Back to Journals » Clinical Ophthalmology » Volume 8
Thyroid-stimulating immunoglobulins as measured in a reporter bioassay are not detected in patients with Hashimoto’s thyroiditis and ophthalmopathy or isolated upper eyelid retraction
Authors Wall J, Lahooti H , El Kochairi I, Lytton S, Champion B
Received 1 May 2014
Accepted for publication 19 June 2014
Published 9 October 2014 Volume 2014:8 Pages 2071—2076
DOI https://doi.org/10.2147/OPTH.S67098
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Jack R Wall,1 Hooshang Lahooti,1 Ilhem El Kochairi,1 Simon D Lytton,2 Bernard Champion1
1Department of Medicine, the University of Sydney, Penrith, NSW, Australia; 2SeraDiaLogistics, Munich, Germany
Abstract: Although ophthalmopathy is mainly associated with Graves’ hyperthyroidism, milder eye changes are also found in about 25% of patients with Hashimoto’s thyroiditis (HT). The recent finding of negative thyrotropin receptor (TSHR) antibodies, as measured in the Thyretain™ thyroid-stimulating immunoglobulin (TSI) reporter bioassay, in patients with euthyroid Graves’ disease raises the possibility that TSHR antibodies are not the cause of ophthalmopathy in all situations. Here, we have tested serum from patients with HT with and without ophthalmopathy or isolated upper eyelid retraction (UER) for TSHR antibodies, using the TSI reporter bioassay and collagen XIII as a marker of autoimmunity against the orbital fibroblast. Study groups were 23 patients with HT with ophthalmopathy, isolated UER, or both eye features and 17 patients without eye signs. Thyretain™ TSI results were expressed as a percentage of the sample-to-reference ratio, with a positive test being taken as a sample-to-reference ratio of more than 140%. Serum collagen XIII antibodies were measured in standard enzyme-linked immunosorbent assay. TSI tests were positive in 22% of patients with HT with no eye signs but in no patient with eye signs. In contrast, TSI tests were positive in 94% of patients with Graves’ ophthalmopathy. Tests were negative in all normal subjects tested. Collagen XIII antibodies were detected in 83% of patients with ophthalmopathy, UER, or both eye features, but in only 30% of patients with no eye signs. Our findings suggest that TSHR antibodies do not play a major role in the pathogenesis of ophthalmopathy or isolated UER in patients with HT. Moreover, the role of TSHR antibodies in the development of ophthalmopathy in patients with Graves’ disease remains to be proven. In contrast, collagen XIII antibodies appear to be a good marker of eye disease in patients with HT.
Keywords: Hashimoto’s thyroiditis, ophthalmopathy, upper eyelid retraction, TSH receptor antibodies, collagen XIII
Introduction
Although ophthalmopathy is mainly associated with Graves’ hyperthyroidism, as Graves’ ophthalmopathy (GO), in general, milder eye changes are also found in about 25% of patients with Hashimoto’s thyroiditis (HT).1 Ophthalmopathy is also associated with transient subacute or silent thyroiditis2 and, in 10% of cases, with the apparent absence of thyroid autoimmunity, so-called euthyroid Graves’ disease.3,4 In general, Graves patients with obvious eye disease have positive thyrotropin receptor (TSHR) antibodies, as measured in TSHR binding inhibiting immunoglobulin assays, such as the well-known thyroid stimulating hormone receptor antibody (TRAB) assay.5–7 However, we have recently found that TSHR antibodies, as measured in a novel chimeric cell-based reporter bioassay for thyroid-stimulating immunoglobulins (TSI), the TSI reporter assay, are not detected in patients with euthyroid Graves’ disease unless they convert to Graves’ hyperthyroidism (Wall and colleagues, unpublished data, 2014). Moreover, there may be other situations in which these antibodies are not detected in patients with “endocrine ophthalmopathy.”8
Although most patients with HT have mild ophthalmopathy, manifested mainly as lid retraction and lag and itchy gritty and watery eyes, a few patients do have more typical ophthalmopathy with eye muscle involvement and inflammatory changes. The eye changes of HT are often missed unless carefully looked for, including testing for upper eyelid retraction (UER). In our studies, we have shown that patients with mild eye disease have antibodies against calsequestrin and collagen XIII, but TSHR antibody tests are usually negative.1,2 In other studies, TSI measured in the TSI reporter assay are more closely associated with ophthalmopathy than the hyperthyroidism in patients with Graves’ disease.9–11 The recent finding of negative TSI antibodies in patients with euthyroid Graves’ disease raises the possibility that TSHR antibodies are not the cause of ophthalmopathy in all situations. Studies of patients with HT are expected to shed further light on the relationship between ophthalmopathy and TSHR antibodies. Here, we have tested serum from patients with HT, with and without classical ophthalmopathy or isolated UER, using the TSI reporter bioassay.
