Back to Journals » Adolescent Health, Medicine and Therapeutics » Volume 11
Thyme Tea and Primary Dysmenorrhea Among Young Female Students
Authors Zeru AB , Muluneh MA
Received 7 September 2020
Accepted for publication 6 October 2020
Published 20 October 2020 Volume 2020:11 Pages 147—155
DOI https://doi.org/10.2147/AHMT.S280800
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Alastair Sutcliffe
Abayneh Birlie Zeru,1 Mikyas Arega Muluneh2
1Department of Public Health, College of Health Science, Debre Berhan University, Debre Berhan, Amhara Regional State, Ethiopia; 2Department of Midwifery, College of Health Science, Debre Berhan University, Debre Berhan, Amhara Regional State, Ethiopia
Correspondence: Abayneh Birlie Zeru
Department of Public Health, College of Health Science, Debre Berhan University, Po. Box: 445, Debre Berhan, Amhara Regional State, Ethiopia
Tel +251919134994
Email [email protected]
Background: Thyme tea, locally known as “tossign tea”, is one of the most popular herbal-tea in Ethiopia used for the medicinal attribute, besides adding aroma and flavor to the tea. Therefore, this study aimed to assess the effect of thyme tea-drinking and other dietary factors of school girls on primary dysmenorrhea.
Methods: An institutional case–control study was conducted from December 2019 to March 2020 in the suburbs of Debre Berhan town, Ethiopia. Data were collected through a face-to-face interview using a pre-tested semi-structured questionnaire on 252 (86 cases and 166 controls) study participants. Data were entered to Epi Data version 3.1 and then exported to IBM SPSS version 24 for analysis. Bivariable and multivariable logistic regressions were carried out to identify factors significantly associated with primary dysmenorrhea.
Results: The mean (±SD) age of cases was 15.98 (± 1.60) years and controls was 15.73 (± 1.35) years. Thyme tea drinking was reported by 19 (22.1%) of cases and 56 (33.7%) of controls. Thyme tea-drinking decreased the risk of primary dysmenorrhea by 63.2% (AOR: 0.368, 95% CI: 0.145– 0.934). Coffee drinking tends to increase the odds of severe dysmenorrhea on young female students. Besides, age, age at menarche, meal frequency, and residence were significantly associated with primary dysmenorrhea.
Conclusion: Thyme tea-drinking, consumption of vegetables and fruits had primary dysmenorrhea related pain-relieving tendency. Delayed onset of menarche decreased the risk of primary dysmenorrhea. Coffee drinking was positively associated with primary dysmenorrhea. Further studies on the effect of thyme tea on primary dysmenorrhea are required.
Keywords: primary dysmenorrhea, thyme tea, tossign, Ethiopia
Introduction
Dysmenorrhea is defined as a recurrent lower abdominal pain during menstruation. It is categorized into primary and secondary. Primary dysmenorrhea is lower abdominal pain associated with the normal ovulatory cycle in the absence of pelvic disease or abnormality. The pain starts a few hours before or after the onset of menstruation and lasts for a few hours to 2–3 days. Secondary dysmenorrhea is also menstrual pain caused by underlying pelvic pathology which usually occurred on relatively older reproductive-age women.1–4
Primary dysmenorrhea has a wide range of severity with significant medical and psychosocial implications.5–7 Due to the lack of a standard method to measure menstrual pain and the subjectivity nature of pain levels across individuals; studies across the world used various methods to measure the amount and severity of primary dysmenorrhea. As a result, the prevalence varies significantly from place to place. It ranges from 41.7% to 93.3% and the severe form of dysmenorrhea accounts for 10% to 20% in the world.7–10 Studies from Ethiopia reported that about 70% of school girls experienced some form of primary dysmenorrhea and up to 12% had severe pain. The prevalence reaches up to 85.4% among university students.5,11–15
Though primary dysmenorrhea is one of the most common gynecologic complaints of adolescent and young females, it is poorly understood by many girls that they simply accept it as a normal part of their menstrual cycle. As a result, primary dysmenorrhea has a medical and psychosocial effect on adolescent girls. It is a cause for female students’ school absenteeism and/or reduced class concentration and participation ability leading to poor school performance.16–18
