Back to Journals » Clinical Interventions in Aging » Volume 13
Thoracopelvic assisted movement training to improve gait and balance in elderly at risk of falling: a case series
Authors Springer S, Friedman I, Ohry A
Received 28 February 2018
Accepted for publication 2 May 2018
Published 20 June 2018 Volume 2018:13 Pages 1143—1149
DOI https://doi.org/10.2147/CIA.S166956
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Walker
Shmuel Springer,1 Itamar Friedman,2 Avi Ohry3,4
1Department of Physical Therapy, Faculty of Health Sciences, Ariel University, Ariel, Israel; 2ProMedoss, Charlotte, NC, USA; 3Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel; 4Reuth Rehabilitation and Medical Center, Tel Aviv, Israel
Background: Age-related changes in coordinated movement pattern of the thorax and pelvis may be one of the factors contributing to fall risk. This report describes the feasibility of using a new thoracopelvic assisted movement device to improve gait and balance in an elderly population with increased risk for falls.
Methods: In this case series, 19 older adults were recruited from an assisted living facility. All had gait difficulties (gait speed <1.0 m/s) and history of falls. Participants received 12 training sessions with the thoracopelvic assisted movement device. Functional performance was measured before, during (after 6 sessions), and after the 12 sessions. Outcomes measures were Timed Up and Go, Functional Reach Test, and the 10-meter Walk Test. Changes in outcomes were calculated for each participant in the context of minimal detectable change (MDC) values.
Results: More than 25% of participants showed changes >MDC in their clinical measures after 6 treatment sessions, and more than half improved >MDC after 12 sessions. Six subjects (32%) improved their Timed Up and Go time by >4 seconds after 6 sessions, and 10 (53%) after 12 sessions. After the intervention, 4 subjects (21%) improved their 10-meter Walk Test velocity from limited community ambulation (0.4–0.8 m/s) to functional community ambulation (>0.8 m/s).
Conclusion: Thoracopelvic assisted movement training that mimics normal walking pattern may have clinical implications, by improving skills that enhance balance and gait function. Additional randomized, controlled studies are required to examine the effects of this intervention on larger cohorts with a variety of subjects.
Keywords: gait, balance, older adults, training
Introduction
Approximately 30% of community-dwelling adults over the age of 65 fall at least once a year.1 Among those with Parkinson’s disease, mild cognitive impairment, or dementia, this percentage increases to 60%–80%.2 Many intrinsic and extrinsic factors can lead to falls among the elderly.3 Thus, developing effective interventions to prevent falls is a challenging task.
Studies have shown age-related changes in coordinated movement pattern of the thorax and pelvis during task performance.4–6 For instance, due to reduced use of thoracolumbar rotation, older adults demonstrate a shorter reach distance while performing 1-arm reaching tasks as compared to younger individuals.4
Reduced motor control while walking may lead to gait impairments. Moreover, most falls occur while walking, and reduced motor control is considered a serious risk factor for falls.1 A common gait impairment among the elderly is reduced trunk and pelvic rotation.5 During axial trunk rotation, older adults move the trunk and pelvis as 1 unit, which negatively affects gait.6
Since control of trunk movement is essential to maintaining balance, changes in trunk movement control may be one of the factors contributing to fall risk.7 Additionally, pelvic rotation is important in adjusting crossing stride and obstacle clearing.8 Thus, improving pelvic and trunk rotation may reduce the risk of falls and increase balance.
Exercise regimens that target pelvis and trunk rotation, such as Tai Ji Quan, are effective in reducing falls among seniors.9 However, practical drawbacks such as lack of adherence and resource intensiveness prevent these exercises from being optimal interventions.10 Exercise in a relaxed, seated position may enhance adherence, especially among individuals with balance disorders. In settings with limited resources, the use of technology may provide a cost-effective approach for training.
Thus, the purpose of this case series feasibility report is to describe the training effects of the Balanseat (Mopair Technologies, Ltd., Givat Nili, Israel), a new thoracopelvic assisted movement device, on gait and balance in an elderly population at high risk for falls.
