Back to Journals » Clinical Ophthalmology » Volume 10
Three-year follow-up of ranibizumab treatment of wet age-related macular degeneration: influence of baseline visual acuity and injection frequency on visual outcomes
Authors Razi F, Haq A, Tonne P, Logendran M
Received 7 October 2015
Accepted for publication 14 January 2016
Published 17 February 2016 Volume 2016:10 Pages 313—319
DOI https://doi.org/10.2147/OPTH.S97775
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Faraz Razi,1 Adnaan Haq,2 Prabhu Tonne,3 Maharatnam Logendran3
1Department of Paediatrics, Basingstoke and North Hampshire Hospital, Basingstoke, UK; 2Department of Ophthalmology, Leicester General Hospital, Leicester, UK; 3Department of Ophthalmology, Northampton General Hospital, Northampton, UK
Purpose: To determine the effect of ranibizumab on visual acuity (VA) following a 3-year treatment period for patients diagnosed with wet age-related macular degeneration. To establish whether baseline VA and injection frequency influence visual outcomes.
Patients and methods: Retrospective review of 70 patients (76 eyes) treated with 0.5 mg intravitreal ranibizumab for 3 consecutive months, and pro re nata thereafter (three + pro re nata protocol), over a 3-year period. VA was measured using Early Treatment Diabetic Retinopathy Study (ETDRS) charts at baseline, 12, 24, and 36 months. The number of injections administered at the end of years 1, 2, and 3 were also recorded. Eyes were stratified according to baseline VA, as well as the number of injections administered at the end of year 1. Linear regression analysis determined the relationship between VA and both baseline VA and injection frequency. P<0.05 was considered statistically significant.
Results: At 36 months, VA improved by a mean of 5.3 ETDRS letters (P=0.002), with 29% of eyes (n=22) demonstrating a clinically significant improvement in VA (gain of ≥15 ETDRS letters). Improvements in VA from baseline to 36 months were inversely proportional to the baseline VA (R=0.414, P=<0.001). A positive correlation was observed between injection frequency and change in VA from baseline to 36 months (R=0.244, P=0.036).
Conclusion: Mean improvement in VA is inversely proportional to baseline VA, and directly proportional to injection frequency.
Keywords: long-term results, Lucentis, neovascular AMD
A Letter to the Editor has been received and published for this article
Introduction
Currently, the preferred treatment of wet age-related macular degeneration (AMD) is through intravitreal injection of ranibizumab (Lucentis; Genentech, Inc., South San Francisco, CA, USA). A recombinant humanized monoclonal antibody, ranibizumab, prevents VEGF-A from binding to its receptors VEGFR-1 and VEGFR-2, in turn preventing the stimulation of cell processes responsible for the progression of wet AMD.1
The efficacy of ranibizumab compared to photodynamic therapy or sham therapy in the treatment of wet AMD was originally assessed in numerous prospective, randomized controlled trials. Pivotal clinical trials, such as the ANCHOR2 and MARINA3 studies, demonstrated that monthly injections of 0.5 mg ranibizumab provided a mean improvement in visual acuity (VA) of 10.7 and 7.2 Early Treatment Diabetic Retinopathy Study (ETDRS) letters after 2 years, respectively.
Such studies have informed clinical practice in the UK and, in August 2008, the National Institute of Clinical Excellence approved the use of ranibizumab in the treatment of wet AMD. Patients eligible for treatment undergo a treatment regimen of three mandatory monthly injections followed by pro re nata (PRN) dosing if active disease continues to be identified on subsequent outpatient visits. Many National Health Service Trusts in the UK have adopted this treatment regimen, yet there are limited data illustrating the real-life outcomes following ranibizumab treatment of longer than 2 years duration. Furthermore, few studies highlight the relationship between visual outcomes in wet AMD and the number of ranibizumab injections administered to patients. Identifying a correlation between the two is important in assessing the cost-effectiveness of ranibizumab, especially in light of the availability of alternative, more economical therapies, such as bevacizumab.
The purpose of this current study is two-fold: first, to determine the effect of ranibizumab on VA following a treatment period of 3 years for a clinical patient cohort diagnosed with wet AMD, and second, to establish whether variables such as baseline VA and injection frequency have any influence on visual outcomes.
Material and methods
Study design
This study was a retrospective review of the case notes pertaining to patients treated with intravitreal ranibizumab following diagnosis of wet AMD. Seeing as the review was an ongoing annual audit conducted internally by Northampton General Hospital, formal ethics application or patient consent was not required. This study was conducted in accordance with the Caldicott Principles and the Data Protection Act 1998.