Clinical subjects and methods
TSI tests were carried out on 40 patients with HT, of whom 23 (seven males and 16 females, aged 13–77 years; mean age, 51 years) had ophthalmopathy or isolated UER and 17 (two males and 15 females, aged 13–76 years; mean age, 47 years) had no eye signs, on 16 patients (four males and twelve females aged 38–77 years; mean age, 60 years) with GO as positive TSI controls, and on thirteen normal subjects (seven females and six males, aged 28–50 years; mean age, 42 years) as negative controls. Collagen XIII antibody tests were carried out in 19 of the patients with HT and eye signs and in 36 patients with no eye signs; namely, 19 of the patients who had TSI testing and an additional 17 patients (five males and 12 females, aged 28–74 years; mean age, 52 years) who did not have TSI testing performed.
Serum was drawn at the patient’s first visit, at which time the great majority were hypothyroid. The diagnosis of HT was made according to the usual clinical features of goiter, tiredness, cold intolerance, and weight gain and was confirmed from thyroid function testing, serum thyroid peroxidase and thyroglobulin antibodies, and real-time thyroid ultrasonography.
Eye assessment
The ophthalmopathy was assessed as Nunery type 1 (without restrictive myopathy) or type 2 (with restrictive myopathy);12 given a modified Clinical Activity Score (0–12) from Mourits et al,13 which is a measure of disease activity; put in one of Werner’s NOSPECS classes;14 and given an UER score, determined from scores of 0–3 for each of UER and upper eyelid lag for each eye (total score, 0–12). The degree of proptosis (in millimeters) was measured using a Hertel exophthalmometer, where a positive reading was defined as more than 18 mm in either eye or a 2 mm or more difference between the eyes. For the purpose of the study, “ophthalmopathy” was taken as a NOSPECS class of 2 or higher, regardless of the Clinical Activity Score, which is a measure of activity but not severity, and with a UER score of 2 or more being taken as significant UER.
Thyretain™ TSI reporter bioassay
The Thyretain™-TSI cyclic adenosine monophosphate luciferase reporter bioassay described previously9–11 was used to assess TSI. Briefly, test serum samples and four controls, consisting of reference standard bovine TSH, normal serum, positive TSI serum, and cells alone, were tested in triplicate. Results were expressed as a percentage of the sample-to-reference ratio (SRR%). The cutoff of the Thyretain-TSI reporter SRR% was historically established as two standard deviations above the reference luminescence, which is set to SRR 100% for each plate, with a positive test being taken as an SRR% of more than 140%. The Nepean Hospital Human Ethics committee approved this retrospective study, and consent forms were not needed.
Measurement of collagen XIII antibodies
Serum collagen XIII antibodies were measured in standard enzyme-linked immunosorbent assay, as described in previous publications from this laboratory.2,15,16 Recombinant human collagen XIII was provided by Dr Taina Pihlajaniemi and Dr Tu Min (Oulu University, Oulu, Finland). Antigen concentration was 0.25 μg/mL, and the optimal serum dilution was 1:25. A positive test was taken as an optical density (OD) greater than the upper limit of normal for 30 healthy males younger than 30 years; namely, 174.
Other tests
Plasma fT4, fT4, and TSH levels and serum thyroid peroxidase and thyroglobulin antibodies were measured by Barratt and Smith Pathology (Sydney, NSW, Australia), according to the manufacturers’ instructions.
Statistical analysis
Differences in the TSI levels between the various patient groups and the control subjects were assessed using the Mann–Whitney test for nonparametric data; a P-value of <0.05 was taken as statistical significance.
Results
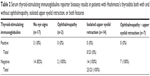
TSI by Thyretain™-report bioassay was tested in 40 patients with HT, 23 of whom had ophthalmopathy, defined as NOSPECS class of 2 or more (n=2); isolated UER, taken as an UER score of 2 or more (n=14); or both ophthalmopathy and UER (n=7). The other 17 patients did not have any eye signs. The demographics, thyroid and orbital antibodies, and eye findings in the 23 patients with HT and ophthalmopathy or isolated UER are summarized in Table 1. The results are summarized in Table 2 and Figure 1. TSI tests were positive in four (22%) of the 17 patients with HT and no eye signs but in none of the 23 patients with ophthalmopathy, UER, or both features (Table 2). TSI tests were positive in 15 (94%) of 16 patients with GO, including one patient with HT who converted to Graves’ disease at the time her TSI test became positive, but TSI tests were negative in all 13 normal patients (Figure 1). TSI values for all groups, as well as their means (± standard error (SE)), are shown in Figure 1. Mean (± SE) values for the three HT groups were not significantly different from the normals (Mann–Whitney test, P = non-specific (NS)), whereas the difference between GO and normals was highly significant (P<0.001).