Current evidence showed that the root cause of primary dysmenorrhea is mainly related to the level of prostaglandins (PGs) production in the uterus during menstruation. The drop in progesterone in the menstrual cycle after ovulation before the start of menses increases the formation of omega-6 fatty acid particularly arachidonic acid in the phospholipids of the cell membrane. PGs are synthesized from arachidonic acid through the cyclooxygenase (COX) pathway. Overproduction of PGs was observed in women with primary dysmenorrhea which is responsible for increased smooth muscle contraction of the uterus and causes painful menstruation.3,19,20
Though effective pharmacological drugs like non-steroid anti-inflammatory drugs (NSAIDs) and hormonal contraceptive pills are widely available,16 most adolescents choose to use home remedies or non-pharmacological remedies for their pain. Non-pharmacologic nutritional measures called nutraceuticals,21 used to control primary dysmenorrhea include: Dietary habit changes and consumption of foods (like fruits, vegetables, eggs, milk, and fish which are rich in vitamins, minerals, and omega-3 fatty acids),4,22,23 and use of herbal products of cinnamon,24 ginger root,25 and Foeniculum vulgare22 were reported effective in reducing the intensity of dysmenorrhea.
Thyme tea is locally known as “tossign tea” is one of the most popular herbal-tea in Ethiopia. Thyme is wild endemic dietary herbs used as spices to flavor various food products and herbal medicine. Though, fresh or dried leaves of Thyme species are used locally as a tea for the medicinal attribute, mostly it is marinated for adding aroma and flavor to the tea. T. schimperi and T. serrulatus are common thymus species found in Ethiopia. However, T. schimperi is a more prevalent highland area of North Shewa, a central part of Ethiopia.26–28
Even though thyme tea is locally used as herbal medicine to treat various health problems like abdominal pain, diarrhea, parasite, cough, asthma, hypertension, and general pain syndrome, it is not used to treat menstrual pain related to primary dysmenorrhea in Ethiopia.26,27 But studies in other countries revealed that thyme is effective herbal medicine in alleviating menstrual pains and treating dysmenorrhea.29,30 According to a study in Iran, oil extracted from T. Vulgaris was as effective as Ibuprofen in reducing the severity of dysmenorrheal pain.31 The extracts of the thyme have anti-oxidant, anti-inflammatory, analgesic, and anti-spasmodic effects.32
Hence, it is hypothesized that thyme tea drinking may probably decrease the risk of severe dysmenorrhea. However, there is no study tried to investigate the association between thyme tea habit and dysmenorrhea. Therefore, this study aimed to assess the effect of thyme tea drinking and other dietary factors of school girls on severe primary dysmenorrhea in the suburbs of Debre Berhan town.
Methods and Materials
Study Design, Period and Setting
This study was conducted from December 2019 to March 2020 in the suburbs of Debre Berhan town. Debre Berhan is a zonal administrative town of North Shewa, Amhara national regional state. It is about 582.44km south east of Bahir Dar and 130km northeast of the capital city Addis Ababa of driving distance. Debre Berhan town is located at a latitude and longitude of 9°41ʹN 39°32ʹE and has an elevation of 2840m. It has a cold agro-climate as part of the highland plateau of North Shewa, central Ethiopia with average maximum and minimum temperature of 20.1°C and 6.5°C respectively.
Study Design and Participants
A case-control study was conducted on post-menarche young female students aged 13 to 19 years old. The participants were recruited from a large study involving 1328 public school adolescent girls that investigated menstruation and menstrual hygiene management practice in Debre Berhan town and surrounding area.
First, each post-menarche students were asked if they experienced one or more menstrual cramp or abdominal pain during the last three consecutive menstrual cycles and asked to rate the intensity of pain using Verbal Multidimensional Scoring System (VMS). VMS considers the impacts of pain on daily activities, systemic symptoms and analgesic requirements to level dysmenorrhea as mild dysmenorrhea (do not limit normal daily activities, and little or no systemic symptoms and analgesic need), moderate (slightly limit normal daily activities, and moderate systemic symptoms and analgesic need) and severe (severely limit normal daily activities such as school absenteeism, and noticeable symptoms and analgesic need).