Methods
Study design and participants
Residents of an assisted living home (Lev Ganim, Netanya, Israel) were enrolled in this rehabilitation protocol. Enrollment criteria included 1) age 65–99 years 2) ability to ambulate at least 10 m on a flat surface, with or without an assistive device, 3) walking difficulty, defined as gait speed slower than 1 m/s (gait speed <1.0 m/s) during 10-meter Walk Test (10MWT),11 and 4) 2 or more falls in the 6 months prior to the intervention. A fall was defined as any unintentional event in which any part of the body above the ankle makes contact with the floor, the ground, or a lower surface. Excluded were any individuals who had a major orthopedic or neurological disorder that could affect gait speed (such as post-stroke or amputation), or other disease that prevented their participation. Twenty-six individuals were approached for eligibility, and 19 subjects met the enrollment criteria and were included in the protocol. Of the participants, 5 were males and 14 were females. The average age was 83.3 (SD 6.2 years). Written informed consent was provided by the participants. Data collection was approved by the institute (Lev Ganim).
Intervention
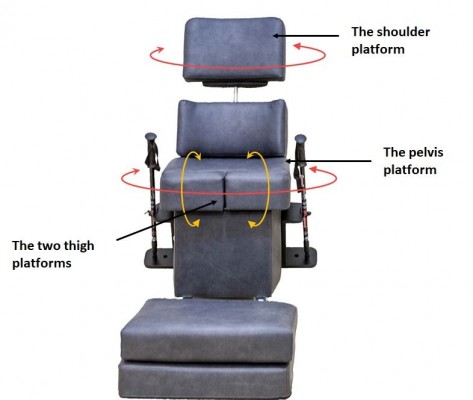
Treatment included 12 sessions of training with the Balanseat thoracopelvic assisted movement training device (Figure 1). The Balanseat is a motorized chair-like device designed to rehabilitate walking and balance. It applies gentle contralateral movement between the trunk, the pelvis, and the thighs to emulate normal human walking pattern.
  | Figure 1 The Balanseat thoracopelvic assisted movement training device. |
The Balanseat’s scientific background is based on 2 concepts. The mechanical concept reflects exertion of a passive movement in a specific plane that may increase the ability of relevant joints to pass through a predetermined range of motion.12 For the motor control concept, the device increases the sensory feedback from mechanosensory afferents to improve the dynamic control of movement.13
Participants received treatment while sitting in the device, which has 4 motor-controlled platforms. The pelvis and shoulder platforms emulate the torso’s contralateral movements, while the 2 thigh platforms move in opposite directions from each other. All platforms move in synchronization to emulate a slow, rotational “walking-like” pattern. When activated, the Balanseat’s pelvis platform moves through an angular range of 8°; the shoulder and thigh platforms range of motion is 4°. The speed of movement can be set according to the individual user’s needs.
Sessions took place twice a week (on the same days), over 6 weeks. Each session included ~30 minutes of training with the Balanseat, followed by 20 minutes of posture gait training. The entire session was supervised by a clinician. The default rotational speed of the device is set to complete the platform’s total range of motion within 5 seconds. During training, the subjects were asked to pay attention to the rotational movement. The system enables the clinician to increase or decrease the default velocity by 25% (down to pause of movement or up to cycle within 2.5 seconds). The 30 minutes of training with the Balanseat were comprised of 7 segments, each lasting 3–5 minutes. The first and last segments were always 25% slower than the default speed. The second and sixth segments were set to the default speed. The 3 remaining segments were tailored to each participant’s gait velocity and subjective feedback (ie, for those with faster walking speeds, the cycle speed was increased to the level they reported they were able to concentrate on the movement sensation). Breaks between movement segments were provided as needed. During the 20 minutes of gait training, participants were encouraged to walk at varied speeds (ie, comfortable and fast), while maintaining a gait pattern that utilized trunk movements similar to those achieved by the Balanseat. No additional instructions or training were provided.
Outcome measures
Outcomes were measured before, during (after 6 sessions), and again after the intervention (12 sessions) and included the Timed Up and Go test (TUG), the Functional reach test (FRT), and the 10MWT. The TUG measures the time it takes a person to stand up from a chair, walk 3 m, turn, and return to sit on the chair. This test was chosen because it is a commonly used, recommended assessment of gait and balance in older people. It is also effective in identifying elderly individuals who are prone to falls.14 The FRT assesses patients’ stability by measuring how far they can reach forward with their arms without taking a step. The FRT is a good indicator to identify those at high risk of falling among elderly individuals.15 The 10MWT measures mobility by assessing walking speed over a short duration. Walking speed is a valid, reliable, and sensitive measure for assessing and monitoring functional status.16 Participants were allowed to use their walking assistive device during the 10MWT and the TUG tests.
