Back to Journals » Journal of Inflammation Research » Volume 15
Thrombo-Inflammatory Prognostic Scores Improve BISAP-Based Risk Stratification in Acute Pancreatitis Patients: A Retrospective Cohort Study
Authors Han T, Cheng T, Liao Y, Lai Q, Tang S, Liu B, He Y, Lei C, Cao Y, Cao Y
Received 18 March 2022
Accepted for publication 28 May 2022
Published 4 June 2022 Volume 2022:15 Pages 3323—3335
DOI https://doi.org/10.2147/JIR.S366246
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Zili You
Tianyong Han,1,* Tao Cheng,1,* Ye Liao,2 Qiang Lai,1 Shiyuan Tang,1 Bofu Liu,1 Yarong He,1 Chenxi Lei,1 Yuling Cao,3 Yu Cao1
1Emergency Department, West China Hospital of Sichuan University, Chengdu, Sichuan, People’s Republic of China; 2Medical Intensive Care Unit, West China Hospital of Sichuan University, Chengdu, Sichuan, People’s Republic of China; 3Operations Management Department, West China Hospital of Sichuan University, Chengdu, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yu Cao, Emergency Department, West China Hospital of Sichuan University, 37 Guoxue Lane, Wuhou District, Chengdu, Sichuan, 610041, People’s Republic of China, Tel +86 28-85422288, Email [email protected]
Purpose: The thrombo-inflammatory prognostic score (TIPS) and the bedside index for severity in acute pancreatitis (BISAP) are both scoring systems that enable the rapid prognostic assessment of early-stage acute pancreatitis (AP) patients, but the overall prognostic utility of these individual systems is limited. This study was thus developed to explore whether a combination of TIPS and BISAP scores would offer better insight to facilitate the risk stratification of AP patients.
Methods: This single-center retrospective cohort research evaluated AP cases referred to the emergency department from January 1, 2017 to September 30, 2017. The ability of TIPS scores to improve BISAP-based AP patient risk stratification was appraised employing the curves of receiver-operating characteristic (ROC) and decision curve analysis (DCA) approaches. The initial endpoint for this research was 28-day mortality, while secondary endpoints comprised intensive care unit admission (AICU) and mechanical ventilation (MV) over a 28-day follow-up period.
Results: Totally, 440 cases enrolled in the current study were divided at a ratio of 1:1 to derivation and validation cohorts. When estimating 28-day mortality, the combination of TIPS and BISAP (T-BISAP) improved the area under the curve (AUC) value in the derivation group from 0.809 to 0.903 (P < 0.05), in addition to similarly improving this AUC value from 0.709 to 0.853 (P < 0.05) in the validation cohort. Moreover, T-BISAP significantly improved the AUC values for 28-day AICU from 0.751 to 0.824 (P < 0.05) and the AUC values for 28-day MV from 0.755 to 0.808 (P < 0.05). A DCA approach revealed T-BISAP to exhibit higher net benefit when used for patient risk stratification as compared to BISAP alone.
Conclusion: The addition of TIPS scores to BISAP scores can enable prediction of 28-day adverse clinical outcomes with AP patients in the ED.
Keywords: acute pancreatitis, prognosis, thrombo-inflammatory prognostic score, TIPS, bedside index for severity in acute pancreatitis, BISAP, risk stratification
Introduction
Acute pancreatitis (AP) is a serious inflammatory circumstance of the pancreas that is able to develop and progress rapidly.1,2 While current understanding of the etiological basis for this condition is increasingly robust, it remains a major threat to global public health.3 The revised Atlanta classification stratifies AP patients into three groups based on overall disease severity: severe AP (SAP), moderately severe AP (MSAP), and mild acute AP (MAP).4 A majority of patients present with MAP or MSAP, with just 15–20% presenting with SAP.5 However, the rapid and accurate risk stratification of AP patients based upon apparent severity at presentation is critical to guiding patient management and has the potential to ensure that appropriate treatments or intensive care be administered so as to maximize the odds of positive outcomes.6,7 Predictive tools that can rapidly and accurately gauge the severity and prognosis of individuals with AP are thus essential to guide patient management efforts.7
Various scoring systems have been developed and leveraged for the prediction of health outcomes in patients with AP, including the Acute Physiology and Chronic Health Evaluation II (APACHE-II), Bedside Index of Severity in Acute Pancreatitis (BISAP), and Ranson’s scoring systems. While the APACHE-II and Ranson’s scores provide a good degree of both specificity and sensitivity, they are time-consuming and difficult to implement, making their use unrealistic for AP patient evaluation in most contexts given the need for rapid clinical decision-making. The BISAP score has been extensively studied as a tool capable of accurate early-stage PA patient risk stratification,8 but it often exhibits suboptimal predictive specificity and sensitivity when utilized at an individual level.
