Back to Journals » Hepatic Medicine: Evidence and Research » Volume 15
Thrombocytopenia (TCP), MELD Score, and Fibrosis Index (FI) Among Hospitalized Patients with Chronic Liver Disease (CLD) in Ma’abar City, Dhamar Governorate, Yemen: A Cross-Sectional Study
Authors Al-Dholae MHH, Salah MK, Al-Ashmali OY , Al Mokdad ASM, Al-Madwami MA
Received 21 October 2022
Accepted for publication 24 April 2023
Published 28 April 2023 Volume 2023:15 Pages 43—50
DOI https://doi.org/10.2147/HMER.S392011
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gerry Lake-Bakaar
Mohammed Haidar Hazaa Al-Dholae,1 Mohammed Kassim Salah,1 Omar Yahya Al-Ashmali,2 Abdul Salam Mohamed Al Mokdad,1 Mohammed Ali Al-Madwami1
1Department of Internal Medicine, Faculty of Medicine & Health Sciences, Thamar University, Dhamar, Yemen; 2Department of Pediatrics, Al-Wahda Teaching Hospital, Thamar University, Ma’abar City, Dhamar Governorate, Yemen
Correspondence: Omar Yahya Al-Ashmali, Department of Paediatrics, Al-Wahda Teaching Hospital, Thamar University, Ma’abar City, Dhamar Governorate, Yemen, Tel +967777638063, Email [email protected]
Purpose: This study sought to assess the prevalence of thrombocytopenia (TCP), underlying aetiologies of chronic liver disease, and the grading and prognostic systems for chronic liver disease (CLD) using non-invasive biomarkers: the Fibrosis index and the Model for End-Stage Liver Disease-Na (MELD-Na) Score, respectively.
Patients and Methods: This was a 15-month multi-centric cross-sectional study of 105 patients with chronic liver disease (CLD). The study was conducted using Sept 2019 to Nov 2020 admission records of CLD patients from Ma’abar City in Dhamar Governorate, Yemen.
Results: A total of 63 (60%) and 42 (40%) patients were identified as thrombocytopenic and non-thrombocytopenic, respectively. The means ± SD of the MELD score and FI were 19 ± 7.302 and 4.1 ± 1.06. TCP prevalence among leukopenic and non-leukopenic patients was 89.5% and 53.5%, respectively (P = 0.004). Likewise, the prevalence of traditional-ultrasonography-diagnosed cirrhotic patients needing liver transplantation (LT) was 82.3% versus 61.3% among corresponding non-cirrhotic patients (P = 0.000).
Conclusion: The prevalence of TCP among the participants of this study was similar to the global rate. However, the prevalence of decompensation was much higher among CLD patients than that found elsewhere, highlighting a need to improve methods for the early diagnosis of CLD in Yemen. This study also identified problems with the diagnostic work-up for non-infectious aetiologies of CLD. The findings suggest the need to improve clinician awareness about effective diagnostic strategies for these aetiologies.
Keywords: liver transplantation, cirrhosis, liver failure, Qat
Introduction
Chronic liver disease (CLD) patients with a platelet count <150 x 10^9/L are diagnosed with thrombocytopenia (TCP), a condition affecting 6–78% of CLD patients globally.1 Mild TCP, defined as a platelet count of 100,000–150,000/μL, and moderate TCP, defined as a platelet count of 50,000–100,000/μL, rarely contraindicate the management of haemostasis issues.2 However, on considering the former measures in CLD patients including investigative invasive ones (such as liver biopsy). There is conflicting data about the platelet count of CLD patients with moderate-to-severe TCP needed to indicate platelet transfusion during invasive procedures.3,4 In general, a platelet count of <50,000/μL indicates transfusion.5
The Child-Turcotte-Pugh score was primarily designed to assess cirrhosis severity in CLD patients to prepare for portal hypertension surgery and subjectively prioritize LT recipients based on the contribution of the parameter, encephalopathy, and the lack of a renal function test.6–8 Meanwhile, the model for end-stage liver disease (MELD) score system was primarily designed to predict 1-month mortality in cirrhotic patients receiving a trans jugular intrahepatic portosystemic shunt (TIPS).9 Serum sodium levels were later added, changing the MELD score into the MELD-Na score, and providing a more accurate estimation of disease severity in organ allocation.10,11 In 2006, Ohta et al developed the fibrosis index (FI) to estimate the histological stage of hepatic fibrosis.12
The current study sought to assess TCP prevalence, including the various underlying aetiologies of CLD, and the grading and prognostic systems using non-invasive biomarkers: the FI and the MELD-Na score, respectively.