Subjects
A total of 79 consecutive patient case notes were initially screened for analysis. Patients who had been diagnosed with choroidal neovascularization of any lesion sub-type for a minimum of 3 years, had a lesion size ≤12 disc areas in greatest linear dimension with absence of permanent damage to the central fovea, received at least one cycle of ranibizumab, underwent optical coherence tomography (Topcon Ltd, Newbury, UK) during each outpatient assessment to confirm diagnosis, and were seen and followed up by a medical retina consultant, were included in the study. Table 1 highlights the prevalence of the different choroidal neovascularization sub-types at baseline. Patients were excluded if their wet AMD had been previously treated with alternative therapies (such as laser photocoagulation, photodynamic therapy, or other anti-VEGF agents), if they received ranibizumab for other diagnoses (such as branch retinal vein occlusion or diabetic retinopathy) or if they did not complete the 3-year follow-up period. Nine patients met the third exclusion criteria, and were discontinued from the study (one patient during year 2 and eight patients during year 3). Reasons for discontinuation included discharge due to repeated non-attendance (n=7) and patient-led decision to withdraw due to deteriorating general health (n=2). Of the remaining 70 patients who completed the 3-year follow-up period, 47% (n=33) were male, and the mean age upon commencing treatment was 81.9 years (range 61 to 93). Six patients received bilateral treatment, increasing the total number of eyes treated to 76.
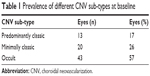  | Table 1 Prevalence of different CNV sub-types at baseline |
Data collection and measurements
Data were collected on treatment-naïve eyes initiated on intravitreal ranibizumab therapy. The data collected were restricted to patient demographics (age and sex), the VA recorded at baseline, 12, 24, and 36 months, the number of injections administered at the end of years 1, 2, and 3, and the number of adverse events (systemic and ocular). Visual outcomes were measured by ophthalmic nurses trained in assessing VA using ETDRS charts at a distance of 4 m from the patient.
Intervention
Eligible patients received ranibizumab by clinicians trained in the administration of intravitreal injections. Patients received an intravitreal injection of 0.5 mg ranibizumab on a monthly basis for 3 consecutive months. After the third mandatory injection, patients were followed up as outpatients once a month, throughout the 3-year follow-up period. Patients received further injections PRN if retreatment criteria were met or at the discretion of the treating clinician. Retreatment criteria included the following: a decrease in VA (≥5 ETDRS letters) in comparison to the last outpatient assessment, or the presence of disease activity on optical coherence tomography, such as persistent, increased or new intra- or sub-retinal fluid, or intra- or sub-retinal hemorrhage. For those patients receiving bilateral treatment, each eye was considered an independent set of data.
Outcome measures
The primary outcome measures were change in VA from baseline at 12, 24, and 36 months, and the number of injections administered by the end of years 1, 2, and 3. Eyes reporting a gain of ≥15 ETDRS letters were classified as demonstrating a clinically significant improvement in VA, while stable VA was defined as a change of ±15 ETDRS letters. Data were stratified to facilitate subgroup analyses. Eyes were stratified into the following subgroups based on baseline VA, in accordance with classification outlined by the International Council of Ophthalmology and International Classification of Diseases, 9th Revision, Clinical Modification:4 those with a VA ≤35 ETDRS letters (profound visual impairment), those with VA between 36 and 54 ETDRS letters (severe visual impairment), and those with a VA ≥55 ETDRS letters (moderate visual impairment). Eyes were also stratified according to the number of injections administered at the end of year 1. Parameters were chosen based on the observations of previous studies investigating the efficacy of ranibizumab administered via a three + PRN regimen. These studies noted that by month 12, a very small number of eyes required only three injections, which was the mandatory minimum. The proportion of eyes requiring no more than four injections, however, was significantly larger.5–7 The authors of the current study wanted to compare the effect on VA between those eyes in receipt of the minimum number of injections against those in receipt of more. In view of historical data, the authors anticipated that a group consisting of eyes in receipt of no more than three injections would be too small for a meaningful comparison. Therefore eyes were stratified into those that received ≤4 injections by the end of year 1, and those that received ≥5.
Statistical analysis
Student’s t-test was used to ascertain whether mean change in VA was statistically significant or not. Linear regression analysis was used to determine the relationship between VA and both baseline VA and injection frequency. Statistical significance was considered to be P<0.05 for all analyses.