Aberrant inflammatory and coagulatory activities are related to the pathogenesis of several diseases.9–11 Our team has found that thrombo-inflammatory prognostic score (TIPS), values, which are established based on the levels of the most abundant inflammatory cytokine of prognostic relevance, interleukin-6 (IL-6), and the thrombotic biomarker prothrombin time (PT) can be applied for the prediction of adverse information in AP cases.12 The BISAP scoring system does not evaluate inflammatory or coagulation-related parameters in AP patients at present, thus potentially leading to the loss of valuable prognostic information.
In light of the above factors, we developed this study to test whether the addition of the TIPS score to the BISAP score would be advantageous in facilitating the more accurate risk stratification of early-stage AP cases.
Materials and Methods
Study Design
The present single-center retrospective cohort research was developed to examine whether TIPS scores would improve BISAP-based AP patient risk stratification during the initial stages of disease for individuals admitted to the emergency department (ED). Included cases were categorized into validation and derivation cohorts at a ratio of 1:1 in order to improve the reliability and accuracy of the resultant analyses. Patients in the derivation cohort were utilized to construct a stratification model based upon a combination of the TIPS and BISAP scoring systems (T-BISAP), while the validation cohort was used to explore the validity of the developed T-BISAP model. This study was confirmed through the Human Ethical Committee of West China Hospital of Sichuan University (No.2019–334) and was consistent with the Declaration of Helsinki. Because of its retrospective nature, the requirement for informed consent to review medical records was waived by the ethical committee. All the clinical information of the patients was confidential, and the data were analyzed anonymously.
The contributors presented the letter of informed satisfaction to take part in.
Study Population
This research retrospectively enrolled AP cases admitted to the West China Hospital of Sichuan University ED between January 1, 2017 and September 30, 2017. The eligible cases for contribution were those fulfilling the following criteria: (1) age ≥18 years, (2) patients met the revised Atlanta Classification (2012) guidelines for AP diagnosis,4 and (3) patients had not been previously diagnosed with AP. Patients were excluded if they had: (1) chronic pancreatitis, (2) AP due to surgery, trauma, or poisoning, (3) a history of malignancies, (4) postoperative pancreatic lesions; (5) AP complicated by chronic hepatic diseases or renal insufficiency, (6) systemic hematological diseases, (7) were pregnant or in the perinatal period, (8) had been treated with anticoagulant drugs prior to admission, (9) exhibited venous thrombosis events upon admission, (10) had incomplete clinical trial indicators, or (11) were missing follow-up data.
Data Collection and Quality Control
To ensure quality control, all individuals responsible for data collection were trained in appropriate data collection protocols at the start of the study so that accurate data would be entered into the study database. Collected data included demographic information, vitals, and laboratory findings, all of which were obtained from patient medical records. Demographics including patient age, sex, etiology, and body mass index (BMI). Vitals at admission included respiratory rate (RR), temperature (T), mean arterial pressure (MBP), and heart rate (HR). Laboratory results included potential of hydrogen (PH), white blood cell (WBC) count, platelet (PLT), interleukin-6 (IL-6), hematocrit (HCT), hemoglobin (HB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), albumin, creatinine (Cr), blood urea nitrogen (BUN), amylase (AMY), cholesterol (CHOL), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), activated partial thromboplastin time (APTT), lipase (LIP), calcium (Ca), prothrombin time (PT), procalcitonin (PCT), C-reactive protein (CRP), and lactate (LAC) measurements. Baseline patient results after admission were used to compute APACHE-II, TIPS, and BISAP scores for these patients.
