Back to Journals » Drug Design, Development and Therapy » Volume 15
Thymoquinone: A Promising Natural Compound with Potential Benefits for COVID-19 Prevention and Cure
Authors Badary OA, Hamza MS , Tikamdas R
Received 28 February 2021
Accepted for publication 13 April 2021
Published 3 May 2021 Volume 2021:15 Pages 1819—1833
DOI https://doi.org/10.2147/DDDT.S308863
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Osama A Badary,1,2 Marwa S Hamza,1 Rajiv Tikamdas1
1Clinical Pharmacy Practice Department, Faculty of Pharmacy, The British University in Egypt, Cairo, Egypt; 2Clinical Pharmacy Department, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt
Correspondence: Osama A Badary
Clinical Pharmacy Practice Department, Faculty of Pharmacy, The British University in Egypt, P.O. Box 43, El-Sherouk City, Cairo, 11837, Egypt
Tel +201064112110
Email [email protected]
Abstract: COVID-19 has caused a major global health crisis, as excessive inflammation, oxidation, and exaggerated immune response in some sufferers can lead to a condition known as cytokine storm, which may progress to acute respiratory distress syndrome (ARDs), which can be fatal. So far, few effective drugs have emerged to assist in the treatment of patients with COVID-19, though some herbal medicine candidates may assist in the fight against COVID-19 deaths. Thymoquinone (TQ), the main active ingredient of black seed oil, possesses antioxidant, anti-inflammatory, antiviral, antimicrobial, immunomodulatory and anticoagulant activities. TQ also increases the activity and number of cytokine suppressors, lymphocytes, natural killer cells, and macrophages, and it has demonstrated antiviral potential against a number of viruses, including murine cytomegalovirus, Epstein-Barr virus, hepatitis C virus, human immunodeficiency virus, and other coronaviruses. Recently, TQ has demonstrated notable antiviral activity against a SARSCoV-2 strain isolated from Egyptian patients and, interestingly, molecular docking studies have also shown that TQ could potentially inhibit COVID-19 development through binding to the receptor-binding domain on the spike and envelope proteins of SARS-CoV-2, which may hinder virus entry into the host cell and inhibit its ion channel and pore forming activity. Other studies have shown that TQ may have an inhibitory effect on SARS CoV2 proteases, which could diminish viral replication, and it has also demonstrated good antagonism to angiotensin-converting enzyme 2 receptors, allowing it to interfere with virus uptake into the host cell. Several studies have also noted its potential protective capability against numerous chronic diseases and conditions, including diabetes, hypertension, dyslipidemia, asthma, renal dysfunction and malignancy. TQ has recently been tested in clinical trials for the treatment of several different diseases, and this review thus aims to highlight the potential therapeutic effects of TQ in the context of the COVID-19 pandemic.
Keywords: thymoquinone, COVID-19, natural, therapeutic benefits
Introduction
COVID-19 Overview
The novel coronavirus that causes COVID-19 was first discovered in 2019 in Wuhan, China. It has since spread globally, resulting in a worldwide pandemic. COVID-19 is an infectious disease that causes severe acute respiratory syndrome, leading to the virus causing it to be formally named SARS-CoV-2. Comorbidities such as chronic diseases and acute organ injuries are strongly correlated with disease severity and mortality among COVID-19 patients,1 though the clinical features of COVID-19 are varied, ranging from asymptomatic states to acute respiratory distress syndrome (ARDS) and multiorgan dysfunction. A fever, coughing, a sore throat, headaches, fatigue, myalgia, and breathlessness are the most common clinical features of COVID-19, however.2 By the end of the first week, in some patients, the disease may progress to pneumonia, respiratory failure, and death.3 This progression is generally associated with an extremely uncontrolled production of pro-inflammatory mediators that leads to ARDS and cytokine storm syndrome.4 Complications thus include acute lung injury, ARDS, shock, and acute kidney injury.
Several clinical trials of possible treatments for COVID-19 are underway, based on those treatments’ antiviral, anti-inflammatory, immunomodulatory, antioxidant or similar activities.5,6 There are also some previously available drugs that have been repurposed for the management of COVID-19, such as remdesivir, hydroxychloroquine, chloroquine, umifenovir, lopinavir, oseltamivir, and favipiravir, as well as adjunctive agents, such as zinc, vitamin D, azithromycin, ascorbic acid, nitric oxide, corticosteroids, and interleukin (IL)-6 antagonists. Growing interest is also developing in the use of new therapeutic methods, such as specific anti-inflammatory molecules (eg tocilizumab), anti-IL-17, and treatment with mesenchymal stromal cells.7 The amplification of anti-2019nCoV-specific T lymphocytes may be another feasible option for treatment.8 In terms of prevention, several COVID-19 vaccines are also now available.9
Alternative Therapies
Although researchers worldwide have worked exhaustively to find a solution, as yet, no entirely adequate therapy for COVID-19 has emerged. Alternative approaches must thus be subject to comprehensive attention, similar to the strategy used in the initial repurposing of conventional therapeutics. An example of such alternative therapy is found in the application of vitamin D, which has been suggested to help reduce the effect of the pandemic on maternal and child health.10 Other speculative suggestions include the idea that vitamin C could help with COVID-19-related symptoms,11 or that honey may have a positive impact on COVID-19 recovery.12 Pharmacological intervention using natural products is considered another example of alternative medicine.13
In the past, herbal medicine has played an important role in managing infectious disease, and a range of herbal medicinal studies on the treatment of a previous SARS coronavirus (SARS-CoV), have provided clinical evidence that herbal medicines have some advantageous effects with regard to the treatment and prevention of epidemics, with several significant results.14 There is also clinical evidence that the use of herbal medicines can have positive consequences in certain COVID-19 treatments.15,16 One systematic review has shown significant impacts on efficacy and improvement of symptoms on combining herbal medicine with Western medicine in the treatment of COVID-19, suggesting that herbal medicine does have a potential role to play in COVID-19 treatment. Further clinical trials are, however, necessary to further confirm the efficacy, and any adverse effects, of herbal medicine as part of COVID-19 treatment.17
Several edible plants are known to act as natural antiviral agents, and these may have the potential to be developed into a COVID-19 nutraceutical. Such a development may offer a supplementary treatment to help people cope with this highly infectious disease and thus protect the global population against the current pandemic.18 In terms of daily diet, herbal preparations with immunomodulatory actions may offer prophylactic therapy to prevent infection and to help contain diseases within communities, as well as encouraging faster post-infection healing.18
Natural Therapeutic Approaches
Some reports have emerged of the beneficial effects of certain traditional herbal medicines with regard to COVID-19. Examples include Ginseng (Panax ginseng), which has a modulatory effect on human immune cells;19 ginger (Zingiber officinale), which has anti-apoptotic, anti-inflammatory, anti-tumor activities, anti-hyperglycemic, antioxidant, and analgesic properties;20 garlic (Allium sativum), which stimulates the immune system;21 and Echinacea extract (Echinacea purpurea (L.) Moench), which has antimicrobial and antioxidant activities.22
Other herbal phyto-constituents have been reported to be effective in reducing infectious conditions, including triterpene glycosides isolated from Heteromorpha23 and extracts from Artemisia annua, Lycoris radiata, Pyrrosia lingua and Lindera aggregate,17,24 while natural inhibitors such as the nsP13 helicase and 3CL protease have been identified, along with myricetin, scutellarein, and phenolic compounds from Isatis indigotica and Torreya nucifera, to be operative against SARS-CoV enzymes.25–27 Moreover, Cinatl et al reported that glycyrrhizin elicited a significant antiviral activity against SARS coronavirus,28 while Nigella sativa (black seed) was reported to have potential for the management of COVID-19 patients’ symptoms.13,29–31
Nigella sativa: An Overview
Nigella sativa (Black seed), from the family Ranunculaceae, have been found in several ancient sites, including Tutankhamun’s tomb. The Persian physician Avicenna, regarded as the father of early modern medicine, described the plant in his Canon of Medicine as offering a treatment for shortness of breath,32 which frequently accompanies pathological conditions such as asthma and pneumonia. Volatile oils and alkaloids are generally associated with biological activity, and the volatile oils of these seeds contain nigellone, thymoquinone (TQ), thymohydroquinone, dithymoquinone, thymol, carvacrol, α and β-pinene, d-limonene, d-citronellol, p-cymene, carvacrol, t-anethole, 4-terpineol and longifolene.33,34 Nigella sativa seeds thus offer a natural product with multiple potential pharmacological activities including antidiabetic, anticancer, immunomodulatory, analgesic, antimicrobial, anti-inflammatory, bronchodilator, renal and gastro-protective, and antioxidant properties.35,36
Thymoquinone
Thymoquinone (2-Isopropyl-5-methylbenzo-1, 4-quinone) is the main active ingredient of the volatile oil of black seed (Figure 1). It was first extracted by El–Dakhakhny,37 and amongst the various different active constituents reported so far, TQ remains the major bioactive principle due to its range of therapeutic benefits including antioxidant,38 anti-inflammatory,39 anti-cancer,40 antibacterial,41 antifungal activity,42 and anticonvulsant activity.43 Furthermore, a more specific effect of the antiviral activity of TQ and black seed fixed oil against murine cytomegalovirus infection model has been reported.44,45 TQ may thus offer integral complementary support in conditions of uncertain core basic needs during COVID-19 treatment. However, the question of whether TQ might act as a distinct therapeutic drug for the control and/or treatment of COVID-19 still remains to be investigated.
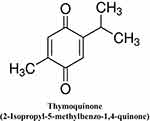 |
Figure 1 Chemical structure of thymoquinone. |
The Aim of the Review
This review aims to focus on the potentially beneficial roles of TQ against COVID-19 pathophysiology in the context of antioxidant, anti-inflammatory, immunomodulatory, epigenetic modulation, antiviral activity, docking studies on anti-COVID-19 activity, antibacterial and anticoagulant effects for the treatment of COVID-19.