Results
Overall change in VA
The mean baseline VA was 37.3 ETDRS letters (range 4 to 68). This increased to a mean VA of 44.9 ETDRS letters (range 2 to 70) by 12 months (Student’s t-test, P<0.001). Further statistically significant improvements in VA, in comparison to baseline VA, were demonstrated at 24 months, with mean VA being 43.5 ETDRS letters (range 10 to 70, P<0.001 compared to baseline), and again at 36 months, with mean VA recorded as 42.6 ETDRS letters (range 0 to 70, P=0.002 compared to baseline). Overall VA improved by a mean of 5.3 ETDRS letters over the course of the 3-year follow-up period.
Percentage of eyes demonstrating stable or improved VA
Overall, 29% of eyes (n=22) demonstrated a clinically significant improvement in VA (gain of ≥15 ETDRS letters) by 36 months. The greatest proportion of eyes reporting this degree in improvement was observed at 12 months (34%, n=26). At 24 months, this had decreased to 30% (n=23), and slightly further by the end of the study. Sixty-three percent of eyes (n=48) reported a change of ±15 ETDRS letters by 36 months, and were therefore classified as stable. Only 8% (n=6) reported a decline in VA (loss of >15 ETDRS letters) by the end of the study.
VA outcomes based on baseline VA
Patients were stratified into subgroups based on their baseline VA. Figure 1 highlights the mean change in VA for each subgroup from baseline to 12, 24, and 36 months. Patients with a baseline VA of ≤35 ETDRS letters reported the largest improvement by 36 months, with a mean gain of 12.1 ETDRS letters. Those patients with a baseline VA of 36 to 54 ETDRS letters experienced more modest gains at 36 months (mean gain of 0.4 ETDRS letters), while the ≥55 ETDRS letters baseline group reported the least significant change in VA by the end of the study (mean gain of 0.2 ETDRS letters). The poorer the baseline VA, the greater the proportion of eyes reporting clinically significant gains at 36 months (49% if ≤35 ETDRS letters, 29% if 36 to 54 ETDRS letters, and 0% if ≥55 ETDRS letters; see Table 2). A statistically significant inversely proportional relationship between baseline VA and visual outcomes was observed (R=0.414, P<0.001).
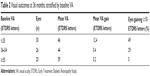  | Table 2 Visual outcomes at 36 months, stratified by baseline VA |
VA outcomes based on number of injections
Patients were stratified into subgroups based on the number of injections they had received by 12 months. Figure 2 shows the mean change in VA for each subgroup from baseline to 12, 24, and 36 months. Patients in receipt of fewer injections (≤4 injections) within the first year continued this trend in subsequent years, in comparison to their counterparts who continuously received more injections (≥5 injections). Patients receiving fewer injections in the first year (36%, n=27) reported a mean loss of 0.52 ETDRS letters at 36 months. In comparison, patients injected ≥5 times in the first year (64%, n=49) observed a modest improvement of 1.89 ETDRS letters by the end of the study. Over the entire patient cohort, the mean number of injections administered by the end of year 1 was 5.6 (range 3 to 9), 3.5 by the end of year 2 (range 0 to 8), and 3.5 by the end of year 3 (range 0 to 10). The correlation between the number of injections and change in VA from baseline to 36 months was found to be statistically significant (R=0.244, P=0.036).
Adverse effects
No ocular adverse effects following administration of ranibizumab were reported throughout the course of the study.
Cost of achieving improved VA
A total of 919 injections were administered throughout the 3-year follow-up period. Patients were treated with a mean of 5.6 injections in year 1 and 3.5 in years 2 and 3. A single ranibizumab injection costs £742.17.8 The mean cost of treatment per patient in year 1 was £4,156.15, which decreased to £2,597.60 by the end of year 2. The cost remained unchanged by the end of year 3. The mean cumulative cost of treatment per patient throughout the 3-year follow-up period was £8,974.40.
Discussion
In the absence of treatment, patients diagnosed with wet AMD can experience a decline in VA equivalent to one logarithm of the maximum angle of resolution line lost by 3 months, three lines by 1 year, and four lines by 2 years.9 This equates to five, 15, and 20 ETDRS letters, respectively.10 Comparisons of monthly dosing and variable dosing regimens of ranibizumab have demonstrated equal efficacy in slowing disease progression in wet AMD.11,12 Major clinical trials investigating the effect of ranibizumab following a variable dosing strategy of three injections plus PRN (three + PRN), such as the SUSTAIN and PrONTO studies, have reported favorable visual outcomes, albeit after only 1 or 2 years of treatment, respectively.5,13 The current study reports that approximately one third of patients experienced a clinically significant improvement in VA (gain of ≥15 ETDRS letters) following 3-year treatment using the three + PRN dosing schedule. This result correlates well with other studies of a similar duration.6,7 Furthermore, with 92% of patients reporting stable or improved VA by month 36, this study demonstrates that longer term administration of ranibizumab via a three + PRN dosing schedule is effective in managing disease progression of wet AMD in a real-life clinical patient cohort.