Materials and Methods
This was a 15-month multi-centric cross-sectional study involving 105 CLD patients. The study was performed on Sept 2019 to Nov 2020 admission registries of CLD patients from Ma’abar City in Dhamar Governorate, Yemen. Descriptive variables, including gender, Qat use habit, aetiology, anaemia, leukopenia, pancytopenia, infectious state, traditional-ultrasonography-diagnosed liver cirrhosis, liver failure, ALT value status, compensation, TCP, and LT listing were obtained and represented as frequency and percentage by MELD- Na score. The MELD-Na score and FI were represented as medians and standard deviations.
Personal history regarding using Qat (khat, miraa, or Catha edulis Forsska), a cathinone-containing alkaloid plant with green leaves that is masticated for its transient stimulant effect,13 was also considered. The survey included general and systemic examinations, as well as blood testing. Patients were diagnosed with decompensated chronic liver disease using the results of both clinical and radiological workups. Clinical parameters included signs of liver failure, while radiological parameters included “traditional-ultrasonography-diagnosed hepatic cirrhosis” with portal hypertension and splenomegaly.
Confidence intervals of 90% for the prevalence of TCP among CLD patients were calculated. Using a confidence level of 90%, a margin of error of 8%, a power of 80%, and a hypothesis of no difference, the minimum required sample size was 103. The demographic and pathological characteristics were compared by thrombocyte count and statistically tested using the Chi-Square test of independence (post-Pearson Chi-square and Fisher’s exact tests). LT listing (according to the MELD-Na score), MELD-Na score, and FI were compared with traditional-ultrasonography-diagnosed liver cirrhosis calculated using the Median test.
Patients were grouped into F4 and F0–F3 fibrosis stages, which represented cirrhosis positivity and negativity according to the histopathological criteria for grading liver fibrosis designated by Desmet et al.14 LT listing was defined according to the MELD-Na score into yes (scores >14) and no (scores ≤14) groups.15 The MELD-Na score and FI were calculated according to the formulas adopted by Kim et al and Ohta et al, respectively,12,16,17 using the website, “MD+CALC”18 (Table 1).
 |
Table 1 Formulas Used to Calculate Non-Invasive CLD Biomarkers |
Ethics Approval and Informed Consent
This study was approved by the Thamar University Medical Ethics Committee (TUMEC-19031) in Dhamar, Yemen, according to the guidelines outlined in the Declaration of Helsinki. National-law-compatible, verbally informed consent for participation was obtained from each respondent. Written informed consents to publish were inapplicable.
Results
Participant distribution by gender, Qat use, underlying etiology, compensation status, traditional-ultrasonography-diagnosed liver fibrosis, splenic size, hepatic failure, anaemia, leukopenia, pancytopenia, raised ALT, and TCP is shown in detail in Table 2 and Table 3. The MELD- Na score, FI, and LT listing (according to MELD Na-score) as a median and standard deviation and frequency and percentage, respectively, are shown in Table 4. Of the 105 participants, 60 (57%) and 45 (43%) were males and females, respectively, 51 (48.57%) and 54 (51.4%) were Qat-using and Qat-not-using cases, respectively, 30 (28%), 29 (27.6%), 24 (22.8%), 14 (13.3%), and 8 (7.6%) were undiagnosable, or had autoimmune hepatitis, HCV hepatitis, HBV hepatitis, and schistosomiasis, respectively, 69 (66%) and 36 (34%) had splenomegaly and normal spleen size, respectively, 88 (84%) and 17 (16%) were cirrhotic and non-cirrhotic, respectively, 95 (90%) and 10 (10%) had or did not have liver failure, respectively, 66 (63%) and 39 (37%) were anaemic and non-anaemic, respectively, 86 (82%) and 19 (18%) were leukopenic and non-leukopenic, respectively, 88 (84%) and 17 (16%) were non-pancytopenic and pancytopenic, respectively, 67 (64%) and 38 (38%) were associated with normal ALT and elevated ALT levels, respectively, and 63 (60%) and 42 (40%) were thrombocytopenic and non- thrombocytopenic, respectively (Table 3). The medians ± SD of the MELD- Na score and FI were 19 ± 7.302 and 4.1 ± 1.06, respectively, and a total of 68 (65%) participants had a MELD-Na score >14, requiring LT listing (Table 4).