Potential Beneficial Effects of Thymoquinone in COVID-19
N sativa, due to its wide range of bioactive components such as TQ and nigellimine, could offer a range of benefits for treating COVID-19, such as blocking the introduction of the virus to pneumocytes; providing ionophores to improve zinc intake, thereby improving the host immune response to SARS-CoV-2; and preventing the virus from replicating.29 TQ is the main bioactive principle in N Sativa, and this has been found to confer a range of therapeutic advantages34 including antioxidant,38 anti-inflammatory,39,46 anticancer,40 antibacterial,41 antifungal,42 anticoagulant,47 anti-sepsis,48 and anticonvulsant activity.43 N Sativa seeds have also demonstrated immunomodulatory effects,49,50 while several studies suggest that N Sativa seeds have some antiviral effects.44,51,52 In addition to its immunomodulatory and antioxidant properties, however, N Sativa and its active constituents have also been noted to provide anti-ischemic effects in several organs, including the brain, kidney, heart, liver, and intestines.53 Such evidence strongly suggests that N. sativa seeds and their active constituents may have significant therapeutic potential against COVID-19 and its complications13,54 (Figure 2).
 |
Figure 2 Multitargeted protective effects of thymoquinone against COVID-19 pathogenesis. |
Antioxidant Effect
Reactive oxygen species (ROS) are formed during normal cellular respiration and as a reaction to xenobiotics.55 They are highly reactive, and thus may harm and change the functions of various cell components, such as lipids, proteins, nucleic acids, and carbohydrates.56 Oxidative stress occurs due to imbalance between oxidants and antioxidants,57 and it is a crucial factor in pathogenesis of many diseases58 such as diabetes,59 inflammation,60 cardiovascular diseases,61 cancer,62 and advanced age.63 A major factor in the excessive immune response seen in some COVID-19 infections may thus be the overwhelming of the antioxidative defense mechanism and the resulting oxidative damage.55
Antioxidant properties require high radical-scavenging capabilities, and this is one of the essential characteristic functions of TQ. TQ works by activating the enzymes that protect cells from cellular damage caused by oxidative stress. Several studies have shown that TQ does this by increasing the expression of mRNA and stimulating various cytoprotective antioxidant enzymes, including catalases, superoxide dismutase, glutathione reductase, and glutathione-S-transferase.64–68 TQ thus offers protection against glucose or methylglyoxal induced loss of superoxide dismutase activity and fragmentation or cross-linking.69
Anti-Inflammatory Effect
While the rapid spread of COVID-19 is concerning, the inflammatory response of the host is an important determinant of the outcome and severity of any infection.70 A cytokine storm represents cytokine overproduction, seen in the most severe cases of COVID-19, a process which includes T cell depletion, pulmonary disease and damage to the lungs.71 Granulocytosis can also lead to strong superoxide explosion,72 the formation of reactive oxygen species (ROS)73 and further production of proinflammatory cytokines.74 The background of anti-inflammatory therapy complementing antiviral therapy must thus be understood in order to manage such symptoms in COVID-19, as treatment should aim to control inflammation without affecting the host’s ability to respond adaptively to the virus. The nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2) can resist oxidative stress,75 and this is always dysregulated in disease states, such as diabetes, liver disease, and inflammatory bowel diseases,76 as well as in severe aging.77 Any such conditions are thus risk factors for COVID-19-induced ARDS.78
Activation of Nrf2 has also been shown to be involved in preserving lung architecture in reactions to inflammatory syndrome, as well as having some therapeutic effects in various lung disorders, including respiratory infections and ARDS.79 Furthermore, Nrf2 is responsible for the transcription of certain macrophage-specific genes involved in the tissue repair that grant protection from viral infections,80 as well as restoring redox homeostasis, which protects against oxidative stress by upregulating thioredoxin reductase, glutathione, peroxiredoxin, and NADPH.81
It has been reported that TQ decreases levels of various proinflammatory mediators, such as IL-1β, IL-6, TNFα, IFNβ, and PGE66 in rats, as well as preventing pulmonary inflammation and improving the resistance of airways to damage induced by diesel exhaust particles. TQ also decreases blood leukocyte and plasma IL-6 levels.82 In a mouse model of allergic asthma, TQ reduced lung eosinophils, increased Th2 cytokines, and decreased mucus-producing goblet cells.46 TQ also inhibits inducible synthase nitric oxide (iNOS) and transforming growth factor-β1 in asthmatic murine experimental models.83–85
The experimental evidence suggests that TQ inhibits cyclooxygenase (COX) and lipoxygenase enzymes, preventing the generation of eicosanoids.86 TQ decreases the synthesis of LTs87 and inhibits prostaglandin and thromboxane synthesis by decreasing COX2 expression, achieved by upregulating IL-1 receptor-associated kinase 1 (IRAK1).88 IRAK1-mediated signal inhibitors also downregulate NF-κB and activator protein 1/AP1 transcriptional activities which are required to activate the COX-2 expression,88 and TQ further downregulates the expression of many other inflammatory cytokines and signals mediators, including interleukin IL-1, IL-6, TNFα, and iNOS.88 These mediators can cause alveolar macrophages and neutrophils to create more damage by increasing pulmonary vascular permeability, releasing oxygen radicals and proteolytic enzymes.89 The anti-oxidant activity of TQ can also help in minimizing cell inflammation, while its ROS generation plays an important role in the synthesis of arachidonic acid based on the activation and/or expression of the basic upstream signaling molecules protein kinase B and NF-κB.90
Immunomodulatory Effect
TQ has several major immunomodulatory effects due to the crosslink between inflammatory and immunomodulatory pathways. TQ could thus potentially suppress inflammation-induced immunosuppression based on its negative effects on proinflammatory eicosanoid synthesis and mediated gene expression in NF-κB.91 TQ can thus modulate many aspects of cellular and humoral immunity by inhibiting the function and expression of various inflammatory cytokines and their effector molecules.92 TQ modulates cell immune responses, including dendritic cell maturity, NK-cells cytotoxicity, phagocytic involvement, chemotaxis, and the activation of T-cells. It also tends to have a context-relating effect on particular cell immune responses: for example, TQ prevents the maturation of lipopolysaccharide-induced dendritic cells by blunting the expression of IL-10, IL-12 and TNFα with enhancement of caspase 3/8 and increasing annexin V binding.93 TQ also improves the survival of CD8 antigen-specific T cells and improves the sustained expression of L-selectin, which may have an important effect on adoptive T cell therapy.94
Epigenetic Modulatory Effect
Various epigenetic pathways are involved in COVID-19 infection, and these pathways may thus be therapeutically utilized.95 Possible targets for host immune response include epigenetic enzymes.96,97 The aberrant genetic expression and protein function that characterize COVID-19 are caused by genetic and epigenetic changes, and natural compounds can target and regulate genetic expression, directly or indirectly, based on their interference with genetic and epigenetic mechanisms.98–100 TQ is thus a promising molecule because it modulates epigenetic properties such as histone acetylation and deacetylation as well as DNA methylation and demethylation.101,102 In addition, TQ plays a role in activating and deactivating noncoding RNA, acting as a potent apoptosis-induced enzyme that causes histone acetylation and deacetylation.103–105
Endogenous miRNA activity has been studied in the field of viral replication for several complex virus mechanisms.106 It has thus been shown that miR34a has an effect on the inactivation of epithelial-mesenchymal transition-transcription factors (EMT-TFs), and epithelial–mesenchymal transition is known to play a crucial role in organ fibrosis and epithelial cell malignancy.107 A promising therapeutic approach against COVID-19 thus stems from the idea of inactivating EMT-TFs using miR34a,108 as a previous study showed that TQ may act as an enhancer of miR34a activity.109 miR146a is another miR involved in the process of inflammatory cytokine inhibition, which acts via the NF-κB pathway.110 It functions as a negative regulator for NF-κB, and it is a well-recognized transcript factor for the IL-6 gene.111 miR-146a-5p transcription is also regulated by NF-κB,112 and patients with COVID-19 have been shown to have higher levels of IL-6 and lower levels of miR-146a-5p than average, suggesting imbalances in the physiological axis of IL-6/miR-146a-5p in the pathogenesis of COVID-19 infections.113 TQ treatment, however, controls miR146a expression and can therefore reduce inflammatory reactions by interfering with NF-kB.114
Antiviral Activity
Several studies support the potential antiviral activity of TQ against various viral infections, which is mainly attributed to its multiple beneficial effects, such as antioxidant, anti-inflammatory, and immunomodulatory effects in addition to possible direct viral eradication.115,116 The antiviral effect of Nigella sativa oil, including its major active component TQ, was demonstrated in a murine cytomegalovirus (MCMV) model; this showed that Nigella sativa significantly reduced the liver and spleen viral loads, which coincided with enhanced IFN-γ production and increased CD4 (+) T cell response.44 TQ has also been shown to significantly inhibit Epstein-Barr virus (EBV) replication in EBV-infected B cells,117 while Nigella sativa has been shown to exhibit antiviral activity against the hepatitis C virus (HCV), as evidenced by reduced viral load and improved liver function in HCV patients who received Nigella sativa at 450 mg, three times a day for three successive months.51 This effect is also supported by observations of the selective inhibition of HCV virus replication by alpha-zam, a Nigella sativa seed formulation.118 Nigella sativa has also been suggested to be effective in controlling human immunodeficiency virus (HIV) infection, with one study reporting that treatment of HIV patients with Nigella sativa for six months resulted in sustained sero-reversion with a significant reduction in viral load and CD4 count elevation.52
Nigella sativa extract containing TQ has also, more specifically, been reported to decrease viral replication and loads in cells infected with some coronaviruses.119 Interestingly, one in vitro study demonstrated that TQ showed significant antiviral activity against a SARSCoV-2 strain isolated from Egyptian patients,120 possibly through blocking the entry of the virus into the cells.121 Overall, the existing studies highlight the immense potential of TQ as an effective antiviral agent against COVID-19, a premise which is highly supported by the molecular docking studies examining TQ’s effects against various virus and host cell targets, which are discussed in more detail in the following section.