Endpoints and Follow-Up
Following ED admission, AP patients were administered conventional treatments. The 28-day outcomes for these patients were determined through medical record review and telephone calls as appropriate. The initial study information was all-cause mortality within 28 days of ED admission. Secondary outcomes included ICU admission (AICU) and mechanical ventilation (MV) within the 28-day follow-up period. AICU was defined based upon patients being admitted to the ICU because of severe dystrophy, unstable vitals, acute hydroelectrolyte imbalance, as well as a need for organ supports. MV was defined by the need for non-invasive or invasive ventilation owing to respiratory failure.13
TIPS and BISAP Score Calculations
TIPS values were calculated based upon IL-6 and PT levels, with patients exhibiting IL-6 levels ≥78 pg/mL and PT ≥ 13.65 s being considered a score of 2, while cases with only 1 or 0 of these values above the indicated thresholds were, respectively, assigned a score of 1 and 0.12 BISAP scores (0–5) were based upon the following variables, each of which was assigned 1 point: elevated BUN (>25 mg/dl), age >60 years, development of systemic inflammatory response syndrome (SIRS), impaired mental status (Glasgow coma scale score <15), and the existence of pleural effusion.14
Statistical Analysis
Initially, data were subjected to filtering and quality control analyses. To determine whether continuous variables were normally distributed, the Kolmogorov–Smirnov test was utilized, with data that did conform to the normal distribution being given as means ±standard deviations and compared with Student’s t-tests, while all other continuous variables were given as medians (interquartile range) and compared using Mann–Whitney U-tests. Categorical variables were given as frequencies (proportions) and compared with χ2 or Fisher’s exact test as appropriate.
We began by comparing TIPS and BISAP scores among patient groups with different adverse clinical outcomes. To determine whether combining TIPS and BISAP scores was associated with more reliable AP patient risk stratification, the T-BISAP model was constructed through correlation analyses conducted through Pearson correlation and logistic regression assessment. The curves of receiver operating characteristic (ROC) were then employed for comparing the predictive value of the T-BISAP and BISAP models on the basis of the area under the ROC curve (AUC) when predictive adverse clinical outcomes within 28 days. These AUC values were compared with the DeLong test. A decision curve analysis (DCA) was additionally executed to explore the clinical benefit associated with the T-BISAP model. A two-sided P < 0.05 was the threshold of significance for the analyses. SPSS v 26.0 (IBM, NY, USA), MedCalc ® Statistical computer program v 19, and R V.3.5.1 (R Foundation for Statistical Computing, Vienna, Austria) were employed for all statistical assessments.
Results
Patient Properties and Clinical Findings
In total, 440 patients (267 male, 60.88%) were considered in the present research, with an average age of 48.48±14.89 years. Over the 28-day follow-up period, 27 patients (6.14%) died. Enrolled cases were divided at a ratio of 1:1 to derivation and validation groups (n = 220 each) with SPSS 26.0, with no substantial discrepancies in baseline patient features, laboratory findings, AP etiology, AP severity, or endpoint outcomes between groups (Table 1).
 |
Table 1 Basic Characteristics of the Derivation Group and the Validation Group |
Derivation Group Participant Characteristics
The average age of the 220 cases in the derivation group (59.1% male) was 48.42±14.33 years. Of these patients, 14 died during the 28-day follow-up period, and baseline characteristics were then compared between patients that did and did not experience mortality during this period as shown in Table 2.