Finally, collagen XIII antibodies were tested for in 18 patients with HT and eye signs and 33 with no eye signs; namely, 16 patients who had TSI testing and an additional 17 patients who had not yet had TSI testing performed. A positive test was taken as an OD ×1,000 of 174 or more. Collagen XIII antibody tests were positive in ten (30%) of 33 patients with no eye signs and in 15 (83%) of 18 patients with eye signs; namely, in all three patients with ophthalmopathy, in five (56%) of nine patients with isolated UER, and in all seven patients (100%) with both ophthalmopathy and UER (Table 3).
Discussion
The eye and orbital disorder usually associated with thyroid auto immunity is a complex disorder because one must explain the unique link between the orbital and thyroid reactions. One commonly held view is that the TSHR antibodies that cause Graves’ hyperthyroidism also cause the ophthalmopathy by cross-reacting with the TSHR in the orbital fibroblasts and adipocytes.5–7,17 Although it is true that TSHR antibody levels tend to be higher in patients with ophthalmopathy and very high in those with severe active disease, and that the hyperthyroidism and eye signs tend to occur together when TSHR antibody levels are first detected,18,19 there are some situations in which these antibodies are not closely associated with ophthalmopathy and the overall evidence is mainly circumstantial.8 We studied patients with HT, using a new TSI reporter bioassay, to address whether TSHR antibodies, as measured in this assay, are associated with ophthalmopathy or isolated UER in this disorder. Recently, we have shown that in patients with so-called euthyroid Graves’ disease, tests were always negative in all thirteen patients tested, with the exception of four patients at the time they converted to Graves’ hyperthyroidism (Lahooti and colleagues, unpublished data, 2014). Because the ophthalmopathy is presumed to be the same as in GO, but not associated with thyroid dysfunction or thyroid autoimmunity, we concluded that TSHR antibodies were unlikely to play any important role in GO.
The eye changes in HT are usually mild, but a few patients do have severe disease with extra ocular muscle damage, as in GO. However, about 25% have mainly upper eyelid signs and symptoms and UER.1 To summarize, TSI tests were positive in 22% of patients with HT with no eye signs but in no patient with ophthalmopathy, isolated UER, or both eye features. As expected, TSI tests were strongly positive in 94% of patients with GO tested and negative in all normal subjects. In contrast, collagen XIII antibodies, which are associated with the congestive ophthalmopathy subtype of “thyroid-associated ophthalmopathy,”20 were detected in 83% of patients with ophthalmopathy or UER, including 100% of patients with both ophthalmopathy and UER, but in only 30% of patients with no eye signs. Collagen XIII is one of only two members of the large collagen family that is expressed on the surface of the orbital fibroblast where it could be seen by the immune system.16,17 Although collagen XIII autoantibodies are not specific for ophthalmopathy, being detected in some normals as well as in patients with Graves’ disease without evident eye signs, as in this study, they seem to be a marker for orbital and upper eyelid inflammation in patients with HT.
Earlier, we showed that TSI tests were always negative in patients with so-called euthyroid Graves’ disease (Lahooti and colleagues, unpublished data, 2014), and here we do not show a significant relationship between the antibodies and the eye changes of HT. In our opinion, the pathogenesis of the ophthalmopathy associated with Graves’ disease remains unknown but is unlikely to involve the TSHR. Future studies should address the role of T-cell reactivity against extraocular muscle and orbital fibroblast autoantigens including calsequestrin, collagen XIII, and the TSHR.
In conclusion, the findings of mainly negative TSI among patients with HT with eye signs but positive tests in two patients with no ophthalmopathy strongly suggest TSHR antibodies do not play a major role in the pathogenesis of ophthalmopathy or isolated UER in patients with HT. In contrast, collagen XIII antibodies may be a better marker of orbital connective tissue inflammation in these patients.
Acknowledgments
This research was supported by grants from Nepean Blue Mountains Local Health District and the Nepean Medical Research Foundation. We thank Dr Jeffrey Houtz for the performance of the TSI tests.
Disclosure
The authors report no conflicts of interest in this work.