Molecular Docking Studies Related to Anti-COVID-19 Activity
Molecular docking is a promising in silico method that may be used to screen various compounds for their antiviral potential by testing the binding affinities of the compounds against different viral or host cell receptor proteins. The molecular targets of SARS-CoV-2 include various viral proteins involved in viral entry, such as spike proteins, and replication, such as viral proteases.122 In addition, host cell targets, such as angiotensin-converting enzyme 2 (ACE2) receptor and cell surface heat shock protein (HSPA5), which are involved in viral entry, may also offer potential therapeutic targets.122 Molecular docking studies have already shown that TQ could potentially inhibit COVID-19 by binding to the receptor-binding domain on the spike protein of SARS-CoV-2, which would hinder virus entry into the host cell.123 Additionally, it may bind to the SARS-CoV-2 envelope protein and inhibit its ion channel and pore formation activity.124 Other studies have shown that TQ might display inhibitory action against the SARS CoV2 protease, which would halt viral replication.120,125–127
TQ has also demonstrated a good affinity against ACE2 receptors, which allows it to interfere with virus uptake into the host cell.121,127 Molecular dynamics simulations have shown that TQ can interfere with the attachment of SARS‐CoV‐2 to host cells by binding to a cell surface, HSPA5, which is recognized by the viral spike protein and upregulated upon viral infection.128,129 These in silico studies indicate a multi-targeted potential for TQ against COVID-19, and thus pave the way for further investigation of such anti-COVID-19 potential through in-vitro and in-vivo studies that may better support translation into clinical practice.
Antibacterial Activity
COVID-19 may also be associated with serious secondary bacterial infections, such as bacterial pneumonia, as well as nosocomial infections resulting from the prolonged hospitalization of critically ill patients, both of which significantly increase morbidity and mortality in COVID-19 patients.130 Moreover, the intensive use of antibiotics in patients suffering from COVID-19 could result in the emergence of multidrug-resistant bacteria, which could further worsen COVID-19 adverse outcomes.131 Interestingly, TQ exerts antibacterial activity against several Gram positive and Gram negative bacteria, including Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli, which could be used to augment antibiotic effects.41,116,132 Furthermore, TQ has demonstrated significant antimicrobial activity against anaerobic bacteria, specifically Clostridium difficile,133 as well as clinical isolates of Mycobacterium tuberculosis,134 alongside antibacterial and resistance modifying activities with regard to methicillin-resistant Staphylococcus aureus (MRSA)135 and Listeria monocytogenes.136
Nigella sativa was also seen to be significantly effective in eradicating Helicobacter pylori in patients with non-ulcer dyspepsia.137 This suggests that TQ could play a significant role in the prevention and management of secondary bacterial infections in COVID-19 patients in addition to its potential value for modifying bacterial resistance and potentiating antibiotic actions.
Anticoagulation Effect
Thrombotic complications have become a major problem in COVID-19 patients. Preliminary COVID-19 studies have shown that infected patients typically develop thrombocytopenia with higher D-dimer levels, while the rates of developing thrombocytopenia in patients with severe COVID-19 are even higher.70 Viral infections often cause systemic inflammatory responses and interfere with the balance of procoagulants and anticoagulants,138 and in severe or critically ill patients, large quantities of inflammatory mediators, hormones and immunoglobulin are released, leading to blood hypercoagulability. The level of interleukins, especially IL-6, IL-7, IL-2, granulocyte colony-=stimulating factor, monocyte chemoattractant protein-1, macrophage inflammatory proteins 1-alpha, and TNFα, has been similarly found to be increased in patients with COVID-19.139
An earlier study found that coagulation factors VII, VIII, II, V, and X were significantly increased in COVID-19 patients.140 TQ, however, interferes with blood clotting by directly decreasing factor Xa activity in the blood coagulation pathway and by down-regulating TNFα, a cytokine that plays a critical role in the link between inflammatory and thrombosis pathways.47
The Effect of Thymoquinone on Comorbidities
The magnitude of COVID-19 infection is increased by a variety of comorbidities. TQ may thus also be helpful in patients infected with COVID-19 where it can relieve some comorbidity.13 Serious COVID-19 complications include ARDS, pneumonia and multi-organ failure, and the risk of all of these is increased in patients with diabetes and cardiovascular diseases.141,142 N. Sativa has been shown to reduce plasma glucose levels and control haemoglobin-A1c,143 while intraperitoneal administration of TQ has been demonstrated to substantially decrease hyperglycemia in streptozotocin-induced diabetes in the rats.144 One study reported that 7% of deaths in COVID-19 patients can be ascribed to circulatory failure in myocarditis, suggesting that cardiovascular disorders play an important role in determining final adverse outcomes.145 TQ can also act centrally as an antihypertensive agent, as well as having a regulatory effect on platelet aggregation and blood clotting,146,147 and TQ protects the heart from injury induced by isoproterenol in rats.148
It is also notable that autoimmune and auto-inflammatory diseases, especially in children, may impact the severity of COVID-19 infection, with overlapping symptoms leading to pediatric inflammatory multisystem syndrome (PIMS) that includes Kawasaki-like diseases.149,150 This complex syndrome has been reported as “Kawa-COVID-19” because of the association with the symptoms of COVID-19 infection.151,152 In patients with Kawa-COVID-19, C-Reactive protein (CRP), IL-6, IL-8, and TNF-α were all significantly raised,153 suggesting that Nigella sativa could play a beneficial role in controlling incidence of PIMS or Kawa-COVID-19 by regulating and modulating immune response and reducing the occurrence of proinflammatory cytokines IL-2, IL-4, IL-5, II-6, IL-12, and IL-13.154
Dual Benefit of Thymoquinone as Adjunctive Therapy
TQ can be used in combination with other therapeutic agents that may be usefully repurposed for the treatment of COVID-19, as well as alongside other supportive treatments. Given the multiple beneficial effects of TQ and its favorable safety profile,155 the adjunct use of TQ with conventional therapeutic agents would have the dual benefit of attenuating drug-induced toxicity and improving therapeutic effectiveness, which could in turn result in reducing the required effective dosage of concomitantly used drugs, thus further minimizing any adverse effects. The potential cardioprotective,156 neuroprotective,157 hepatoprotective,158 nephroprotective,159 and gastroprotective160 effects of TQ may thus be employed in counteracting a range of drug-associated toxicities;161 currently, various supportive treatments such as acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs) render COVID-19 patients at increased risk of liver and kidney toxicity.162,163
TQ has been shown to counteract acetaminophen-induced hepatotoxicity164,165 as well as NSAIDs-associated nephrotoxicity and gastrointestinal side effects.166 TQ can also act synergistically with corticosteroids to protect the lungs by mitigating the inflammatory response and resulting cytokine storm; this would allow the use of lower steroid doses, thus reducing the risk of potential adverse effects.167,168 TQ has further demonstrated significant protective effects against the renal toxicity associated with antibiotics, such as vancomycin used in COVID-19 patients with secondary bacterial respiratory infections.169 TQ could also potentially counteract the toxic effects of various repurposed drugs,170,171 such as the cardiotoxicity risk associated with chloroquine and azithromycin161,172,173 and the potential liver and kidney toxicities associated with antivirals such as remdesivir and lopinavir.155,161,170 TQ can also exert gastroprotective effects160 against gastric ulceration, which is associated with the IL-6 antagonist, tocilizumab,174 in addition to potentiating its anti-inflammatory effect.175
Clinical Applicability of Thymoquinone
The high hydrophobic and lipophilic characters of TQ lead to poor solubility, low bioavailability, and difficulty in formulation.176 The various pharmacokinetics of TQ have been reported in detail,177–179 and TQ has poor oral bioavailability based on its low aqueous solubility and dissolution rate.180 Moreover, TQ shows rapid polyexponential decline following intravenous dosing,178 as well as binding with bovine serum albumin and alpha-1 acid glycoprotein.181–183 This poor solubility and limited bioavailability are the two main problems for developing TQ for clinical use, and several chemical derivatives and novel nanoformulations have thus been developed to improve the pharmacokinetic behaviors of TQ to increase bioavailability.184,185 TQ has, for example, been successfully encapsulated into nanolipid carriers.186–188
TQ in different dose ranges shows beneficial effects with negligible toxicity in animal models of different diseases.156–159,189–195 TQ is a well-tolerated drug in rodents, and numerous studies have been done to determine the toxicological properties of TQ in vitro and in vivo.196–198 Even mice treated with 0.03% TQ in their drinking water for three months showed no signs of toxicity.196 Moreover, TQ has demonstrated a high safety profile in rats based on high doses using oral and intraperitoneal administration.199,200
TQ compounds are currently used in clinical trials for the treatment of various types of cancer and other diseases.201,202 In a Phase I safety and clinical activity study of TQ in patients with advanced refractory malignant disease, TQ was well tolerated at doses ranging from 75 mg/day to 2600 mg/day, with neither toxicities nor therapeutic responses reported.203 This absence of side effects in humans is in agreement with the extremely low toxicity of oral TQ administration in experimental animals.196
Prospects and Limitations
Despite the numerous molecular docking studies on potential anti-COVID-19 activity of TQ, experimental studies on the effects of TQ against COVID-19 and its associated complications remain limited. The multi-targeted beneficial effects of TQ and its favorable safety profile do, however, appear to warrant in-vivo investigations and clinical trials on its anti-COVID-19 potential to support the translation into clinical practice to treat COVID-19 patients either alone or in combination with other potential therapies. TQ could also provide the additional benefits of ameliorating comorbidities and attenuating certain drug-induced adverse effects, as well as improving the therapeutic effectiveness of some other therapies. Novel formulations of TQ nanoparticles may, however, be required to overcome the poor bioavailability and the pharmacokinetic limitation of this compound in terms of clinical use.