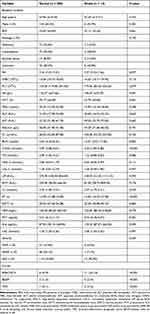 |
Table 2 Characteristics of Patients in the Derivation Group |
Comparison of BISAP and TIPS Scores in AP Patients That Experienced Clinical Adverse Outcomes
In the derivation cohort, rates of adverse clinical outcomes rose with rising TIPS and BISAP scores. When comparing these scores between patients that did and did not die during the follow-up period, both the TIPS and BISAP scores were greater in the deceased patient group relative to the surviving patient group (Figure 1A and B, P < 0.05). We additionally divided AP patients into AICU and non-AICU groups based upon whether or not they were admitted to the ICU within a 28-day period. The results of these analyses indicated that both TIPS and BISAP scores were considerably greater in the AICU group relative to the non-AICU group (Figure 1C and D, P < 0.05). Consistently, these two scores were notably greater in the MV group relative to the non-MV group (Figure 1E and F, P < 0.05).
Correlations Between the T-BISAP Scores and Adverse Clinical Outcomes
Correlation assessments were next executed to examine the association between T-BISAP scores and AP patient clinical outcomes using Pearson χ2-tests. A substantial positive association was detected between T-BISAP scores and 28-day mortality (r = 0.389, P < 0.001), AICU (r = 0.436, P < 0.001), and MV (r = 0.395, P < 0.001), with this correlation being stronger than that for either TIPS or BISAP scores individually (Table 3).
 |
Table 3 Correlation Analysis |
Logistic Regression Analyses
In univariate logistic regression assessments, T-BISAP scores were significantly associated with the risk of adverse AP patient clinical outcomes. To confirm the relevance of this relationship, a multivariate analysis was conducted after adjusting for potential confounding variables, revealing T-BISAP scores on admission to be independent predictors of adverse AP patient outcomes including 28-day mortality (OR = 2.894, 95% CI 1.705–4.914, P < 0.001), AICU (OR = 3.040, 95% CI 2.022–4.570, P < 0.001), and MV (OR = 3.018, 95% CI 1.989–4.579, P < 0.001). For further details regarding these results, see Table 4.
 |
Table 4 Univariable and Multivariable Logistic Regression Analyses of the T-BISAP Associated with the Clinical Adverse Outcomes in Patients with AP |
The Value of T-BISAP Scores as Predictors of AP Patient 28-Day Mortality
The curves of ROC were next constructed to assess the capability of T-BISAP scores to predict AP patient 28-day mortality in the derivation cohort (Figure 2). This analysis revealed that the addition of TIPS scores to BISAP scores led to a significant improvement in AUC values from 0.809 to 0.903 (P = 0.0088). While BISAP scores exhibited good specificity (84.47%) but worse sensitivity (57.14%), T-BISAP scores exhibited excellent specificity and sensitivity values of 83.50% and 85.71%, respectively (Table 5).
 |
Table 5 ROC Curve Analysis of Predicting Prognosis of Patients with AP in the Derivation Group |
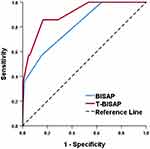 |
Figure 2 ROC curves analysis for predicting 28-day mortality. |
Validation of the Value of T-BISAP Scores as Predictors of AP Patient 28-Day Mortality
Next, we calculated AUC values for patients in the validation group to greater confirm the clinical usefulness of adding the TIPS and BISAP scores when predicting AP patient 28-day mortality. Relative to the BISAP scores, T-BISAP scores were associated with higher AUC values when predicting 28-day mortality (AUC: 0.853 vs 0.709, ∆AUC = 0.144, P = 0.0265, Figure 3A), AICU (AUC: 0.824 vs 0.751, ∆AUC = 0.073, P = 0.0113, Figure 3B), and MV (0.808 vs 0.755, ∆AUC = 0.053, P = 0.0277, Figure 3C) relative to BISAP alone. A DCA approach further confirmed that T-BISAP was associated with greater net benefit than BISAP scores over a wide range of threshold probabilities (Figure 4).