For this study, primary dysmenorrhea was defined as abdominal crampy menstrual pain which starts immediately before or after the onset of menses and lasts for 12 hours to 3 days. Absence from school by young female students due to dysmenorrhea was used to classify severe primary dysmenorrhea. Accordingly, those who had severe dysmenorrhea and associated school absenteeism were recruited as “cases” and those who did not have experience of any menstrual cramp were taken as comparison group “controls”. The eligibility criteria: being age ≤19 years was used for inclusion and students who had lower abdominal pain in non-menses days, history of abdominal surgery, history of modern family planning use, and history of pregnancy were excluded from the study.
The sample size was computed using Epi Info version 7.1.5.0 software. We considered 95% confidence level, 80% power, and 2:1 control-to-case ratio for an unmatched case-control study. By taking 63.0% and 43.4% prevalence of coffee drinking among dysmenorrheic and non-dysmenorrheic women respectively from a study in Shanghai china33 we get a sample size of 248. Adding a 10% non-response rate gave the final sample size of 273 (cases 91, controls 182).
Data Collection Procedures and Tools
Data were collected by trained female nurses using a semi-structured questionnaire through a face-to-face interview. The interview was conducted at school during free class time and it took on average 25–30 minutes. The questionnaire was developed by reviewing different pieces of literature and contextually adapted to socio-cultural norms and agro-climatic conditions of the area. The questionnaire was divided into four parts: socio-demographic characteristics, menstrual conditions, dietary practices (diet diversity and meal frequency including thyme tea consumption), and anthropometric measurements.
Socio-demographic characteristics include age, residence, religion, family size, and parental education status, and occupation. Menstrual conditions addressed age at menarche, menstrual regularity, menstrual cycle length, and bleeding duration. Menstrual regularity was by asking the question: “Is your menstrual cycle length is regular in the last six menstrual cycles?” If yes, participants were asked the average length of the menstrual cycle in days. If no, participants were asked to recall the shortest and the longest length of the menstrual cycle in days, and menstrual irregularity was considered if the difference between the shortest and longest menstrual length was greater or equal to 8 days. The intensity of dysmenorrhea was again measured by Verbal Multidimensional Scoring System (VMS) both on cases and controls. From the controls, one participant who failed to fulfill the control definitions was excluded from the analysis.
Dietary factors such as; meal frequency, diet diversity, frequency of vegetables, fruits, and dairy consumption per week were assessed. The 24 hours recall diet diversity score (DDS) was measured by adapting the called Minimum Diet Diversity for Women (MDD-W) tool which was developed by the FANTA project. The tool was developed to reflect the micronutrient adequacy of women’s diet. The minimum food groups required to get adequate micronutrient was five out of ten defined food groups. Therefore, DDS of ≥5 was considered adequate.34
Information about thyme tea and other caffeinated drinks like; tea and coffee consumptions were collected. Participants were asked whether they drink thyme tea, normal tea, and coffee in their everyday life or not. If yes, they were asked to quantify the amount and frequency of consumption using the number of cups per day per week.
Anthropometric measurements: Height was measured to the nearest 0.1cm with barefoot, and weight was measured to the nearest 0.1kg with light clothing and barefoot. Nutritional status was assessed by Body Mass Index (BMI), which was calculated by dividing weight in kilograms by height in meters square. Study participants with BMI <18.5kg/m2, 18.5–25kg/m2 and >25kg/m2 were classified as underweight, normal and overweight respectively.
Data Quality Assurance
The English version of the questionnaire was translated into the local language (Amharic), the consistency was check by re-translated back to English and compared with the original version. The Amharic version of the questionnaire was pre-tested on 25 post-menarche female students to check its understandability by study participant, response rate to each question, and determine the time required per questionnaire. One-day training was given for three female data collectors and one supervisor. Daily monitoring and supervision of the data collection, checking for completeness and consistency of collected data was carried out by supervisors. The principal investigator also examined the collected data for completeness and consistency before data entry.