Conclusion
This article examined the concept that certain natural compounds may target the molecular mechanisms of COVID-19, as well as potentially assisting with overcoming the diverse health complications associated with the repeated use or withdrawal of conventional therapeutics. TQ, the main active ingredient of Black seed oil, is an easy, cost-effective natural source of anti-inflammatory, antioxidant, immune stimulant, antibacterial, anticoagulant, and antiviral properties. TQ use may thus be expected to improve COVID-19 comorbidities and to protect against certain antiviral drug-induced side effects and toxicities. TQ appears to be a promising therapeutic option for managing COVID-19 and its complications, and clinical trials in COVID-19 patients to examine the beneficial effects of TQ are thus highly recommended.
Abbreviations
ACE, angiotensin-converting enzyme; ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease-2019; COX, cyclooxygenase; EBV, Epstein-Barr virus; EMT-TFs, epithelial–mesenchymal transition–transcription factors; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HSP, heat shock protein; IL, interleukin; IRAK1, interleukin-1 receptor-associated kinase 1; MCMV, murine cytomegalovirus; Nrf2, the nuclear factor erythroid 2 (NFE2)-related factor 2; ROS, reactive oxygen species; SARS-CoV-2, severe acute respiratory syndrome; TQ, thymoquinone.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang X, Fang X, Cai Z, et al. Comorbid chronic diseases and acute organ injuries are strongly correlated with disease severity and mortality among COVID-19 patients: a systemic review and meta-analysis. Research (Wash D C). 2020;2020:2402961. doi:10.34133/2020/2402961
2. Wu D, Wu T, Liu Q, Yang Z. The SARS-CoV-2 outbreak: what we know. Int J Infect Dis. 2020;94:44–48. doi:10.1016/j.ijid.2020.03.004
3. Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. 2020;80(6):e14–e18. doi:10.1016/j.jinf.2020.03.005
4. Felsenstein S, Herbert JA, McNamara PS, Hedrich CM. COVID-19: immunology and treatment options. Clin Immunol. 2020;215:108448. doi:10.1016/j.clim.2020.108448
5. Zhang T, He Y, Xu W, Ma A, Yang Y, Xu KF. Clinical trials for the treatment of Coronavirus disease 2019 (COVID-19): a rapid response to urgent need. Sci China Life Sci. 2020;63(5):774–776. doi:10.1007/s11427-020-1660-2
6. Manhas S, Anjali A, Mansoor S, et al. Covid-19 pandemic and current medical interventions. Arch Med Res. 2020;51(6):473–481. doi:10.1016/j.arcmed.2020.05.007
7. Horie S, Gonzalez HE, Laffey JG, Masterson CH. Cell therapy in acute respiratory distress syndrome. J Thorac Dis. 2018;10(9):5607–5620. doi:10.21037/jtd.2018.08.28
8. Zumla A, Hui DS, Azhar EI, Memish ZA, Maeurer M. Reducing mortality from 2019-nCoV: host-directed therapies should be an option. Lancet (London, England). 2020;395(10224):e35–e36. doi:10.1016/s0140-6736(20)30305-6
9. Forni G, Mantovani A, Forni G, et al. COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differ. 2021;28(2):626–639. doi:10.1038/s41418-020-00720-9
10. Ahmed N, Araf Y, Ullah M. Potential roles of vitamin D in the treatment of COVID-19 patient and improving maternal and child health during pandemic. J Adv Biotechnol Exp Ther. 2021;4:133. doi:10.5455/jabet.2021.d114
11. Farjana M, Moni A, Sohag AAM, et al. Repositioning vitamin C as a promising option to alleviate complications associated with COVID-19. Infect Chemother. 2020;52(4):461–477. doi:10.3947/ic.2020.52.4.461
12. Hossain KS, Hossain MG, Moni A, et al. Prospects of honey in fighting against COVID-19: pharmacological insights and therapeutic promises. Heliyon. 2020;6(12):e05798. doi:10.1016/j.heliyon.2020.e05798
13. Islam MN, Hossain KS, Sarker PP, et al. Revisiting pharmacological potentials of Nigella sativa seed: a promising option for COVID-19 prevention and cure. Phytother Res. 2021. doi:10.1002/ptr.6895
14. Yang Y, Islam MS, Wang J, Li Y, Chen X. Traditional Chinese Medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int J Biol Sci. 2020;16(10):1708–1717. doi:10.7150/ijbs.45538
15. Chan KW, Wong VT, Tang SCW. COVID-19: an update on the epidemiological, clinical, preventive and therapeutic evidence and guidelines of integrative Chinese-Western Medicine for the Management of 2019 Novel Coronavirus Disease. Am J Chin Med. 2020;48(3):737–762. doi:10.1142/s0192415x20500378
16. Khanna K, Kohli SK, Kaur R, et al. Herbal immune-boosters: substantial warriors of pandemic Covid-19 battle. Phytomedicine. 2020:153361. doi:10.1016/j.phymed.2020.153361
17. Ang L, Song E, Lee HW, Lee MS. Herbal medicine for the treatment of Coronavirus Disease 2019 (COVID-19): a Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Clin Med. 2020;9(5):1583. doi:10.3390/jcm9051583
18. Patel B, Sharma S, Nair N, Majeed J, Goyal RK, Dhobi M. Therapeutic opportunities of edible antiviral plants for COVID-19. Mol Cell Biochem. 2021. doi:10.1007/s11010-021-04084-7
19. Kang S, Min H. Ginseng, the ‘Immunity Boost’: the effects of panax ginseng on immune system. J Ginseng Res. 2012;36(4):354–368. doi:10.5142/jgr.2012.36.4.354
20. Shahrajabian MH, Sun W, Cheng Q. The power of natural Chinese medicine, ginger and ginseng root in an organic life. Middle East J Sci Res. 2019;27:64–71.
21. Daliri EB-M, Kim S-H, Park B-J, et al. Effects of different processing methods on the antioxidant and immune stimulating abilities of garlic. Food Sci Nutr. 2019;7(4):1222–1229. doi:10.1002/fsn3.942
22. Sharifi-Rad M, Mnayer D, Morais-Braga MFB, et al. Echinacea plants as antioxidant and antibacterial agents: from traditional medicine to biotechnological applications. Phytother Res. 2018;32(9):1653–1663. doi:10.1002/ptr.6101
23. Cheng PW, Ng LT, Chiang LC, Lin CC. Antiviral effects of saikosaponins on human coronavirus 229E in vitro. Clin Exp Pharmacol Physiol. 2006;33(7):612–616. doi:10.1111/j.1440-1681.2006.04415.x
24. Li SY, Chen C, Zhang HQ, et al. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res. 2005;67(1):18–23. doi:10.1016/j.antiviral.2005.02.007
25. Lin C-W, Tsai F-J, Tsai C-H, et al. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antiviral Res. 2005;68(1):36–42. doi:10.1016/j.antiviral.2005.07.002
26. Ryu YB, Jeong HJ, Kim JH, et al. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CL(pro) inhibition. Bioorg Med Chem. 2010;18(22):7940–7947. doi:10.1016/j.bmc.2010.09.035
27. Yu MS, Lee J, Lee JM, et al. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorg Med Chem Lett. 2012;22(12):4049–4054. doi:10.1016/j.bmcl.2012.04.081
28. Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet (London, England). 2003;361:2045–2046. doi:10.1016/S0140-6736(03)13615-X
29. Rahman MT. Potential benefits of combination of Nigella sativa and Zn supplements to treat COVID-19. J Herb Med. 2020;23:100382. doi:10.1016/j.hermed.2020.100382
30. Maideen NMP. Prophetic medicine-Nigella Sativa (Black cumin seeds)-potential herb for COVID-19? J Pharmacopunct. 2020;23(2):62–70. doi:10.3831/kpi.2020.23.010
31. Kulyar MF-EA, Li R, Mehmood K, Waqas M, Li K, Li J. Potential influence of Nigella sativa (Black cumin) in reinforcing immune system: a hope to decelerate the COVID-19 pandemic. Phytomedicine. 2020;153277. doi:10.1016/j.phymed.2020.153277
32. Bakhtiar L, Gruner OC, Shah MH, Crook JR, Nasr SH. Avicenna. In: The Canon of Medicine (Al-Qanunfi’l-Tibb); 1999: Great Books of the Islamic World, Chicago, IL : KAZI Publications 1999-2014.
33. Ghahramanloo KH, Kamalidehghan B, Akbari Javar H, Teguh Widodo R, Majidzadeh K, Noordin MI. Comparative analysis of essential oil composition of Iranian and Indian Nigella sativa L. extracted using supercritical fluid extraction and solvent extraction. Drug Des Devel Ther. 2017;11:2221–2226. doi:10.2147/dddt.s87251
34. Haseena S, Aithal M, Das K, Saheb S. Phytochemical analysis of Nigella sativa and its effect on reproductive system. J Pharm Sci Res. 2015;7:514–517.
35. Tavakkoli A, Ahmadi A, Razavi BM, Hosseinzadeh H. Black seed (Nigella Sativa) and its constituent thymoquinone as an antidote or a protective agent against natural or chemical toxicities. Iran J Pharm Res. 2017;16(Suppl):2–23.
36. Ahmad A, Husain A, Mujeeb M, et al. A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac J Trop Biomed. 2013;3(5):337–352. doi:10.1016/S2221-1691(13)60075-1
37. El–Dakhakhny M. Studies on the Chemical Constitution of Egyptian Nigella Sativa L. Seeds. II1) the essential oil. Planta Med. 1963;11(04):465–470. doi:10.1055/s-0028-1100266
38. Hosseinzadeh H, Taiari S, Nassiri-Asl M. Effect of thymoquinone, a constituent of Nigella sativa L., on ischemia–reperfusion in rat skeletal muscle. Naunyn-Schmiedeberg Arch Pharmcol. 2012;385(5):503–508. doi:10.1007/s00210-012-0726-2
39. El Gazzar M, El Mezayen R, Marecki JC, Nicolls MR, Canastar A, Dreskin SC. Anti-inflammatory effect of thymoquinone in a mouse model of allergic lung inflammation. Int Immunopharmacol. 2006;6(7):1135–1142. doi:10.1016/j.intimp.2006.02.004
40. Gali-Muhtasib H, Ocker M, Kuester D, et al. Thymoquinone reduces mouse colon tumor cell invasion and inhibits tumor growth in murine colon cancer models. J Cell Mol Med. 2008;12(1):330–342. doi:10.1111/j.1582-4934.2007.00095.x
41. Halawani E. Antibacterial activity of thymoquinone and thymohydroquinone of Nigella sativa L. and their interaction with some antibiotics. Adv Biol Res. 2009;3:148–152.