 |
Table 2 Demographic and Pathological Characteristics of the Study Population |
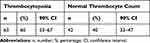 |
Table 3 Distribution of Thrombocytopenia |
 |
Table 4 MELD Score, Fibrosis Index, and Urgent LT Listing by MELD Score Compared with Traditional-Ultrasonography- Diagnosed Liver Fibrosis |
A comparison of the cases by each descriptive variable was determined for those with and without TCP (Table 5). A total of 89.5% and 55.8% of the leukopenic patients with CLD and participants with hepatic failure had TCP, respectively. The median ± SD of the MELD-Na score and FI was 19 ± 7.302 and 4.1 ± 1.06. Leukopenic and non-leukopenic participants had an 89.5% and 53.5% prevalence of TCP, respectively (Table 5; P = 0.004). Likewise, traditional-ultrasonography-diagnosed cirrhotic and non-cirrhotic participants had an LT listing of 82.4% and 61.3%, respectively (Table 4; P = 0.000).
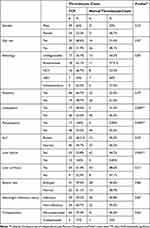 |
Table 5 Patient Demographic and Pathological Characteristics by Thrombocyte Count |
Discussion
The distribution of CLD by gender was similar between this and prior studies. This study assessed the influence of Qat use, a habit that is unique to Yemeni people, hypothesizing that using Qat would have no impact on the development of CLD or the prevalence of TCP among CLD patients. The findings showed that the proportion of participants who used Qat was slightly higher than the percentage who did not, however, this difference was not significant (P = 0.767).
The prevalence of decompensation in this study was significantly higher than previously reported, perhaps due to fewer early diagnoses of CLD in Yemen. While approximately 4–12% of cirrhotic patients develop one or more manifestations of decompensation each year, including ascites, variceal bleeding, and hepatic encephalopathy,19–23 96% of the participants in our study were decompensated (P = 0.000).
The aetiology of CLD could not be determined for most of the participants in this study, and 28% of cases were designated as undiagnosable. This group is analogous to the global designation of non-alcoholic fatty liver disease (NAFLD), which accounts for most CLD cases worldwide.24,25 About 24% of CLD patients are diagnosed with NAFLD globally, with significant geographic heterogeneity ranging from 13.5% in Africa to 30.5% in South America, 31.8% in the Middle East, and 33.9% in Asia.24,25
TCP was highly prevalent in this and prior worldwide studies. While the current study reported that 60% of the participants had TCP (95% CI, 53–67%), other studies have reported that 76% of CLD patients have TCP.15 In contrast, the median FI and MELD-Na scores were significantly higher in the current than in prior studies,12,16,26 likely due to lack of early diagnoses. The current study also showed no significant difference between the median FI of traditional-ultrasonography-diagnosed cirrhotic participants and corresponding non-cirrhotic patients. This may be due to the low sensitivity of traditional ultrasonography, which is dependent on liver parenchyma echogenicity and surface, for cirrhotic significant liver fibrosis (ie, F4 grade) and lack of use of newer elastographic ultrasonography and elastographic MRI.27
Prior studies have proposed that TCP results from platelet sequestration in the enlarged spleen due to portal hypertension,28,29 however, this study showed no significant difference in the occurrence of TCP between splenomegaly-associated CLD cases and those with normal spleen size (P = 0.86). These findings, and those of other studies, suggest that the aetiology of TCP may be multifactorial.30 In addition, neither conservative nor surgical management of portal hypertension is shown to correct this condition.31,32 It is worth noting that TCP can occur as a result of pancytopenia, which affected up to 16% of participants in the current study. TCP may also be associated with splenic sequestration, defective platelet production that is either secondary to viral hepatitis or alcohol toxicity, or anti-platelet antibodies.33–36 In addition, TCP correlates with CLD stage.15 The current study found a higher prevalence of TCP among CLD cases with full-blown liver failure than those without (100% vs 53%). Similarly, Gallus et al identified a higher prevalence of TCP among acute hepatitis patients with liver failure than those without (52% vs 16%) (1972).37
