10.3389/fmicb.2020.589234
8. Geisinger E, Huo W, Hernandez-Bird J, et al. Acinetobacter baumannii: envelope determinants that control drug resistance, virulence, and surface variability. Annu Rev Microbiol. 2019;73:481–506. doi:10.1146/annurev-micro-020518-115714
9. Kobayashi SD, Malachowa N, DeLeo FR. Neutrophils and bacterial immune evasion. J Innate Immun. 2018;10:432–441. doi:10.1159/000487756
10. Sato Y, Unno Y, Miyazaki C, et al. Multidrug-resistant Acinetobacter baumannii resists reactive oxygen species and survives in macrophages. Sci Rep. 2019;9:17462. doi:10.1038/s41598-019-53846-3
11. Russo TA, Luke NR, Beanan JM, et al. The K1 capsular polysaccharide of Acinetobacter baumannii strain 307-0294 is a major virulence factor. Infect Immun. 2010;8:3993–4000. doi:10.1128/IAI.00366-10
12. Kamoshida G, Kikuchi-Ueda T, Nishida S, et al. Pathogenic bacterium Acinetobacter baumannii inhibits the formation of neutrophil extracellular traps by suppressing neutrophil adhesion. Front Immunol. 2018;9:178. doi:10.3389/fimmu.2018.00178
13. Kamoshida G, Kikuchi-Ueda T, Tansho-Nagakawa S, et al. Acinetobacter baumannii escape from neutrophil extracellular traps (NETs). J Infect Chemother. 2015;1:43–49. doi:10.1016/j.jiac.2014.08.032
14. Russo TA, Beanan JM, Olson R, et al. The K1 capsular polysaccharide from Acinetobacter baumannii is a potential therapeutic target via passive immunization. Infect Immun. 2013;81:915–922. doi:10.1128/IAI.01184-12
15. Nielsen TB, Yan J, Luna BM, et al. Monoclonal antibody requires immunomodulation for efficacy against Acinetobacter baumannii infection. J Infect Dis. 2021;224:2133–2147. doi:10.1093/infdis/jiab265
16. Talyansky Y, Nielsen TB, Yan J, et al. Capsule carbohydrate structure determines virulence in Acinetobacter baumannii. PLoS Pathog. 2021;17:e1009291. doi:10.1371/journal.ppat.1009291
17. Osei Sekyere J, Govinden U, Bester LA, et al. Colistin and tigecycline resistance in carbapenemase-producing Gram-negative bacteria: emerging resistance mechanisms and detection methods. J Appl Microbiol. 2016;121:601–617. doi:10.1111/jam.13169
18. Cai Y, Chai D, Wang R, et al. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J Antimicrob Chemother. 2012;67:1607–1615. doi:10.1093/jac/dks084
19. Foong WE, Wilhelm J, Tam HK, et al. Tigecycline efflux in Acinetobacter baumannii is mediated by TetA in synergy with RND-type efflux transporters. J Antimicrob Chemother. 2020;75:1135–1139. doi:10.1093/jac/dkaa015
20. Yaghoubi S, Zekiy AO, Krutova M, et al. Tigecycline antibacterial activity, clinical effectiveness, and mechanisms and epidemiology of resistance: narrative review. Eur J Clin Microbiol Infect Dis. 2021;5:1–20.