42. Abdel Azeiz A, Darweesh M, Amin A. American science efficacy of thymoquinone against vaginal candidiasis in prednisolone-induced immunosuppressed mice. J Am Sci. 2013;9:155.
43. Hosseinzadeh H, Parvardeh S. Anticonvulsant effects of thymoquinone, the major constituent of Nigella sativa seeds, in mice. Phytomedicine. 2004;11(1):56–64. doi:10.1078/0944-7113-00376
44. Salem ML, Hossain MS. Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection. Int J Immunopharmacol. 2000;22(9):729–740. doi:10.1016/s0192-0561(00)00036-9
45. Mahboubi M. Natural therapeutic approach of Nigella sativa (Black seed) fixed oil in management of Sinusitis. Integr Med Res. 2018;7(1):27–32. doi:10.1016/j.imr.2018.01.005
46. El Gazzar M, El Mezayen R, Nicolls MR, Marecki JC, Dreskin SC. Downregulation of leukotriene biosynthesis by thymoquinone attenuates airway inflammation in a mouse model of allergic asthma. Biochim Biophys Acta. 2006;1760(7):1088–1095. doi:10.1016/j.bbagen.2006.03.006
47. Muralidharan-Chari V, Kim J, Abuawad A, Naeem M, Cui H, Mousa SA. Thymoquinone modulates blood coagulation in vitro via its effects on inflammatory and coagulation pathways. Int J Mol Sci. 2016;17(4):474. doi:10.3390/ijms17040474
48. Alkharfy KM, Ahmad A, Jan BL, Raish M. Thymoquinone reduces mortality and suppresses early acute inflammatory markers of sepsis in a mouse model. Biomed Pharmacother. 2018;98:801–805. doi:10.1016/j.biopha.2018.01.028
49. Haq A, Abdullatif M, Lobo PI, Khabar KS, Sheth KV, al-Sedairy ST. Nigella sativa: effect on human lymphocytes and polymorphonuclear leukocyte phagocytic activity. Immunopharmacology. 1995;30(2):147–155. doi:10.1016/0162-3109(95)00016-m
50. Haq A, Lobo PI, Al-Tufail M, Rama NR, Al-Sedairy ST. Immunomodulatory effect of Nigella sativa proteins fractionated by ion exchange chromatography. Int J Immunopharmacol. 1999;21(4):283–295. doi:10.1016/s0192-0561(99)00010-7
51. Barakat EM, El Wakeel LM, Hagag RS. Effects of Nigella sativa on outcome of hepatitis C in Egypt. World J Gastroenterol. 2013;19(16):2529–2536. doi:10.3748/wjg.v19.i16.2529
52. Onifade AA, Jewell AP, Adedeji WA. Nigella sativa concoction induced sustained seroreversion in HIV patient. Afr J Tradit Complement Altern Med. 2013;10(5):332–335.
53. Oskouei Z, Akaberi M, Hosseinzadeh H. A glance at black cumin (Nigella sativa) and its active constituent, thymoquinone, in ischemia: a review. Iran J Basic Med Sci. 2018;21(12):1200–1209. doi:10.22038/ijbms.2018.31703.7630
54. Ahmad A, Rehman M, Ahmad P, Alkharfy K. Covid-19 and thymoquinone: connecting the dots. Phytother Res. 2020;34(11):2786–2789. doi:10.1002/ptr.6793
55. Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24(5):981–990. doi:10.1016/j.cellsig.2012.01.008
56. Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9–19. doi:10.1097/WOX.0b013e3182439613
57. Betteridge DJ. What is oxidative stress? Metabolism. 2000;49(2Suppl 1):3–8. doi:10.1016/s0026-0495(00)80077-3
58. Hagen TM. Oxidative stress, redox imbalance, and the aging process. Antioxid Redox Signal. 2003;5(5):503–506. doi:10.1089/152308603770310149
59. Wright E
60. Di Virgilio F. New pathways for reactive oxygen species generation in inflammation and potential novel pharmacological targets. Curr Pharm Des. 2004;10(14):1647–1652. doi:10.2174/1381612043384727
61. Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL. Oxidative stress in atherosclerosis. Curr Atheroscler Rep. 2017;19(11):42. doi:10.1007/s11883-017-0678-6
62. Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi:10.1186/1477-3163-5-14
63. Romano AD, Serviddio G, de Matthaeis A, Bellanti F, Vendemiale G. Oxidative stress and aging. J Nephrol. 2010;23(Suppl 15):S29–36.
64. Mahmoud YK, Abdelrazek HMA. Cancer: thymoquinone antioxidant/pro-oxidant effect as potential anticancer remedy. Biomed Pharmacother. 2019;115:108783. doi:10.1016/j.biopha.2019.108783
65. Leisegang K, Almaghrawi W, Henkel R. The effect of Nigella sativa oil and metformin on male seminal parameters and testosterone in Wistar rats exposed to an obesogenic diet. Biomed Pharmacother. 2021;133:111085. doi:10.1016/j.biopha.2020.111085
66. Umar S, Zargan J, Umar K, Ahmad S, Katiyar CK, Khan HA. Modulation of the oxidative stress and inflammatory cytokine response by thymoquinone in the collagen induced arthritis in Wistar rats. Chem Biol Interact. 2012;197(1):40–46. doi:10.1016/j.cbi.2012.03.003
67. Kassab RB, El-Hennamy RE. The role of thymoquinone as a potent antioxidant in ameliorating the neurotoxic effect of sodium arsenate in female rat. Egypt J Basic Appl Sci. 2017;4(3):160–167. doi:10.1016/j.ejbas.2017.07.002
68. Badary OA, Taha RA, Gamal el-Din AM, Abdel-Wahab MH. Thymoquinone is a potent superoxide anion scavenger. Drug Chem Toxicol. 2003;26(2):87–98. doi:10.1081/dct-120020404
69. Khan MA, Anwar S, Aljarbou AN, et al. Protective effect of thymoquinone on glucose or methylglyoxal-induced glycation of superoxide dismutase. Int J Biol Macromol. 2014;65:16–20. doi:10.1016/j.ijbiomac.2014.01.001
70. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi:10.1056/NEJMoa2002032
71. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
72. Galani IE, Andreakos E. Neutrophils in viral infections: current concepts and caveats. J Leukoc Biol. 2015;98(4):557–564. doi:10.1189/jlb.4VMR1114-555R
73. Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21(7):363–383. doi:10.1038/s41580-020-0230-3
74. DeDiego ML, Nieto-Torres JL, Regla-Nava JA, et al. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J Virol. 2014;88(2):913–924. doi:10.1128/jvi.02576-13
75. Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi:10.1146/annurev.pharmtox.46.120604.141046
76. Rabbani PS, Soares MA, Hameedi SG, et al. Dysregulation of Nrf2/Keap1 redox pathway in diabetes affects multipotency of stromal cells. Diabetes. 2019;68(1):141–155. doi:10.2337/db18-0232
77. Schmidlin CJ, Dodson MB, Madhavan L, Zhang DD. Redox regulation by NRF2 in aging and disease. Free Radic Biol Med. 2019;134:702–707. doi:10.1016/j.freeradbiomed.2019.01.016
78. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus Disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi:10.1001/jamainternmed.2020.0994
79. Liu Q, Gao Y, Ci X. Role of Nrf2 and its activators in respiratory diseases. Oxid Med Cell Longev. 2019;2019:7090534. doi:10.1155/2019/7090534
80. Saddawi-Konefka R, Seelige R, Gross ET, et al. Nrf2 induces IL-17D to mediate tumor and virus surveillance. Cell Rep. 2016;16(9):2348–2358. doi:10.1016/j.celrep.2016.07.075
81. Brüne B, Dehne N, Grossmann N, et al. Redox control of inflammation in macrophages. Antioxid Redox Signal. 2013;19(6):595–637. doi:10.1089/ars.2012.4785
82. Nemmar A, Al-Salam S, Zia S, et al. Contrasting actions of diesel exhaust particles on the pulmonary and cardiovascular systems and the effects of thymoquinone. Br J Pharmacol. 2011;164(7):1871–1882. doi:10.1111/j.1476-5381.2011.01442.x
83. Ammar El SM, Gameil NM, Shawky NM, Nader MA. Comparative evaluation of anti-inflammatory properties of thymoquinone and curcumin using an asthmatic murine model. Int Immunopharmacol. 2011;11(12):2232–2236. doi:10.1016/j.intimp.2011.10.013
84. Berger P, Girodet P-O, Manuel Tunon-de-lara J. Mast cell myositis: a new feature of allergic asthma? Allergy. 2005;60(10):1238–1240. doi:10.1111/j.1398-9995.2005.00898.x
85. Alkharfy K. Nitric oxide pathway as a potential therapeutic target in COVID-19. FARMACIA. 2020;68:966–969. doi:10.31925/farmacia.2020.6.2
86. Houghton PJ, Zarka R, de Las Heras B, Hoult JR. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995;61(1):33–36. doi:10.1055/s-2006-957994
87. Mansour M, Tornhamre S. Inhibition of 5-lipoxygenase and leukotriene C4 synthase in human blood cells by thymoquinone. J Enzyme Inhib Med Chem. 2004;19(5):431–436. doi:10.1080/14756360400002072
88. Hossen MJ, Yang WS, Kim D, Aravinthan A, Kim J-H, Cho JY. Thymoquinone: an IRAK1 inhibitor with in vivo and in vitro anti-inflammatory activities. Sci Rep. 2017;7(1):42995. doi:10.1038/srep42995
89. Sharma AK, Fernandez LG, Awad AS, Kron IL, Laubach VE. Proinflammatory response of alveolar epithelial cells is enhanced by alveolar macrophage-produced TNF-alpha during pulmonary ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2007;293(1):L105–13. doi:10.1152/ajplung.00470.2006
90. Korbecki J, Baranowska-Bosiacka I, Gutowska I, Chlubek D. The effect of reactive oxygen species on the synthesis of prostanoids from arachidonic acid. J Physiol Pharmacol. 2013;64(4):409–421.