Data Analysis Procedures
Collected data were entered into Epi Data version 3.1 software and exported to IBM SPSS Statistics for Windows, Version 24 (Corp Armonk, New York, USA) for analysis. Both bi-variable and multivariable binary logistic regression analyses were carried out to see the association between severe dysmenorrhea and thyme tea consumption and other dietary habits of young female students. Odds ratios with 95% confidence intervals were computed as a measure of association and a p-value of <0.05 was used to declare the presence of a statistically significant association between the dependent variable and covariates.
Ethical Considerations
Ethical clearance was obtained from the Ethical Review Committee of Debre Berhan University. Permission to proceed with the study was obtained from school directors. Study participants were adequately informed about the importance of the study and participate in the study was based on volunteerism. Based on the Ethical Review Committee’s approval, oral consent was obtained by ≥15years old study participants during data collection. A day prior to the interview, <15years aged participants were informed to get verbal consent from their parents besides their oral assent to participate in the study. School teachers also consented for their students’ participation in the study. For confidentiality purpose, the names of participants and other personal identifiers related questions were excluded in the questionnaire.
Results
Socio-Demographic and Menstrual Characteristics of Participants
Data collected from 252 (cases 86, controls 166) study participants, and the response rate was 92.3%. Table 1 describes the socio-demographic and menstrual characteristics as well as the nutritional status of cases and controls.
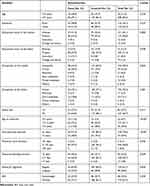 |
Table 1 Socio-Demographic and Menstrual Characteristics of Study Participants |
Almost all 247 (98.0%) and 230 (91.3%) of study participants had a habit of tea and coffee drinking, respectively. Thyme tea drinking was reported by 75 (29.8%) study participants, and it was 19 (22.1%) among cases and 56 (33.7%) among controls (Table 2).
 |
Table 2 Bivariable Analysis of Thyme Tea Drinking and Its Frequency on Primary Dysmenorrhea |
Meal frequency of >3 meals per day was higher 75 (45.2%) among controls compared to 27 (31.4%) among cases. The mean (±SD) 24hours DDS of cases and controls was 4.27 (±1.87) and 4.44 (±1.69), respectively. Those who consumed at least 5 food groups (the minimum required food groups) in the last 24 hours were 30 (34.9%) of cases and 77 (46.4%) of controls (Table 3).
 |
Table 3 Factors Associated with Primary Dysmenorrhea Among Young Female Students |
Risk Factors of Dysmenorrhea
Table 2 described the bi-variable analysis of thyme tea drinking and the frequency of thyme tea drinking on primary dysmenorrhea. Even if, the association was not significant at p-value <0.05, thyme tea-drinking tended to decrease the risk of primary dysmenorrhea. Concerning the frequency of thyme tea consumption, those who drank thyme tea at least once per day had a lesser risk of primary dysmenorrhea by over 70% (COR: 0.286 and 95% CI: 0.095–0.862).
As shown in Table 3 below, the adjusted effect of thyme tea drinking on primary dysmenorrhea was computed by involving dietary, menstrual, and socio-demographic related variables in the final model. Variables found to have significant association on bi-variable analysis: meal frequency, frequency of dairy consumption, frequency of vegetable and fruit consumption, age at menarche and post-menarche duration, and other clinically important variables such as; age, BMI, menstrual regularity, DDS, and frequency of coffee drinking were included in the multivariable analysis.
Accordingly, thyme tea drinking was significantly associated with primary dysmenorrhea. Thyme tea decreased the risk of primary dysmenorrhea by 63.2% (AOR: 0.368 and 95% CI: 0.145–0.934). In this study, coffee drinking tends to increase the odds of primary dysmenorrhea on young female students. Compared to those who drink coffee once per day, those who drink coffee less than once per day (or rarely per week) and coffee non-drinkers had 0.14 and 0.12 times less likely to experience primary dysmenorrhea respectively (Table 3).