Discussion
Acute pancreatitis remains a growing threat to global public health, with an estimated 300,000 ED visits annually in the USA alone and rising incidence rates.6,15 Prior research has demonstrated that AP patient outcomes are influenced by the ability to effectively stratify these patients according to risk levels.6 Accordingly, the Rason’s, BISAP, and APACHE-II scoring systems were developed to differentiate between those patients that are and are not likely to develop SAP in order to guide clinical patient management.16 Each of these scales, however, is subject to certain limitations.17 An optimal prognostic scale must be inexpensive, extremely accurate, rapid, and reproducible.18 There thus remains a major unmet need to design a risk stratification system capable of identifying high-risk AP patients so as to more effectively guide their clinical treatment.
In prior reports, systemic changes in inflammatory and coagulatory activity have been observed in AP patients and closely linked to high rates of mortality in this patient population. We have previously conducted a retrospective single-center analysis of value of TIPS scores, which are on the basis of the inflammatory and thrombotic biomarkers, for the prediction of the negative AP patient outcomes upon admission to the ED.12 This analysis revealed that TIPS scores could effectively gauge the elevated risk of multiple adverse clinical outcomes over a 28-day period in this patient population.
Japanese guidelines currently recommend that BISAP scores be utilized to grade AP severity.19 Indeed, multiple studies have shown these BISAP scores to be accurate predictors of elevated mortality risk among AP patients.20,21 One key advantage of the BISAP scoring system is that these values can be rapidly calculated to permit efficient patient stratification based upon disease severity in the clinic.22 Silva-Vaz et al20 calculated AUC values of 0.786 and 0.970 for the BISAP score at admission and 48 h, respectively, when used to predict mortality, suggesting that it is a less reliable predictor upon patient admission. Other studies have also noted that BISAP scores are limited by their inability to differentiate among individuals suffering from temporary organ dysfunction and those exhibiting long-term organ dysfunction, potentially overestimating disease severity.23
In prior reports, combinations of scoring systems have similarly been shown to increase their prognostic utility when assessing AP patients to gauge the risk of adverse clinical outcomes. We found both BISAP and TIPS scores to be independently related to the odds of 28-day mortality in individuals with AP, and we confirmed that the odds of adverse clinical outcomes rose as these scores increased. The TIPS score consists of biomarkers of inflammation (IL-6) and coagulation (PT), both of which have the potential to predict poor AP patient outcomes yet are absent from the BISAP scoring system. No study to our knowledge has previously evaluated the prognostic utility of combining TIPS and BISAP scores when evaluating patients with AP. We therefore hypothesized that TIPS may offer value as a means of improving our ability to estimate adverse clinical information among AP cases admitted to the ED.
Consistent with our hypothesis, we found that TIPS scores significantly improved the ability of BISAP scores to gauge the risk of adverse outcomes among AP patients, significantly improving the AUC value for predicting 28-day mortality over that for BISAP scores alone in our derivation cohort. Moreover, we found T-BISAP scores to improve sensitivity relative to BISAP scores alone while maintaining excellent specificity when evaluating this primary study endpoint.
To confirm the accuracy of our findings, we repeated these analyses in our validation cohort and found that this did not result in any change in our overall conclusions. When comparing the relative performance of T-BISAP and BISAP systems as a means of predicting patient 28-day mortality, T-BISAP exhibited significantly better predictive performance as evidenced by an improved AUC value (P < 0.05). We additionally used a DCA approach to evaluate the utility of this model as a tool for estimating AP patient mortality, and found T-BISAP to exhibit a higher net benefit than BISAP alone at all threshold probability levels. This thus confirms the clear clinical utility of T-BISAP as a tool for predicting survival outcomes among individuals with AP that is superior to BISAP alone.
Patients with SAP often exhibit high mortality rates tied to multiple organ failure.24 The early admission to the ICU can provide improved odds of survival to those SAP patients necessitating organ support.25 Given limitations in ICU space, it is critical that the patients most in need of admission be quickly identified.26 We found that the T-BISAP tool was better capable to estimate AICU in AP cases relative to the scores of BISAP alone, as evidenced by a significant improvement in the corresponding AUC value.