91. Shaterzadeh-Yazdi H, Noorbakhsh MF, Hayati F, Samarghandian S, Farkhondeh T. Immunomodulatory and anti-inflammatory effects of thymoquinone. Cardiovasc Hematol Disord Drug Targets. 2018;18(1):52–60. doi:10.2174/1871529x18666180212114816
92. Majdalawieh AF, Fayyad MW. Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: a comprehensive review. Int Immunopharmacol. 2015;28(1):295–304. doi:10.1016/j.intimp.2015.06.023
93. Xuan NT, Shumilina E, Qadri SM, Götz F, Lang F. Effect of thymoquinone on mouse dendritic cells. Cel Physiol Biochem. 2010;25(2–3):307–314. doi:10.1159/000276563
94. Mohany M, El-Feki M, Refaat I, Garraud O, Badr G. Thymoquinone ameliorates the immunological and histological changes induced by exposure to imidacloprid insecticide. J Toxicol Sci. 2012;37(1):1–11. doi:10.2131/jts.37.1
95. El Baba R, Herbein G. Management of epigenomic networks entailed in coronavirus infections and COVID-19. Clin Epigenetics. 2020;12(1):118. doi:10.1186/s13148-020-00912-7
96. Chlamydas S, Papavassiliou AG. Epigenetic mechanisms regulating COVID-19 infection. Epigenetics. 2020;1–8. doi:10.1080/15592294.2020.1796896
97. Chai P, Yu J, Ge S, Jia R, Fan X. Genetic alteration, RNA expression, and DNA methylation profiling of coronavirus disease 2019 (COVID-19) receptor ACE2 in malignancies: a pan-cancer analysis. J Hematol Oncol. 2020;13(1):43. doi:10.1186/s13045-020-00883-5
98. Stefanska B, Karlic H, Varga F, Fabianowska-Majewska K, Haslberger A. Epigenetic mechanisms in anti-cancer actions of bioactive food components–the implications in cancer prevention. Br J Pharmacol. 2012;167(2):279–297. doi:10.1111/j.1476-5381.2012.02002.x
99. Patra SK, Szyf M. DNA methylation-mediated nucleosome dynamics and oncogenic Ras signaling: insights from FAS, FAS ligand and RASSF1A. FEBS J. 2008;275(21):5217–5235. doi:10.1111/j.1742-4658.2008.06658.x
100. Kar S, Parbin S, Deb M, et al. Epigenetic choreography of stem cells: the DNA demethylation episode of development. Cell Mol Life Sci. 2014;71(6):1017–1032. doi:10.1007/s00018-013-1482-2
101. Paluszczak J, Krajka-Kuźniak V, Baer-Dubowska W. The effect of dietary polyphenols on the epigenetic regulation of gene expression in MCF7 breast cancer cells. Toxicol Lett. 2010;192(2):119–125. doi:10.1016/j.toxlet.2009.10.010
102. Arafa E-SA, Zhu Q, Shah ZI, et al. Thymoquinone up-regulates PTEN expression and induces apoptosis in doxorubicin-resistant human breast cancer cells. Mutat Res. 2011;706(1):28–35. doi:10.1016/j.mrfmmm.2010.10.007
103. Parbin S, Shilpi A, Kar S, et al. Insights into the molecular interactions of thymoquinone with histone deacetylase: evaluation of the therapeutic intervention potential against breast cancer. Article. Mol Biosyst. 2016;12(1):48–58. doi:10.1039/c5mb00412h
104. Lau AW, Liu P, Inuzuka H, Gao D. SIRT1 phosphorylation by AMP-activated protein kinase regulates p53 acetylation. Am J Cancer Res. 2014;4(3):245–255.
105. Nin V, Escande C, Chini CC, et al. Role of deleted in breast cancer 1 (DBC1) protein in SIRT1 deacetylase activation induced by protein kinase A and AMP-activated Protein Kinase*. J Biol Chem. 2012;287(28):23489–23501. doi:10.1074/jbc.M112.365874
106. Bruscella P, Bottini S, Baudesson C, Pawlotsky J-M, Feray C, Trabucchi M. Viruses and miRNAs: more friends than foes. Front Microbiol. 2017;8(824). doi:10.3389/fmicb.2017.00824
107. Sohal SS. Epithelial and endothelial cell plasticity in chronic obstructive pulmonary disease (COPD). Respir Investig. 2017;55(2):104–113. doi:10.1016/j.resinv.2016.11.006
108. Centa A, Fonseca AS, Ferreira S, et al. Deregulated miRNA expression is associated with endothelial dysfunction in post-mortem lung biopsies of COVID-19 patients. Am J Physiol Lung Cell Mol Physiol. 2021;320:L405–L412. doi:10.1152/ajplung.00457.2020
109. Imani S, Wei C, Cheng J, et al. MicroRNA-34a targets epithelial to mesenchymal transition-inducing transcription factors (EMT-TFs) and inhibits breast cancer cell migration and invasion. Oncotarget. 2017;8(13):21362–21379. doi:10.18632/oncotarget.15214
110. Feng Y, Chen L, Luo Q, Wu M, Chen Y, Shi X. Involvement of microRNA-146a in diabetic peripheral neuropathy through the regulation of inflammation. Drug Des Devel Ther. 2018;12:171–177. doi:10.2147/DDDT.S157109
111. Su YL, Wang X, Mann M, et al. Myeloid cell-targeted miR-146a mimic inhibits NF-κB-driven inflammation and leukemia progression in vivo. Blood. 2020;135(3):167–180. doi:10.1182/blood.2019002045
112. Qiong L, Zhen R, Linlin Z, et al. Involvement of microRNA-146a in the inflammatory response of status epilepticus rats. CNS Neurol Disorders Drug Targets. 2017;16(6):686–693. doi:10.2174/1871527316666170505123956
113. Sabbatinelli J, Giuliani A, Matacchione G, et al. Decreased serum levels of the inflammaging marker miR-146a are associated with clinical non-response to tocilizumab in COVID-19 patients. Mech Ageing Dev. 2021;193:111413. doi:10.1016/j.mad.2020.111413
114. Khan MA, Tania M, Fu J. Epigenetic role of thymoquinone: impact on cellular mechanism and cancer therapeutics. Drug Discov Today. 2019;24(12):2315–2322. doi:10.1016/j.drudis.2019.09.007
115. Sommer A, Försterling H-D, Naber K. Thymoquinone: shield and sword against SARS-CoV-2. Precis Nanomed. 2020. doi:10.33218/001c.12984
116. Forouzanfar F, Bazzaz BSF, Hosseinzadeh H. Black cumin (Nigella sativa) and its constituent (thymoquinone): a review on antimicrobial effects. Iran J Basic Med Sci. 2014;17(12):929–938.
117. Zihlif MA, Mahmoud IS, Ghanim MT, et al. Thymoquinone efficiently inhibits the survival of EBV-infected B cells and alters EBV gene expression. Integr Cancer Ther. 2013;12(3):257–263. doi:10.1177/1534735412458827
118. Oyero OG, Toyama M, Mitsuhiro N, et al. Selective inhibition of hepatitis C virus replication by alpha-zam, A Nigella sativa seed formulation. Afr J Tradit Complement Altern Med. 2016;13(6):144–148. doi:10.21010/ajtcam.v13i6.20
119. Ulasli M, Gurses SA, Bayraktar R, et al. The effects of Nigella sativa (Ns), Anthemis hyalina (Ah) and Citrus sinensis (Cs) extracts on the replication of coronavirus and the expression of TRP genes family. Mol Biol Rep. 2014;41(3):1703–1711. doi:10.1007/s11033-014-3019-7
120. Seadawy MG, Gad AF, Elhoseny MF, et al. In vitro: natural compounds (Thymol, Carvacrol, Hesperidine, and Thymoquinone) against Sars-Cov2 strain isolated from Egyptian patients. bioRxiv. 2020. doi:10.1101/2020.11.07.367649
121. Xu H, Liu B, Xiao Z, et al. Computational and experimental studies reveal that thymoquinone blocks the entry of coronaviruses into in vitro cells. Infect Dis Ther. 2021;10(1):483–494. doi:10.1007/s40121-021-00400-2
122. Senger MR, Evangelista TCS, Dantas RF, et al. COVID-19: molecular targets, drug repurposing and new avenues for drug discovery. Mem Inst Oswaldo Cruz. 2020;115. doi:10.1590/0074-02760200254
123. Shaikh YI, Sameerâ shaikh ÂVS, Ahmed K, Nazeruddin GM, Pathan ÂHM. The revelation of various compounds found in Nigella sativa L. (Black Cumin) and their possibility to inhibit COVID-19 infection based on the molecular docking and physical properties. Eng Sci. 2020;11:31–35. doi:10.30919/es8d1127
124. Sultan Mohideen AK. Molecular docking analysis of phytochemical thymoquinone as a therapeutic agent on SARS-Cov-2 envelope protein. Biointerface Res Appl Chem. 2021;11:8389–8401. doi:10.33263/BRIAC111.83898401
125. Bouchentouf S, Noureddine M Identification of compounds from Nigella Sativa as new potential inhibitors of 2019 Novel Coronavirus (Covid-19): Molecular Docking Study; 2020.