Besides, age, age at menarche, meal frequency, and residence were significantly associated with primary dysmenorrhea. As the age of young female students’ increases, the probability of experiencing primary dysmenorrhea increase by 66.5%. Late menarche (at or after 15 years of age) girls were 0.09 times less likely to have primary dysmenorrhea as compared to girls who experience menarche at 13–14 years of age. Having ≥4meals/day decreased the odds of primary dysmenorrhea by 67.3% than having 3meals/day. Primary dysmenorrhea was 3.5 times more common among rural residents than urban (Table 3).
Discussion
In this study, thyme tea-drinking showed the tendency of alleviating severe form of dysmenorrhea among young female students. Thyme tea consumers were 63% less likely to experience primary dysmenorrhea than their counterparts. The dysmenorrhea risk reduction effect of thyme tea might be due to the antioxidant and analgesic capacities of bioactive phytochemicals of thyme leaves used in the tea. According to Dagne et al, the essential oil of T. schimperi leaves, thyme species found in this study area is rich in phenolic compounds that have significant antioxidant activities.28 Thyme tea drinking could have a role in reducing PGs production by suppressing the COX-2 pathway and prevent oxidative stress by scavenging lipid peroxide radicals (index of oxidative stress) in dysmenorrhea women. Several studies35–37 showed the presence of increased lipid peroxide in dysmenorrheic women compared to normal women. Overproduction and release of PGs especially PGF2α in the secretary endometrium20,38,39 lead to myometrium over activation and subsequent uterine hypoxic-ischemia. This tissue damage in the uterus activates phospholipase A2 that hydrolysis cell membrane phospholipids, which further propagate the lipid peroxide radicals and arachidonic acid (precursor of prostaglandin syntheses) production, and exacerbate the severity of primary dysmenorrhea.40,41 Phenolic compounds of the thyme leaves in the tea might inhibit the activity of phospholipase A2 enzyme and subsequence arachidonic acid production. A randomized and triple-blind clinical trial in Iran,31 revealed that taking Thyme Vulgaris essential oil was as effective as Ibuprofen in reducing the severity of dysmenorrheal pain.
To rule out whether the protective effect of thyme tea was related to the caffeine constituent of thyme tea, we examined the effect of caffeinated beverages such as coffee drinking on dysmenorrhea. Also, by including into the final model we observed that coffee drinking had an independent direct association with primary dysmenorrhea. Coffee non-drinkers and those who drink rarely per week had 88% and 86% lesser odds of primary dysmenorrhea, respectively compared to daily coffee drinkers. Drinking coffee more than once per day was not significantly associated with primary dysmenorrhea. Similarly, previous studies in Ethiopia,14 Turkey,42 and Kuwait43 showed that caffeinated beverages exacerbate the risk of primary dysmenorrhea. Though the mechanism of how coffee aggravates dysmenorrhea is not clearly understood, one possible mechanism is the caffeine vasoconstriction effect which decreases the blood flow to the uterus exacerbating the severity of dysmenorrhea.
Concerning dietary habits, young female students who had had four or more meals per day were 67.3% less likely to experience primary dysmenorrhea than those who had three meals per day. Meal frequency of twice per day or less had no association with primary dysmenorrhea, maybe this is due to the small number of this study population who had had ≤2 meals/day.
The frequency of fruit or vegetable intake was inversely associated with primary dysmenorrhea. Those who had frequent fruits or vegetable consumption (≥4times/week) had around 68% lesser odds of dysmenorrhea compared to those who never had one. This is consistent with previous studies report of increased consumption of fruits and vegetables as the sources of vitamins and minerals are associated with decreased menstrual pain.44–46 Micronutrients such as vitamins (vitamin C, vitamin E, and β-carotene) and mineral (zinc) are non-enzymatic dietary antioxidants. Hence, frequent consumption of fruits and vegetables is important to prevent the occurrence of oxidative stress secondary to increased lipid peroxide in primary dysmenorrhea.47,48
Other risk factors associated with primary dysmenorrhea were age and age at menarche of young female students. As the age of young female students approaches to late teens, the probability of experiencing primary dysmenorrhea also increased. Late menarche girls had a decreased risk of primary dysmenorrhea. Several previous studies6,7,11,15,49 conducted on adolescent girls also revealed that the peak prevalence of primary dysmenorrhea occurs in late teens, and the onset of menarche at an early age is associated with increased risk of primary dysmenorrhea.