Baseline VA
Previous studies have shown that gains in VA tend to be more significant in patients with poorer baseline VA. A study led by Shona et al reported mean gains to be greatest amongst patients classified as having poor and intermediate baseline VA.14 Williams and Blyth also demonstrated VA gain to be inversely proportional to baseline VA.15 Such observations mirror the findings in the current study. By month 36, patients with a baseline VA of ≤35 ETDRS letters gained 11.9 ETDRS letters more than patients with a baseline VA of ≥55 ETDRS letters. Unlike the current study, however, no other has assessed the influence of baseline VA past a follow-up period of 2 years.16–19 By evaluating visual outcomes over a longer period, we have been able to provide a more accurate reflection of how the efficacy of ranibizumab is influenced by baseline VA. Furthermore, by identifying “poor responders” based on longer term data, our findings may prompt clinicians to investigate whether patients with similar baseline VA may respond more favorably to other ranibizumab regimens.
Number of injections
Data exist suggesting that increasing the frequency of ranibizumab injections can lead to superior visual outcomes. Following stratification of patients into subgroups receiving fewer or more injections, Dadgostar et al observed VA gains to be greater in the latter cohort.19 Outcomes were assessed in the short term, after 12±4.3 months. Recent work of a longer duration led by Rofagha et al (SEVEN-UP study) demonstrated greater gains in VA for those patients in receipt of more injections.20 The results of the current study are comparable (R=0.244, P=0.036). At the end of the 3-year follow-up period, the difference in VA between those receiving ≤4 injections and those receiving ≥5 was 2.4 ETDRS letters, in favor of the latter. Despite observing visual outcomes after 7 years of treatment, the SEVEN-UP study, unlike our own, does not quantify the strength of the relationship between injection frequency and VA. That said, we note that this study assessed a small number of eyes (n=76). Calculation of R is known to be influenced by several factors, all of which are exacerbated by small sample size.21 With this in mind, cautious interpretation of the correlation obtained, albeit positive, is advised.
Implications for clinical practice
There are two main learning points from this study, both of which have the potential to inform clinical practice. The first is that baseline VA is inversely related to improvement in visual outcomes. Eyes with better baseline VA exhibited the poorest gains at the end of the study. The eyes were treated using ranibizumab administered via a three + PRN regimen. Given that another study applying an identical treatment protocol of similar duration has mirrored these findings,12 the authors query whether eyes with superior baseline VA may respond more favorably to alternative treatment regimens. It may be prudent for clinicians to conduct trials in which cohorts of patients with a baseline VA ≥36 ETDRS letters are treated with differing ranibizumab regimens. If more efficacious treatment schedules are identified, clinical practice could change from treating every patient with the now standardized three + PRN regimen, to providing bespoke treatment programs based on a patient’s baseline physiology.
The second noteworthy point is that the clinical benefit acquired from receiving more ranibizumab injections is negligible. Indeed, eyes in receipt of ≥5 injections by the end of year 1 reported greater gains in VA at the end of the study. However, the difference in the percentage of eyes reporting clinically significant gains between the two subgroups was minimal (13% if receiving ≤4 injections, 16% if receiving ≥5). Perhaps there exists a limit to the number of injections that can provide VA gains of clinical significance. Further study investigating changes in VA using narrower injection frequency subgroups is encouraged. If data can be acquired to suggest that clinically significant gains are unobtainable after a certain number of injections, clinicians could be justified in providing a finite number of repeat doses to patients. This potential change in practice could have far reaching benefits, such as a reduction in the National Health Service’s substantial annual expenditure on ranibizumab.
Limitations
One limitation of this study is the relatively small sample size. Although this can influence the generalizability of results, the number of patients available for screening, and therefore inclusion in the study, was restricted by the size of the population surrounding the semi-rural district general hospital. The second limitation of note was the lack of data pertaining to patients who did not complete the study. Such information is necessary to provide a comprehensive picture of real-life outcomes. This study, however, was concerned with recording visual outcomes upon completion of 36 months of treatment. To incorporate incomplete data into our analysis would have been contradictory to our objective.