126. Youness K, Mohammed M, Houda F. In silico investigation of the SARS CoV2 protease with thymoquinone, the Major Constituent of Nigella Sativa. Curr Drug Discov Technol. 2020;17:1–4. doi:10.2174/1570163817666200712164406
127. Sekiou O, Bouziane I, Bouslama Z, Djemel A In-silico identification of potent inhibitors of COVID-19 Main Protease (Mpro) and Angiotensin Converting Enzyme 2 (ACE2) from natural products: quercetin, hispidulin, and cirsimaritin exhibited better potential inhibition than hydroxy-chloroquine against COVID-19 main protease active site and ACE2; 2020.
128. Elfiky AA. Natural products may interfere with SARS-CoV-2 attachment to the host cell. J Biomol Struct Dyn. 2020;1–10. doi:10.1080/07391102.2020.1761881
129. Ibrahim IM, Abdelmalek DH, Elshahat ME, Elfiky AA. COVID-19 spike-host cell receptor GRP78 binding site prediction. J Infect. 2020;80(5):554–562. doi:10.1016/j.jinf.2020.02.026
130. Hendaus MA, Jomha FA. Covid-19 induced superimposed bacterial infection. J Biomol Struct Dyn. 2020;1–7. doi:10.1080/07391102.2020.1772110
131. Vaillancourt M, Jorth P. The unrecognized threat of secondary bacterial infections with COVID-19. mBio. 2020;11(4):e01806–20. doi:10.1128/mBio.01806-20
132. Chaieb K, Kouidhi B, Jrah H, Mahdouani K, Bakhrouf A. Antibacterial activity of Thymoquinone, an active principle of Nigella sativa and its potency to prevent bacterial biofilm formation. BMC Complement Altern Med. 2011;11:29. doi:10.1186/1472-6882-11-29
133. Randhawa MA, Alenazy AK, Alrowaili MG, Basha J. An active principle of Nigella sativa L., thymoquinone, showing significant antimicrobial activity against anaerobic bacteria. J Intercult Ethnopharmacol. 2017;6(1):97–101. doi:10.5455/jice.20161018021238
134. Randhawa MA. In vitro antituberculous activity of thymoquinone, an active principle of Nigella sativa. J Ayub Med Coll Abbottabad. 2011;23(2):78–81.
135. Hariharan P, Paul-Satyaseela M, Gnanamani A. In vitro profiling of antimethicillin-resistant Staphylococcus aureus activity of thymoquinone against selected type and clinical strains. Lett Appl Microbiol. 2016;62(3):283–289. doi:10.1111/lam.12544
136. Mouwakeh A, Telbisz Á, Spengler G, Mohácsi-Farkas C, Kiskó G. Antibacterial and resistance modifying activities of Nigella sativa essential oil and its active compounds against listeria monocytogenes. In Vivo (Brooklyn). 2018;32(4):737–743. doi:10.21873/invivo.11302
137. Salem EM, Yar T, Bamosa AO, et al. Comparative study of Nigella Sativa and triple therapy in eradication of Helicobacter Pylori in patients with non-ulcer dyspepsia. Saudi J Gastroenterol. 2010;16(3):207–214. doi:10.4103/1319-3767.65201
138. Subramaniam S, Scharrer I. Procoagulant activity during viral infections. Front Biosci. 2018;23:1060–1081. doi:10.2741/4633
139. Aggarwal S, Gollapudi S, Gupta S. Increased TNF-alpha-induced apoptosis in lymphocytes from aged humans: changes in TNF-alpha receptor expression and activation of caspases. J Immunol. 1999;162(4):2154–2161.
140. Masi P, Hékimian G, Lejeune M, et al. Systemic inflammatory response syndrome is a major contributor to COVID-19-associated coagulopathy: insights from a Prospective, Single-Center Cohort Study. Circulation. 2020;142(6):611–614. doi:10.1161/circulationaha.120.048925
141. Guo LP, Liu SX, Yang Q. Effect of thymoquinone on acute kidney injury induced by sepsis in BALB/c mice. Biomed Res Int. 2020;2020:1594726. doi:10.1155/2020/1594726
142. Gupta R, Ghosh A, Singh AK, Misra A. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes MetabSyndr. 2020;14(3):211–212. doi:10.1016/j.dsx.2020.03.002
143. Kaatabi H, Bamosa AO, Badar A, et al. Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabetes mellitus: placebo controlled participant blinded clinical trial. PLoS One. 2015;10(2):e0113486. doi:10.1371/journal.pone.0113486
144. Sangi SMA, Sulaiman MI, El-Wahab MFA, Ahmedani EI, Ali SS. Antihyperglycemic effect of thymoquinone and oleuropein, on streptozotocin-induced diabetes mellitus in experimental animals. Pharmacogn Mag. 2015;11(Suppl 2):S251–S257. doi:10.4103/0973-1296.166017
145. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi:10.1007/s00134-020-05991-x
146. El Tahir KEH, Ashour MMS, Al-Harbi MM. The cardiovascular actions of the volatile oil of the black seed (Nigella sativa) in rats: elucidation of the mechanism of action. Gen Pharmacol. 1993;24(5):1123–1131. doi:10.1016/0306-3623(93)90359-6
147. Enomoto S, Asano R, Iwahori Y, et al. Hematological studies on black cumin oil from the seeds of Nigella sativa L. Biol Pharm Bull. 2001;24(3):307–310. doi:10.1248/bpb.24.307
148. Randhawa MA, Alghamdi MS, Maulik SK. The effect of thymoquinone, an active component of Nigella sativa, on isoproterenol induced myocardial injury. Pak J Pharm Sci. 2013;26(6):1215–1219.
149. Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol. 2020;16(8):413–414. doi:10.1038/s41584-020-0448-7
150. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771–1778. doi:10.1016/S0140-6736(20)31103-X
151. Pouletty M, Borocco C, Ouldali N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020;79(8):999–1006. doi:10.1136/annrheumdis-2020-217960
152. Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the COVID-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi:10.1136/bmj.m2094
153. Waltuch T, Gill P, Zinns LE, et al. Features of COVID-19 post-infectious cytokine release syndrome in children presenting to the emergency department. Am J Emerg Med. 2020;38(10):
154. Koshak A, Koshak E, Heinrich M. Medicinal benefits of Nigella sativa in bronchial asthma: a literature review. Saudi Pharm J. 2017;25(8):1130–1136. doi:10.1016/j.jsps.2017.07.002
155. Goyal SN, Prajapati CP, Gore PR, et al. Therapeutic potential and pharmaceutical development of thymoquinone: a multitargeted molecule of natural origin. Front Pharmacol. 2017;8(656). doi:10.3389/fphar.2017.00656
156. al-Shabanah OA, Badary OA, Nagi MN, al-Gharably NM, al-Rikabi AC, al-Bekairi AM. Thymoquinone protects against doxorubicin-induced cardiotoxicity without compromising its antitumor activity. J Exp Clin Cancer Res. 1998;17(2):193–198.
157. Isaev NK, Chetverikov NS, Stelmashook EV, Genrikhs EE, Khaspekov LG, Illarioshkin SN. Thymoquinone as a potential neuroprotector in acute and chronic forms of cerebral pathology. Biochemistry (Mosc). 2020;85(2):167–176. doi:10.1134/s0006297920020042
158. Nagi MN, Alam K, Badary OA, al-Shabanah OA, al-Sawaf HA, al-Bekairi AM. Thymoquinone protects against carbon tetrachloride hepatotoxicity in mice via an antioxidant mechanism. Biochem Mol Biol Int. 1999;47(1):153–159. doi:10.1080/15216549900201153
159. Badary OA, Nagi MN, al-Shabanah OA, al-Sawaf HA, al-Sohaibani MO, al-Bekairi AM. Thymoquinone ameliorates the nephrotoxicity induced by cisplatin in rodents and potentiates its antitumor activity. Can J Physiol Pharmacol. 1997;75(12):1356–1361. doi:10.1139/y97-169
160. Arslan S, Ethem G, Coskun O, Gürel A, Sayan H, Celik I. The protective effect of thymoquinone on ethanol-induced acute gastric damage in the rat. Nutr Res. 2005;25:673–680. doi:10.1016/j.nutres.2005.06.004
161. Alrashedi M. The protective role of thymoquinone against drugs toxicity: a review. J Pharm Res Int. 2018;1–11. doi:10.9734/JPRI/2018/44944
162. Tittarelli R, Pellegrini M, Scarpellini MG, et al. Hepatotoxicity of paracetamol and related fatalities. Eur Rev Med Pharmacol Sci. 2017;21(1 Suppl):95–101.
163. Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. BiochemPharmacol. 2020;180:114147. doi:10.1016/j.bcp.2020.114147
164. Nagi MN, Almakki HA, Sayed-Ahmed MM, Al-Bekairi AM. Thymoquinone supplementation reverses acetaminophen-induced oxidative stress, nitric oxide production and energy decline in mice liver. Food Chem Toxicol. 2010;48(8–9):2361–2365. doi:10.1016/j.fct.2010.05.072
165. Aycan IÖ, Tüfek A, Tokgöz O, et al. Thymoquinone treatment against acetaminophen-induced hepatotoxicity in rats. Int J Surg. 2014;12(3):213–218. doi:10.1016/j.ijsu.2013.12.013
166. Aycan İÖ, Elpek Ö, Akkaya B, et al. Diclofenac induced gastrointestinal and renal toxicity is alleviated by thymoquinone treatment. Food Chem Toxicol. 2018;118:795–804. doi:10.1016/j.fct.2018.06.038
167. Isik AF, Kati I, Bayram I, Ozbek H. A new agent for treatment of acute respiratory distress syndrome: thymoquinone. An experimental study in a rat model. Eur J Cardiothorac Surg. 2005;28(2):301–305. doi:10.1016/j.ejcts.2005.04.012
168. Ahmad A, Alkharfy KM, Jan BL, et al. Thymoquinone treatment modulates the Nrf2/HO-1 signaling pathway and abrogates the inflammatory response in an animal model of lung fibrosis. Exp Lung Res. 2020;46(3–4):53–63. doi:10.1080/01902148.2020.1726529
169. Basarslan F, Yilmaz N, Ates S, et al. Protective effects of thymoquinone on vancomycin-induced nephrotoxicity in rats. Hum Exp Toxicol. 2012;31(7):726–733. doi:10.1177/0960327111433185
170. Wong A. COVID-19 and toxicity from potential treatments: panacea or poison. Emerg Med Australas. 2020;32(4):697–699. doi:10.1111/1742-6723.13537
171. Chary MA, Barbuto AF, Izadmehr S, Hayes BD, Burns MM. COVID-19: therapeutics and their toxicities. J Med Toxicol. 2020;16(3):284–294. doi:10.1007/s13181-020-00777-5
172. Ojha S, Azimullah S, Mohanraj R, et al. Thymoquinone protects against myocardial ischemic injury by mitigating oxidative stress and inflammation. Evid Based Complement Alternat Med. 2015;2015:143629. doi:10.1155/2015/143629
173. Xiao J, Ke ZP, Shi Y. The cardioprotective effect of thymoquinone on ischemia-reperfusion injury in isolated rat heart via regulation of apoptosis and autophagy. J Cell Biochem. 2018;119(9):7212–7217. doi:10.1002/jcb.26878
174. Bruce-Hickman D, Sajeed SM, Pang YH, Seow CS, Chen W, Gulati Kansal M. Bowel ulceration following tocilizumab administration in a COVID-19 patient. BMJ Open Gastroenterol. 2020;7(1):e000484. doi:10.1136/bmjgast-2020-000484
175. Cobourne-Duval MK, Taka E, Mendonca P, Soliman KFA. Thymoquinone increases the expression of neuroprotective proteins while decreasing the expression of pro-inflammatory cytokines and the gene expression NFκB pathway signaling targets in LPS/IFNγ -activated BV-2 microglia cells. J Neuroimmunol. 2018;320:87–97. doi:10.1016/j.jneuroim.2018.04.018
176. Odeh F, Ismail SI, Abu-Dahab R, Mahmoud IS, Al Bawab A. Thymoquinone in liposomes: a study of loading efficiency and biological activity towards breast cancer. Drug Deliv. 2012;19(8):371–377. doi:10.3109/10717544.2012.727500
177. Alkharfy KM, Ahmad A, Khan RMA, Al-Asmari M. High-performance liquid chromatography of thymoquinone in rabbit plasma and its application to pharmacokinetics. J Liq Chromatogr Relat Technol. 2013;36(16):2242–2250. doi:10.1080/10826076.2012.717062
178. Alkharfy KM, Ahmad A, Khan RM, Al-Shagha WM. Pharmacokinetic plasma behaviors of intravenous and oral bioavailability of thymoquinone in a rabbit model. Eur J Drug Metab Pharmacokinet. 2015;40(3):319–323. doi:10.1007/s13318-014-0207-8
179. Alkharfy KM, Ali FA, Alkharfy MA, et al. Effect of compromised liver function and acute kidney injury on the pharmacokinetics of thymoquinone in a rat model. Xenobiotica. 2020;50(7):858–862. doi:10.1080/00498254.2020.1745319
180. Kalam MA, Raish M, Ahmed A, et al. Oral bioavailability enhancement and hepatoprotective effects of thymoquinone by self-nanoemulsifying drug delivery system. Mater Sci Eng C Mater Biol Appl. 2017;76:319–329. doi:10.1016/j.msec.2017.03.088
181. Lupidi G, Scire A, Camaioni E, et al. Thymoquinone, a potential therapeutic agent of Nigella sativa, binds to site I of human serum albumin. Phytomedicine. 2010;17(10):714–720. doi:10.1016/j.phymed.2010.01.011
182. El-Najjar N, Ketola RA, Nissilä T, et al. Impact of protein binding on the analytical detectability and anticancer activity of thymoquinone. J Chem Biol. 2011;4(3):97–107. doi:10.1007/s12154-010-0052-4
183. Lupidi G, Camaioni E, Khalifé H, et al. Characterization of thymoquinone binding to human α1-acid glycoprotein. J Pharm Sci. 2012;101(7):2564–2573. doi:10.1002/jps.23138
184. Wirries A, Breyer S, Quint K, Schobert R, Ocker M. Thymoquinone hydrazone derivatives cause cell cycle arrest in p53-competent colorectal cancer cells. Exp Ther Med. 2010;1(2):369–375. doi:10.3892/etm_00000058
185. Elmowafy M, Samy A, Raslan MA, et al. Enhancement of bioavailability and pharmacodynamic effects of thymoquinone via Nanostructured Lipid Carrier (NLC) formulation. AAPS PharmSciTech. 2016;17(3):663–672. doi:10.1208/s12249-015-0391-0
186. Ng WK, Saiful Yazan L, Yap LH, Wan NorHafiza WA, How CW, Abdullah R. Thymoquinone-loaded nanostructured lipid carrier exhibited cytotoxicity towards breast cancer cell lines (MDA-MB-231 and MCF-7) and cervical cancer cell lines (HeLa and SiHa). Biomed Res Int. 2015;2015:263131. doi:10.1155/2015/263131
187. Ong YS, Saiful Yazan L, Ng WK, et al. Acute and subacute toxicity profiles of thymoquinone-loaded nanostructured lipid carrier in BALB/c mice. Int J Nanomedicine. 2016;11:5905–5915. doi:10.2147/IJN.S114205
188. El-Far AH, Al Jaouni SK, Li W, Mousa SA. Protective roles of thymoquinone nanoformulations: potential nanonutraceuticals in human diseases. Nutrients. 2018;10(10):1369. doi:10.3390/nu10101369
189. Badary OA. Thymoquinone attenuates ifosfamide-induced Fanconi syndrome in rats and enhances its antitumor activity in mice. J Ethnopharmacol. 1999;67(2):135–142. doi:10.1016/s0378-8741(98)00242-6
190. Badary OA, Abdel-Naim AB, Abdel-Wahab MH, Hamada FM. The influence of thymoquinone on doxorubicin-induced hyperlipidemic nephropathy in rats. Toxicology. 2000;143(3):219–226. doi:10.1016/s0300-483x(99)00179-1
191. Awad AS, Kamel R, Sherief MA. Effect of thymoquinone on hepatorenal dysfunction and alteration of CYP3A1 and spermidine/spermine N-1-acetyl-transferase gene expression induced by renal ischaemia-reperfusion in rats. J Pharm Pharmacol. 2011;63(8):1037–1042. doi:10.1111/j.2042-7158.2011.01303.x
192. Bai T, Yang Y, Wu YL, et al. Thymoquinone alleviates thioacetamide-induced hepatic fibrosis and inflammation by activating LKB1-AMPK signaling pathway in mice. Int Immunopharmacol. 2014;19(2):351–357. doi:10.1016/j.intimp.2014.02.006
193. ElKhoely A, Hafez HF, Ashmawy AM, et al. Chemopreventive and therapeutic potentials of thymoquinone in HepG2 cells: mechanistic perspectives. J Nat Med. 2015;69(3):313–323. doi:10.1007/s11418-015-0895-7
194. Gholamnezhad Z, Havakhah S, Boskabady MH. Preclinical and clinical effects of Nigella sativa and its constituent, thymoquinone: a review. J Ethnopharmacol. 2016;190:372–386. doi:10.1016/j.jep.2016.06.061
195. Abulfadl YS, El-Maraghy NN, Ahmed AAE, Nofal S, Badary OA. Protective effects of thymoquinone on D-galactose and aluminum chloride induced neurotoxicity in rats: biochemical, histological and behavioral changes. Neurol Res. 2018;40(4):324–333. doi:10.1080/01616412.2018.1441776
196. Badary OA, Al-Shabanah OA, Nagi MN, Al-Bekairi AM, Elmazar MMA. Acute and subchronic toxicity of thymoquinone in mice. Drug Dev Res. 1998;44(2‐3):56–61. doi:10.1002/(SICI)1098-2299(199806/07)44:2/3<56::AID-DDR2>3.0.CO;2-9
197. Qadri SM, Mahmud H, Föller M, Lang F. Thymoquinone-induced suicidal erythrocyte death. Food Chem Toxicol. 2009;47(7):1545–1549. doi:10.1016/j.fct.2009.03.037
198. Khader M, Bresgen N, Eckl PM. In vitro toxicological properties of thymoquinone. Food Chem Toxicol. 2009;47(1):129–133. doi:10.1016/j.fct.2008.10.019
199. Al-Ali A, Alkhawajah AA, Randhawa MA, Shaikh NA. Oral and intraperitoneal LD50 of thymoquinone, an active principle of Nigella sativa, in mice and rats. J Ayub Med Coll Abbottabad. 2008;20(2):25–27.
200. Abukhader MM. The effect of route of administration in thymoquinone toxicity in male and female rats. Indian J Pharm Sci. 2012;74(3):195–200. doi:10.4103/0250-474x.106060
201. Tavakkoli A, Mahdian V, Razavi BM, Hosseinzadeh H. Review on Clinical Trials of Black Seed (Nigella sativa) and its Active Constituent, Thymoquinone. J Pharmacopunct. 2017;20(3):179–193. doi:10.3831/kpi.2017.20.021
202. Asaduzzaman Khan M, Tania M, Fu S, Fu J. Thymoquinone, as an anticancer molecule: from basic research to clinical investigation. Oncotarget. 2017;8(31):51907–51919. doi:10.18632/oncotarget.17206
203. Alamri A, Bamosa A. Phase I Safety and Clinical Activity Study of Thymoquinone in patients with advanced refractory malignant disease. Shiraz Med J. 2009;10:107–111.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