Limitations that could affect the finding of this study are: first, there could be recall bias since study variables were measured through participants’ self-report. Second, in the study area thyme leaves sometimes used as food additives and spices to flavor a wide range of food products. The amount and frequency the thyme leaves consumed by the study participants in the form of food additive or spices were not measured in the study. This may dilute and lead to underestimating the magnitude of the association between thyme tea and primary dysmenorrhea. Third, other important variables that might affect the occurrence of primary dysmenorrhea including alcohol drinking, stress, and physical exercise were not considered in the study. However, this study gives new insight into the possible role of the local herbal tea, thyme tea, drinking in reducing dysmenorrhea.
Conclusion
Thyme tea drinking and consumption of vegetables and fruits had primary dysmenorrhea related pain-relieving tendency. Delayed onset of menarche decreased the risk of primary dysmenorrhea. Coffee drinking was positively associated with primary dysmenorrhea. Further investigations with a clinical trial on the effect of thyme tea drinking on dysmenorrhea are required to enrich epidemiological evidence and mechanism of action.
Acknowledgment
The authors acknowledge the school directors for their consent and assistance. Most of all we are sincerely grateful for the study participants and data collectors for their full involvement in the data collection process. We thank Debre Berhan University for the material.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Dawood YM. Dysmenorrhea. Glob Libr Womens Med. 2008. doi:10.3843/GLOWM.10009.
2. Smith RP, Kaunitz AM. Dysmenorrhea in Adult Women: Treatment; 2020. Available from: https://www.uptodate.com/contents/dysmenorrhea-in-adult-women-treatment.
3. Dharmapuri S. Dysmenorrhea in adolescents. Pediatr Med. 2019;2:34 I. doi:10.21037/pm.22019.21006.21009.
4. Preedy VR, Hunter L-A, Patel VB, eds. Diet Quality: An Evidence-Based Approach, Volume 1, Nutrition and Health.
5. Mohammed H, Hassen N, Musa A. Dysmenorrhea and associated factors among secondary school students in East Hararghe Zone, Eastern Ethiopia. East Afr J Health Biomed Sci. 2019;3(1):39–48.
6. Acheampong K, Baffour-Awuah D, Ganu D, et al. Prevalence and predictors of dysmenorrhea, its effect, and coping mechanisms among adolescents in Shai Osudoku District, Ghana. Obstet Gynecol Int. 2019;2019:5834157. Article ID 5834159. doi:10.5831155/5832019/5834159.
7. Armour M, Parry K, Manohar N, et al. The prevalence and academic impact of dysmenorrhea in 21,573 young women: a systematic review and meta-analysis. J Womens Health. 2019;28(8):1161–1171. doi:10.1089/jwh.2018.7615.
8. Hu Z, Tang L, Chen L, Chiwanga A, Xu H. Prevalence and risk factors associated with primary dysmenorrhea among Chinese female university students: a cross-sectional study. Pediatr Adolesc Gynecol. 2020;33:15–22. doi:10.1016/j.jpag.2019.09.004
9. Abdul-Razzak KK, Ayoub NM, Abu-Taleb AA, Obeidat BA. Influence of dietary intake of dairy products on dysmenorrhea. J Obstet Gynaecol Res. 2010;36(2):377–383. doi:10.1111/j.1447-0756.2009.01159.x
10. Kizilirmak A, Kartal B, Calpbinici P. Prevalence of dysmenorrhea in young women and their coping methods. Med Sci. 2019. doi:10.5455/medscience.2018.5407.8937.
11. Azagew AW, Kassie DG, Walle TA. Prevalence of primary dysmenorrhea, its intensity, impact and associated factors among female students’ at Gondar town preparatory school, Northwest Ethiopia. BMC Womens Health. 2020;20:5. doi:10.1186/s12905-019-0873-4
12. Shiferaw MT, Wubshet M, Tegabu D. Menstrual problems and associated factors among students of Bahir Dar University, Amhara National Regional State, Ethiopia: a cross-sectional survey. Pan Afr Med J. 2014;17:246. doi:10.11604/pamj.12014.11617.11246.12230.