Conclusion
Ranibizumab is effective in stabilizing vision over a 36-month period if administered in a three + PRN dosing protocol. Poorer baseline VA contributes to superior visual gains. Patients with superior baseline VA experience limited improvement, and whether this could be improved using alternative ranibizumab regimens merits further investigation. Moreover, the extent of managing disease progression in wet AMD is directly proportional to the frequency of injections administered. However, clinically significant gains are limited to the minority of patients, and given the cost of ranibizumab, further assessment of alternative therapies following a comparable study design is warranted.
Acknowledgment
Participating investigator: Dr Nilesh Sunnasy – collected data.
Disclosure
The authors report no conflicts of interest in this work.
References
Novartis Pharmaceuticals. Lucentis® ranibizumab (rbe). Novartis Pharmaceuticals; 2014. Available from: http://www.novartis.com.au/pi_pdf/luc.pdf. Accessed January 21, 2016. | ||
Brown DM, Kaiser PK, Michels M, et al; ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. | ||
Rosenfeld PJ, Brown DM, Heier JS, et al; MARINA Study Group. Ranibizumab for Neovascular Age-Related Macular Degeneration. N Engl J Med. 2006;355(14):1419–1431. | ||
International Council of Ophthalmology. Visual Standards - Aspects and Ranges of Visual Loss with an Emphasis on Population Surveys. International Council of Ophthalmology; 2002. Available from: http://www.icoph.org/downloads/visualstandardsreport.pdf. Accessed January 21, 2016. | ||
Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009;148(1):43–58. | ||
Muniraju R, Ramu J, Sivaprasad S. Three-year visual outcome and injection frequency of intravitreal ranibizumab therapy for neovascular age-related macular degeneration. Ophthalmologica. 2013;230(1):27–33. | ||
Frennesson CI, Nilsson SE. A three-year follow-up of ranibizumab treatment of exudative AMD: impact on the outcome of carrying forward the last acuity observation in drop-outs. Acta Ophthalmol. 2014; 92(3):216–220. | ||
NICE. BNF Lucentis® (Novartis). NICE. Available from: http://www.evidence.nhs.uk/formulary/bnf/current/11-eye/118-miscellaneous-ophthalmic-preparations/1182-ocular-diagnostic-and-peri-operative-preparations-and-photodynamic-treatment/subfoveal-choroidal-neovascularisation/ranibizumab/lucentis. Accessed January 21, 2016. | ||
Mitchell P, Korobelnik JF, Lanzetta P, et al. Ranibizumab (Lucentis) in neovascular age-related macular degeneration: evidence from clinical trials. Br J Ophthalmol. 2010;94(1):2–13. | ||
Carlson N, Kurtz D. Clinical Procedures for Ocular Examination. 3rd ed. New York: McGraw-Hill Education; 2003. | ||
Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012; 119(7): 1388–1398. | ||
Kodjikian L, Souied EH, Mimoun G, et al. Ranibizumab versus Bevacizumab for Neovascular Age-related Macular Degeneration: Results from the GEFAL Noninferiority Randomized Trial. Ophthalmology. 2013;120(11):2300–2309. | ||
Holz FG, Amoaku W, Donate J, et al. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology. 2011;118(4):663–671. | ||
Shona O, Gupta B, Vemala R, Sivaprasad S. Visual acuity outcomes in ranibizumab-treated neovascular age-related macular degeneration; stratified by baseline vision. Clin Experiment Ophthalmol. 2011;39(1):5–8. | ||
Williams TA, Blyth CP. Outcome of ranibizumab treatment in neovascular age related macula degeneration in eyes with baseline visual acuity better than 6/12. Eye (Lond). 2011;25(12):1617–1621. | ||
Bandukwala T, Muni RH, Schwartz C, Eng KT, Kertes P. Effectiveness of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration in a Canadian retina practice: a retrospective review. Can J Ophthalmol. 2010;45(6):590–595. | ||
Kaiser PK, Brown DM, Zhang K, et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007;144(6): 850–857. | ||
Boyer DS, Antoszyk AN, Awh CC, et al. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114(2):246–252. | ||
Dadgostar H, Ventura AA, Chung JY, Sharma S, Kaiser PK. Evaluation of injection frequency and visual acuity outcomes for ranibizumab monotherapy in exudative age-related macular degeneration. Ophthalmology. 2009;116(9):1740–1747. | ||
Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K; SEVEN-UP Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013;120(11):2292–2299. | ||
Goodwin LD, Leech NL. Understanding Correlation: Factors That Affect the Size of r. The Journal of Experimental Education. 2006;74(3): 249–266. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