21. Skinner C, Zhang G, Patfield S, et al. An in vitro combined antibiotic-antibody treatment eliminates toxicity from Shiga toxin-producing Escherichia coli. Antimicrob Agents Chemother. 2015;59:5435–5444. doi:10.1128/AAC.00763-15
22. Sato Y, Unno Y, Ubagai T, et al. Sub-minimum inhibitory concentrations of colistin and polymyxin B promote Acinetobacter baumannii biofilm formation. PLoS One. 2018;13:e0194556. doi:10.1371/journal.pone.0194556
23. Navidifar T, Amin M, Rashno M. Effects of sub-inhibitory concentrations of meropenem and tigecycline on the expression of genes regulating pili, efflux pumps and virulence factors involved in biofilm formation by Acinetobacter baumannii. Infect Drug Resist. 2019;12:1099–1111. doi:10.2147/IDR.S199993
24. La Scola B, Gundi VA, Khamis A, et al. Sequencing of the rpoB gene and flanking spacers for molecular identification of Acinetobacter species. J Clin Microbiol. 2006;44:827–832. doi:10.1128/JCM.44.3.827-832.2006
25. Ubagai T, Sato Y, Kamoshida G, et al. Immunomodulatory gene expression analysis in LPS-stimulated human polymorphonuclear leukocytes treated with antibiotics commonly used for multidrug-resistant strains. Mol Immunol. 2021;129:39–44. doi:10.1016/j.molimm.2020.11.012
26. Ono Y, Kunii O, Kobayashi K, et al. Evaluation of opsonophagocytic dysfunctions in severely burned patients by luminol-dependent chemiluminescence. Microbiol Immunol. 1993;37:563–571. doi:10.1111/j.1348-0421.1993.tb01678.x
27. Unno Y, Sato Y, Fukuda H, et al. Resolvin E1, but not resolvins E2 and E3, promotes fMLF-induced ROS generation in human neutrophils. FEBS Lett. 2018;592:2706–2715. doi:10.1002/1873-3468.13215
28. Mollinedo F. Neutrophil degranulation, plasticity, and cancer metastasis. Trends Immunol. 2019;40:228–242. doi:10.1016/j.it.2019.01.006
29. Qiu H, Kuolee R, Harris G, et al. Role of NADPH phagocyte oxidase in host defense against acute respiratory Acinetobacter baumannii infection in mice. Infect Immun. 2009;77:1015–1021. doi:10.1128/IAI.01029-08
30. McKenna E, Mhaonaigh AU, Wubben R, et al. Neutrophils: need for Standardized Nomenclature. Front Immunol. 2021;12:602963. doi:10.3389/fimmu.2021.602963
31. Galkina SI, Fedorova NV, Serebryakova MV, et al. Mold alkaloid Cytochalasin D modifies the morphology and secretion of fMLP-, LPS-, or PMA-stimulated neutrophils upon adhesion to fibronectin. Mediators Inflamm. 2017;2017:4308684. doi:10.1155/2017/4308684
32. Sengstock DM, Thyagarajan R, Apalara J, et al. Multidrug-resistant Acinetobacter baumannii: an emerging pathogen among older adults in community hospitals and nursing homes. Clin Infect Dis. 2010;50:1611–1616. doi:10.1086/652759
33. van Faassen H, KuoLee R, Harris G, et al. Neutrophils play an important role in host resistance to respiratory infection with Acinetobacter baumannii in mice. Infect Immun. 2007;75:5597–5608. doi:10.1128/IAI.00762-07
34. Sato Y, Tansho-Nagakawa S, Ubagai T, et al. Analysis of immune responses in Acinetobacter baumannii-infected klotho knockout mice: a mouse model of Acinetobacter baumannii infection in aged hosts. Front Immunol. 2020;11:601614. doi:10.3389/fimmu.2020.601614
35. Jovcic B, Novovic K, Dekic S, et al. Colistin resistance in environmental isolates of Acinetobacter baumannii. Microb Drug Resist. 2021;27:328–336. doi:10.1089/mdr.2020.0188
36. Kumar S, Anwer R, Azzi A. Virulence potential and treatment options of multidrug-resistant (MDR) Acinetobacter baumannii. Microorganisms. 2021;9:2104. doi:10.3390/microorganisms9102104
37. Geisinger E, Isberg RR. Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog. 2015;11:e1004691. doi:10.1371/journal.ppat.1004691
38. Xu Q, Chen T, Yan B, et al. Dual role of gnaA in antibiotic resistance and virulence in Acinetobacter baumannii. Antimicrob Agents Chemother. 2019;63:e00694–19. doi:10.1128/AAC.00694-19
39. Wyres KL, Cahill SM, Holt KE, et al. Identification of Acinetobacter baumannii loci for capsular polysaccharide (KL) and lipooligosaccharide outer core (OCL) synthesis in genome assemblies using curated reference databases compatible with Kaptive. Microb Genom. 2020;6:e000339.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.