13. Yesuf TA, Eshete NA, Sisay EA. Dysmenorrhea among University Health Science Students, Northern Ethiopia: impact and Associated Factors. Hindawi Int J Reprod Med. 2018;2018:
14. Hailemeskel S, Demissie A, Assefa N. Primary dysmenorrhea magnitude, associated risk factors, and its effect on academic performance: evidence from female university students in ethiopia. Int J Womens Health. 2016;8:489–496. doi:10.2147/IJWH.S112768
15. Muluneh AA, Nigussie T, Gebreslasie KZ, Anteneh KT, Kassa ZY. Prevalence and associated factors of dysmenorrhea among secondary and preparatory school students in Debremarkos town, North-West Ethiopia. BMC Womens Health. 2018;18:57. doi:10.1186/s12905-018-0552-x
16. Iacovides S, Avidon I, Baker FC. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update. 2015;21(6):762–778.
17. Derseh B, Afessa N, Temesgen M, et al. Prevalence of dysmenorrhea and its effects on school performance: a cross-sectional study. J Women’s Health Care. 2017;6:361. doi:10.4172/2167-0420.1000361.
18. Sidi I, Hounkpatin B, Obossou A, et al. Primary dysmenorrhea in the schools of parakou: prevalence, impact and therapeutic approach. Gynecol Obstet (Sunnyvale). 2016;6:376. doi:10.4172/2161-0932.1000376.
19. Sanctis D, Ashraf S, Sergio B, et al. Definition and self-reported pain intensity in adolescents with dysmenorrhea: a debate report. J Pediatr Child Health Care. 2016;1(1):1006.
20. Smith P. R. Dysmenorrhea and Menorrhagia: A Clinician’s Guide. Switzerland: Springer; 2018. doi:10.1007/978-3-319-71964-1_1.
21. Franciscis PD, Colacurci N, Riemma G, et al. A nutraceutical approach to menopausal complaints. Medicina. 2019;55:544. doi:10.3390/medicina55090544
22. Mirabi P, Alamolhoda SH, Esmaeilzadeh S, Mojab F. Effect of medicinal herbs on primary dysmenorrhoea- a systematic review. Iran J Pharm Res. 2014;13(3):757–767.
23. Ferna´ndez-Martı´nez E, Onieva-Zafra M, Parra-Ferna´ndez M. Lifestyle and prevalence of dysmenorrhea among Spanish female university students. PLoS One. 2018;13:8. doi:10.1371/journal.pone.0201894
24. Jahangirifar M, Taebi M, Dolatian M. The effect of Cinnamon on primary dysmenorrhea: A randomized, double-blind clinical trial. Complement Ther Clin Pract. 2018;33:56–60. doi:10.1016/j.ctcp.2018.08.001
25. Kafash-Farkhad N, Asadi-Samani M, Rafieian-Kopaei M. A review on phytochemistry and pharmacological effects of Prangosferulacea (L.) Lindl. Life Sci J. 2013;10(SUPPL):360–7p.
26. Damtie D, Mekonnen Y. Thymus species in Ethiopia: distribution, medicinal value, economic benefit, current status and threatening factors. Ethiop J Sci Technol. 2015;8(2):81–92. doi:10.4314/ejst.v8i2.3
27. d’Avigdor E, Wohlmuth H, Asfaw Z, Awas T. The current status of knowledge of herbal medicine and medicinal plants in Fiche, Ethiopia. J Ethnobiol Ethnomed. 2014;10:38. doi:10.1186/1746-4269-10-38
28. Dagne E, Hailu S, Bisrat D, Worku T. Constituents of the essential oil of Thymus Schimperi. Bull Chem Soc Ethiop. 1998;12(1):79–82.
29. Uritu CM, Mihai CT, Stanciu G-D, et al. Medicinal plants of the family lamiaceae in pain therapy: a review. Pain Res Manag. 2018;2018:44. Article ID 7801543. doi:10.1155/2018/7801543
30. Staughton J. Thyme Tea: Benefits and How to Make It; 2020. Available from: https://www.organicfacts.net/health-benefits/herbs-and-spices/thyme-tea.html.