Acute respiratory distress syndrome (ARDS) is a common complication among individuals suffering from SAP,27 potentially leading to the need for mechanical ventilation. The ability to recognize the potential for such respiratory failure and the provision of MV in a timely manner when necessary has the potential to improve patient survival. In this analysis, we examined the ability of our T-BISAP model to predict the need for MV, confirming that TIPS scores significantly enhanced the risk stratification capabilities of BISAP scores when predicting the requirement for MV during the early stages of AP following ED admission.
We additionally compared other common prognostic scoring systems including the APACHE II score to confirm the reliability of the developed T-BISAP approach to AP patient risk stratification. Relative to APACHE II scores, the T-BISAP system yielded similar prognostic abilities with respect to its ability to predict 28-day mortality, AICU, and MV. As the APACHE II system necessitates more parameters and is very time-consuming, it cannot generally be utilized in an ED context. Overall, our results thus suggest that the combined T-BISAP approach can more readily and accurately identify high-risk AP patients admitted to the ED during the initial stages of disease, making it a suitable tool for patient management.
Limitations
There exist some restrictions to this study. For one, this was a single-center analysis of a relatively homogeneous study population, thus introducing inevitable bias despite our best efforts to minimize these effects. Second, the TIPS and BISAP scores were computed upon ED admission, but the changes in these scores in patients over time may have impacted their associated prognostic utility. Thirdly, we did not conduct any external validation, and further large-scale multi-center trials will be essential to validate these findings in the future in a prospective fashion.
Conclusions
The present analysis revealed that high TIPS and high BISAP scores are both independent predictors of AP patient prognosis. The addition of TIPS to BISAP score improved the overall prognostic value of the latter scale when used to conduct an assessment of the risk of negative outcomes in AP patients during the early phase.
Acknowledgment
We would like to thank all the volunteers who took part in this study and all the participants for their contribution to data collection and analysis.
Funding
This work was supported by: Key R&D Project of Sichuan Provincial Department of Science and Technology (2021YFS0023); Technology Innovation Project of Key R & D Support Plans of Chengdu Science and Technology Municipality (2020-YF05-00074-SN); Project of the Beijing Medical and Health Foundation (YWJKJJHKYJJ-B184096-Q26).
Disclosure
The authors have no potential conflicts of interest to disclose.
References
1. Luo Y, Li Z, Ge P, et al. Comprehensive mechanism, novel markers and multidisciplinary treatment of severe acute pancreatitis-associated cardiac Injury-A narrative review. J Inflamm Res. 2021;14:3145–3169. doi:10.2147/JIR.S310990
2. Qu B, Chu Y, Zhu F, et al. Granulocyte colony-stimulating factor enhances the therapeutic efficacy of bone marrow mesenchymal stem cell transplantation in rats with experimental acute pancreatitis. Oncotarget. 2017;8(13):21305–21314. doi:10.18632/oncotarget.15515
3. Xiao AY, Tan ML, Wu LM, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1(1):45–55. doi:10.1016/S2468-1253(16)30004-8
4. Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. doi:10.1136/gutjnl-2012-302779
5. Tenner S, Baillie J, DeWitt J, et al; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):
6. Mederos MA, Reber HA, Girgis MD. Acute pancreatitis: a review. JAMA. 2021;325(4):382–390. doi:10.1001/jama.2020.20317
7. Juneja D, Gopal PB, Ravula M. Scoring systems in acute pancreatitis: which one to use in intensive care units? J Crit Care. 2010;25(2):