References
Tjiang H, Lahooti H, McCorquodale T, Parmar KR, Wall JR. Eye and eyelid abnormalities are common in patients with Hashimoto’s thyroiditis. Thyroid. 2010;20(3):287–290. | ||
Gopinath B, Musselman R, Adams C, Tani J, Beard N, Wall JR. Study of serum antibodies against three eye muscle antigens and the connective tissue antigen collagen XIII in patients with Graves’ disease with and without ophthalmopathy – correlation with clinical features. Thyroid. 2006;16:967–974. | ||
Salvi M, Zhang ZG, Haegert D, et al. Patients with endocrine ophthalmopathy not associated with overt thyroid disease have multiple thyroid immunological abnormalities. J Clin Endocrinol Metab. 1990;70(1):89–94. | ||
McCorquodale T, Lahooti H, Gopinath B, Wall JR. Long-term follow-up of seven patients with ophthalmopathy not associated with thyroid autoimmunity: heterogeneity of autoimmune ophthalmopathy. Clin Ophthalmol. 2012;6:1063–1071. | ||
Eckstein AK, Plicht M, Lax H, et al. Thyrotropin receptor autoantibodies are independent risk factors for Graves’ ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab. 2006;91(9):3464–3470. | ||
Paschke R, Vassart G, Ludgate M. Current evidence for and against the TSH receptor being the common antigen in Graves’ disease and thyroid associated ophthalmopathy. Clin Endocrinol (Oxf). 1995;42(6): 565–569. | ||
Bahn RS. Clinical review 157: Pathophysiology of Graves’ ophthalmopathy: the cycle of disease. J Clin Endocrinol Metab. 2003;88(5):1939–1946. | ||
Wall JR. The TSH-Receptor and Thyroid-Associated Ophthalmopathy – a Convenient Hypothesis with too many Exceptions to be true. Int J Endocrinol Metab. 2007;2:49–51. | ||
Lytton SD, Li Y, Olivo PD, Kohn LD, Kahaly GJ. Novel chimeric thyroid-stimulating hormone-receptor bioassay for thyroid-stimulating immunoglobulins. Clin Exp Immunol. 2010;162(3):438–446. | ||
Lytton SD, Ponto KA, Kanitz M, Matheis N, Kohn LD, Kahaly GJ. A novel thyroid stimulating immunoglobulin bioassay is a functional indicator of activity and severity of Graves’ orbitopathy. J Clin Endocrinol Metab. 2010;95(5):2123–2131. | ||
Lytton SD, Kahaly GJ. Bioassays for TSH-receptor autoantibodies: an update. Autoimmun Rev. 2010;10(2):116–122. | ||
Nunery WR, Martin RT, Heinz GW, Gavin TJ. The association of cigarette smoking with clinical subtypes of ophthalmic Graves’ disease. Ophthal Plast Reconstr Surg. 1993;9(2):77–82. | ||
Mourits MP, Koornneef L, Wiersinga WM, Prummel MF, Berghout A, van der Gaag R. Clinical criteria for the assessment of disease activity in Graves’ ophthalmopathy: a novel approach. Br J Ophthalmol. 1989;73(8):639–644. | ||
Werner SC. Classification of the eye changes of Graves’ disease. Am J Ophthalmol. 1969;68(4):646–648. | ||
Gopinath B, Ma G, Wall JR. Eye signs and serum eye muscle and collagen XIII antibodies in patients with transient and progressive thyroiditis. Thyroid. 2007;17(11):1123–1129. | ||
De Bellis A, Sansone D, Coronella C, et al. Serum antibodies to collagen XIII: a further good marker of active Graves’ ophthalmopathy. Clin Endocrinol (Oxf). 2005;62(1):24–29. | ||
Gopinath B, Musselman R, Adams CL, Tani J, Beard N, Wall JR. Study of serum antibodies against three eye muscle antigens and the connective tissue antigen collagen XIII in patients with Graves’ disease with and without ophthalmopathy: correlation with clinical features. Thyroid. 2006;16(10):967–974. | ||
Weetman AP. Graves’ disease. N Engl J Med. 2000;343(17): 1236–1248. | ||
Gerding MN, van der Meer JW, Broenink M, Bakker O, Wiersinga WM, Prummel MF. Association of thyrotrophin receptor antibodies with the clinical features of Graves’ ophthalmopathy. Clin Endocrinol (Oxf). 2000;52(3):267–271. | ||
Burch HB, Wartofsky L. Graves’ ophthalmopathy: current concepts regarding pathogenesis and management. Endocr Rev. 1993;14(6):747–793. | ||
De Bellis A, Sansone D, Coronella C, et al. Serum antibodies to collagen XIII: a further good marker of active Graves’ ophthalmopathy. Clin Endocrinol (Oxf). 2005;62(1):24–29. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.