31. Salmalian H, Saghebi R, Moghadamnia AA, et al. Comparative effect of thymus vulgaris and ibuprofen on primary dysmenorrhea: A triple-blind clinical study. Caspian J Intern Med. 2014;5(2):82–88.
32. Price S, Price L, eds. Aromatherapy for Health Professionals.
33. Zhang X, Zhang R, Chen D, et al. Association of tea drinking and dysmenorrhoea among reproductive-age women in Shanghai, China (2013–2015): a cross-sectional study. BMJ Open. 2019;9:e026643. doi:10.1136/bmjopen-2018-026643.
34. FAO, FHI360. Minimum Dietary Diversity for Women: A Guide for Measurement. Rome: FAO; 2016.
35. Shundo H, Karaca I, Sevinc L, et al. Role of ischemia and oxidative stress in primary dysmenorrhea pathogenesis. Sakarya Med J. 2017;7(4):205–210.
36. Rao SV, Kiran. VSR, Vijayasree. M. Oxidative stress and antioxidant status in primary dysmenorrhea. J Clin Diagn Res. 2011;5(3):509–511.
37. Orimadegun B, Awolude O, Agbedana E. Markers of lipid and protein peroxidation among Nigerian university students with dysmenorrhea. Niger J Clin Pract. 2019;22:174–180.
38. Zhou SF, Wang HY. One review on the latest etiology research progress of primary dysmenorrhea. Reprod Dev Med. 2018;2:171–177. doi:10.4103/2096-2924.248489
39. Antao V, Black A, Burnett M, Feldman K, Lea R, Robert M. Primary dysmenorrhea consensus guideline. SOGC clinical practice guideline. J Obstet Gynaecol Can. 2005;27:1117–1130.
40. Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Ind J Clin Biochem. 2015;30(1):11–26.
41. Harel Z. Dysmenorrhea in adolescents and young adults: etiology and management. J Pediatr Adolesc Gynecol. 2006;19:363–371. doi:10.1016/j.jpag.2006.09.001
42. Unsal A, Ayranci U, Tozun M, Arslan G, Calik E. Prevalence of dysmenorrhea and its effect on quality of life among a group of female university students. Ups J Med Sci. 2010;115:138–145. doi:10.3109/03009730903457218
43. Al-Matouq S, Al-Mutairi H, Al-Mutairi O, et al. Dysmenorrhea among high-school students and its associated factors in Kuwait. BMC Pediatr. 2019;19:80.
44. Balbi C, Musone R, Menditto A, et al. Influence of menstrual factors and dietary habits on menstrual pain in adolescence age. Eur J Obstet Gynecol Reprod Biol. 2000;91:143–148. doi:10.1016/S0301-2115(99)00277-8
45. Tavallaee M, Joffres MR, Corber SJ, Bayanzadeh M, Rad MM. The prevalence of menstrual pain and associated risk factors among Iranian women. J Obstet Gynaecol Res. 2011;37(5):442–451. doi:10.1111/j.1447-0756.2010.01362.x
46. Bajalan Z, Alimoradi Z, Moafi F. Nutrition as a potential factor of primary dysmenorrhea: a systematic review of observational studies. Gynecol Obstet Invest. 2019;84:209–224. doi:10.1159/000495408.
47. Naz MSG, Kiani Z, Fakari FR, Ghasemi V, Abed M, Ozgoli G. The effect of micronutrients on pain management of primary dysmenorrhea: a systematic review and meta‐analysis. J Caring Sci. 2020;9(1):47–56. doi:10.34172/jcs.32020.34008.
48. Pramanik P, Banerjee SB, Saha P. Primary dysmenorrhea in school going adolescent girls—is it related to deficiency of antioxidant in diet? Int J Life Sci Pharm Res. 2015;5(2):54–63.
49. Mathew A, Varghese DM, Shaju MV, Joseph N, Tamrakar A. Dysmenorrhea among adolescent girls in selected schools at mangalore with view to develop and distribute an information booklet. IOSR-JNHS. 2015;4(1):34–39.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