8. Gao W, Yang HX, Ma CE. The value of BISAP score for predicting mortality and severity in acute pancreatitis: a systematic review and meta-analysis. PLoS One. 2015;10(6):e0130412. doi:10.1371/journal.pone.0130412
9. Li D, Ye L, Yu J, et al. Significance of the thrombo-inflammatory status-based novel prognostic score as a useful predictor for in-hospital mortality of patients with type B acute aortic dissection. Oncotarget. 2017;8(45):79315–79322. doi:10.18632/oncotarget.18105
10. Hudzik B, Szkodziński J, Wasilewski J, et al. A novel simplified thrombo-inflammatory score portends poor outcome in diabetic patients following myocardial infarction. Biomark Med. 2016;10(11):1129–1139. doi:10.2217/bmm-2016-0145
11. Li D, Zhou Y, Yu J, et al. Evaluation of a novel prognostic score based on thrombosis and inflammation in patients with sepsis: a retrospective cohort study. Clin Chem Lab Med. 2018;56(7):1182–1192. doi:10.1515/cclm-2017-0863
12. Han T, Cheng T, Liao Y, et al. Development and validation of a novel prognostic score based on thrombotic and inflammatory biomarkers for predicting 28-day adverse outcomes in patients with acute pancreatitis. J Inflamm Res. 2022;15:395–408. doi:10.2147/JIR.S344446
13. Liu B, Li D, Cheng Y, et al. Development and internal validation of a simple prognostic score for early sepsis risk stratification in the emergency department. BMJ Open. 2021;11(7):e046009. doi:10.1136/bmjopen-2020-046009
14. Wu BU, Johannes RS, Sun X, et al. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut. 2008;57(12):1698–1703. doi:10.1136/gut.2008.152702
15. Ouyang G, Pan G, Liu Q, et al. The global, regional, and national burden of pancreatitis in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. BMC Med. 2020;18(1):388. doi:10.1186/s12916-020-01859-5
16. Hines OJ, Pandol SJ. Management of severe acute pancreatitis. BMJ. 2019;367:l6227. doi:10.1136/bmj.l6227
17. Silva-Vaz P, Abrantes AM, Castelo-Branco M, et al. Multifactorial scores and biomarkers of prognosis of acute pancreatitis: applications to research and practice. Int J Mol Sci. 2020;21(1):338. doi:10.3390/ijms21010338
18. Windsor JA. Search for prognostic markers for acute pancreatitis. Lancet. 2000;355(9219):1924–1925. doi:10.1016/S0140-6736(00)02317-5
19. Yokoe M, Takada T, Mayumi T, et al. Japanese guidelines for the management of acute pancreatitis: Japanese guidelines 2015. J Hepatobiliary Pancreat Sci. 2015;22(6):405–432. doi:10.1002/jhbp.259
20. Silva-Vaz P, Abrantes AM, Morgado-Nunes S, et al. Evaluation of prognostic factors of severity in acute biliary pancreatitis. Int J Mol Sci. 2020;21(12):4300. doi:10.3390/ijms21124300
21. Yang YX, Li L. Evaluating the ability of the bedside index for severity of acute pancreatitis score to predict severe acute pancreatitis: a meta-analysis. Med Princ Pract. 2016;25(2):137–142. doi:10.1159/000441003
22. Senapati D, Debata PK, Jenasamant SS, et al. A prospective study of the Bedside Index for Severity in Acute Pancreatitis (BISAP) score in acute pancreatitis: an Indian perspective. Pancreatology. 2014;14(5):335–339. doi:10.1016/j.pan.2014.07.007
23. Kim TY, Kim SJ, Kim YS, et al. Delta neutrophil index as an early predictive marker of severe acute pancreatitis in the emergency department. United European Gastroenterol J. 2019;7(4):488–495. doi:10.1177/2050640619838359
24. Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet. 2015;386(9988):85–96. doi:10.1016/S0140-6736(14)60649-8
25. Rello J. Demographics, guidelines, and clinical experience in severe community-acquired pneumonia. Crit Care. 2008;12(Suppl 6):S2. doi:10.1186/cc7025
26. Duan J, Bai L, Zhou L, et al. Resource use, characteristics and outcomes of prolonged non-invasive ventilation: a single-centre observational study in China. BMJ Open. 2018;8(12):e019271. doi:10.1136/bmjopen-2017-019271
27. Schmandt M, Glowka TR, Kreyer S, et al. Secondary ARDS following acute pancreatitis: is extracorporeal membrane oxygenation feasible or futile? J Clin Med. 2021;10(5):1000. doi:10.3390/jcm10051000
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