Conclusion
The prevalence of TCP among CLD patients in the Ma’abar District of Dhamar Governorate, Yemen, was similar to that reported worldwide. However, this population had a much higher prevalence of decompensation than previously reported, highlighting the need for early diagnosis of CLD patients in Yemen. While Qat use was not found to be a precipitating factor for TCP among CLD patients, additional cohort studies are needed to further study the potential relationship between Qat use and chronic liver disease. The current study also showed that the work-up used to diagnose non-infectious aetiologies of CLD is ineffective. Thus, it would be advised to build a program that could improve clinician awareness about the diagnostic strategies needed to identify CLD aetiologies. It would also be worth considering the use of highly sensitive Doppler ultrasound techniques, such as the splenic artery pulsatile and portal vein congestion indexes, in place of traditional liver parenchyma echogenicity ones, hiring newer imaging modalities, such as elastography ultrasound and elastography MRI, to evaluate liver fibrosis, establishing hepatology specialized centres dedicated to LT, and creating an MPHP-organized organ allocating system for LT listing that includes both deceased- and living-donation options.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mitchell O, Feldman DM, Diakow M, Sigal SH. The pathophysiology of thrombocytopenia in chronic liver disease. Hepat Med. 2016;8:39–50. PMID: 27186144. doi:10.2147/HMER.S74612
2. Gallo P, Terracciani F, Di Pasquale G, Esposito M, Picardi A, Vespasiani-Gentilucci U. Thrombocytopenia in chronic liver disease: physiopathology and new therapeutic strategies before invasive procedures. World J Gastroenterol. 2022;28(30):4061–4074. doi:10.3748/wjg.v28.i30.4061
3. Northup PG, Garcia-Pagan JC, Garcia-Tsao G, et al. Vascular liver disorders, portal vein thrombosis, and procedural bleeding in patients with liver disease: 2020 practice guidance by the American Assoc. iation for the study of liver diseases. Hepatology. 2021;73:366–413. PMID: 33219529. doi:10.1002/hep.31646]
4. Ronca V, Barabino M, Santambrogio R, et al. Impact of platelet count on perioperative bleeding in patients with cirrhosis undergoing surgical treatments of liver cancer. Hepatol Commun. 2022;6:423–434. PMID: 34716696. doi:10.1002/hep4.1806]
5. Ben-Menachem T, Decker GA, Early DS, et al; ASGE Standards of Practice Committee. Adverse events of upper GI endoscopy. Gastrointest Endosc. 2012;76:707–718. PMID: 22985638. doi:10.1016/j.gie.2012.03.252
6. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi:10.1002/bjs.1800600817
7. Cholongitas E, Marelli L, Shusang V, et al. A system- atic review of the performance of the model for end-stage liver disease (MELD) in the setting of liver transplantation. Liver Transpl. 2006;12:1049–1061. doi:10.1002/lt.20824
8. Wiesner RH. Patient selection in an era of donor liver shortage: current US policy. Nat Clin Pract Gastroenterol Hepatol. 2005;2:24–30. doi:10.1038/ncpgasthep0070
9. Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi:10.1053/he.2000.5852
10. Kalra A, Wedd JP, Biggins SW. Changing prioritization for transplantation: MELD-Na, hepatocellular carcinoma, exceptions and more. Curr Opin Organ Transpl. 2016;21(2):120–126. doi:10.1097/MOT.0000000000000281
11. Sharma P, Schaubel DE, Goodrich NP, et al. Serum sodium and survival benefit of liver transplantation. Liver Transpl. 2015;21(3):308–313. PubMed: 25504743. doi:10.1002/lt.24063
12. Ohta T, Sakaguchi K, Fujiwara A, et al. Simple surrogate index of the fibrosis stage in chronic hepatitis C patients using platelet count and serum albumin level. Acta Med Okayama. 2006;60(2):77–84. doi:10.18926/AMO/30729
13. Kennedy JG. The Flower of Paradise: The Institutionalized Use of the Drug Qat in North Yemen. Dordrecht, The Netherlands: D Reidel Publishing Company; 1987.
14. Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19(6):1513–1520. doi:10.1002/hep.1840190629
15. Giannini EG. Review article: thrombocytopenia in chronic liver disease and pharmacologic treatment options. Aliment Pharmacol Ther. 2006;23(8):1055–1065. PMID: 16611265. doi:10.1111/j.1365-2036.2006.02889.x
16. Díaz LA, Norero B, Lara B, et al. Prioritization for liver transplantation using the MELD score in Chile: inequities generated by MELD exceptions: a collaboration between the Chilean Liver Transplant Programs, the Public Health Institute and the National Transplant Coordinator. Ann Hepatol. 2019;18(2):325–330.
17. Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359(10):1018–1026. PubMed:18768945. doi:10.1056/NEJMoa0801209
18. Graham Walker 2005, MD Calc Website, Evidence-Based Medicine journal https://mdcalc.com.
19. Mellinger JL, Shedden K, Winder GS, et al. The high burden of alcoholic cirrhosis in privately insured persons in the United States. Hepatology. 2018;68(3):872–882. doi:10.1002/hep.29887
20. D’Amico G, Morabito A, D’Amico M, et al. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68(3):563–576. doi:10.1016/j.jhep.2017.10.020
21. D’Amico G, Pasta L, Morabito A, et al. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther. 2014;39(10):1180–1193. doi:10.1111/apt.12721
22. Ratib S, Fleming KM, Crooks CJ, et al. Causes of death in people with liver cirrhosis in England compared with the general population: a population-based cohort study. Am J Gastroenterol. 2015;110(8):1149–1158. doi:10.1038/ajg.2015.191
23. Fleming KM, Aithal GP, Card TR, et al. The rate of decompensation and clinical progression of disease in people with cirrhosis: a cohort study. Aliment Pharmacol Ther. 2010;32(11–12):1343–1350. doi:10.1111/j.1365-2036.2010.04473.x
24. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease–meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi:10.1002/hep.28431
25. Li J, Zou B, Yeo YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4:389–398. doi:10.1016/S2468-1253(19)30039-1
26. Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–470. PMID: 1117235. doi:10.1053/jhep.2001.22172
27. Yeom SK. Prediction of liver cirrhosis, using diagnostic imaging tools. World J Hepatol. 2015;7(17):2069–2079. doi:10.4254/wjh.v7.i17.2069
28. Realdi G, Fattovich G, Hadziyannis S, et al. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. J Hepatol. 1994;21(4):656–666. doi:10.1016/S0168-8278(94)80115-0
29. Liangpunsakul S, Ulmer BJ, Chalasani N. Predictors and implications of severe hypersplenism in patients with cirrhosis. Am J Med Sci. 2003;326(3):111–116. doi:10.1097/00000441-200309000-00001
30. Afdhal N, McHutchison J, Brown R, et al. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48(6):1000–1007. PMID: 18433919. doi:10.1016/j.jhep.2008.03.009
31. Mutchnik MG, Lerner E, Conn HO. Effect of portacaval anastomosis on hypersplenism. Dig Dis Sci. 1980;25(12):929–938. doi:10.1007/BF01308044
32. Jabbour N, Zajiko A, Orons P, Irish W, Fung JJ, Selby RR. Does transjugular intrahepatic portosystemic shunt (TIPS) resolve thrombocytopenia associated with cirrhosis? Dig Dis Sci. 1998;43:2459–2462. doi:10.1023/A:1026634215918
33. Ballard HS. Hematological complications of alcoholism. Alcohol Clin Exp Res. 1989;13:706–720. doi:10.1111/j.1530-0277.1989.tb00408.x
34. Bordin G, Ballare M, Zigrossi P, et al. A laboratory and thrombokinetic study of HCV-associated thrombocytopenia: a direct role of HCV in bone marrow exhaustion? ClinExpRheumatol. 1995;13(Suppl. 13):S39–S43.
35. Nagamine T, Ohtuka T, Takehara K, Arai T, Takagi H, Mori M. Thrombocytopenia associated with hepatitis C viral infection. J Hepatol. 1996;24:135–140. doi:10.1016/S0168-8278(96)80021-3
36. Pockros PJ, Duchini A, McMillan R, Nyberg LM, McHutchison J, Viernes E. Immune thrombocytopenic purpura in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 2002;97:2040–2045. doi:10.1111/j.1572-0241.2002.05845.x
37. Gallus AS, Lucas CR, Hirsch J. Coagulation studies in patients with acute infectious hepatitis. Br J Haematol. 1972;22:761–767. doi:10.1111/j.1365-2141.1972.tb05720.x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