Data analysis
All changes in outcome measures were evaluated using each participant as his or her own control. The changes were compared with the minimal detectable change (MDC) values for comparable patient populations. The MDC is defined as the minimum change that is not likely to be attributable to a chance variation in measurement.17 In addition, previously established cut-off scores were adopted for the current analysis.
For the TUG, the MDC was set at 4 seconds based on values established in adults with Alzheimer’s disease18 and Parkinson’s disease.19 A score of ≥13.5 seconds was used as a cut-off value to identify those at increased risk of falls.20
For the FRT, the MDC was set at 4.3 cm based on values established in adults with Parkinson’s disease.21 A cut-off value <18.5 cm was used to indicate fall risk.15
For the 10MWT, the MDC was set at 0.1 m/s. Among older adults without specific impairments, as well as adults after a hip fracture, a change in gait velocity >0.1 m/s has been determined as a minimal clinically important difference.22,23
Patients were also grouped according to the 3 common categories of ambulation: limited household ambulators (gait velocity <0.4 m/s), limited community ambulators (0.4–0.8 m/s), and functional community ambulators (>0.8 m/s). Transitioning to a higher ambulation category is associated with substantially better function and quality of life, especially with regard to mobility and community participation.24
To describe the treatment effect after 12 sessions, the results were further analyzed according to the following 5 categories: A = no improvement, B = minor improvement (up to 10% change), C = moderate improvement (10%–20% change), D = substantial improvement, (20%–30% change), and E = extensive improvement (>30% change).
Results
Participant characteristics and the clinical outcome results (TUG, FRT, and 10MWT) before treatment (Baseline), after 6 sessions (Mid), and after 12 sessions (End) are presented in Table 1. Table 2 shows the number and percentage of the participants whose clinical outcomes improved >MDC values, and/or exceeded the cut-off points during the Mid and End sessions, as compared to Baseline.
As depicted in Table 2, >25% of subjects showed changes >MDC in clinical measures after 6 sessions of treatment, and >50% improved >MDC after 12 sessions. For example, 6 subjects (32%) improved their TUG time by >4 seconds after 6 sessions, and this increased to 10 (53%) after 12 sessions. When the results of all outcome measures were combined, 4 subjects (21%) improved >MDC in all 3 outcomes.
It should also be noted that some subjects enhanced their performance above the cut-off scores for fall risk and category of ambulation. For example, after 12 sessions, 4 subjects (21%) improved their 10MWT velocity from limited community ambulation to functional community ambulation.
Figure 2 presents the analysis of the categories describing the treatment effect after 12 sessions. Most participants experienced minor to extensive improvement (>10%): TUG 11 (58%), FRT 16 (84%), and 10MWT 13 (68%). At least 32% of participants exhibited extensive improvement (>30%): TUG 6 (32%), FRT 13 (68%), and 10MWT 9 (47%).
Discussion
In this case series, an intervention with a thoracopelvic assisted movement training device designed to improve gait and balance was found feasible for the treatment of elderly people with gait difficulties and history of falls. Both walking and balance improved in a cohort of residents of an assisted living community. Some participants progressed from high fall-risk and limited in-house walking capabilities to a better category of ambulation. Improvements were demonstrated across all 3 functional tests. These results may emphasize the therapeutic value of the investigated intervention. No adverse side effects were observed, regardless of a participant’s results. Furthermore, though not included as an outcome measure, it is important to mention that all participants expressed a desire to continue with training.
The present results are consistent with previous evidence reporting that older adults can gain crucial adaptive skills for resisting falls through training.25,26 The ability achieved, as reflected by improvements in clinical outcomes, was not examined on a long-term basis. Yet, there is evidence that older adults can retain newly acquired fine motor skills for years without retraining.27,28
Another important aspect is the method of training. In this case series, training was performed primarily in the seated position. The current results agree with those of previous studies that showed benefits of chair-based exercises for frail older people.29 Two reasons may support this method of training. Fall prevention programs that involve exercises performed only while standing and walking unassisted might be too challenging for older people with compromised balance and mobility. In addition, practicing and acquiring a motor skill may be easier while performed in a relaxed position. Yet, it should be emphasized that the current protocol also involved gait training intended to reinforce the gait pattern and trunk movement training provided while in a seated position. Thus, the beneficial effects could be related to both types of training.
In this case series, functional performance was measured by 3 different outcomes, the TUG, FRT, and 10MWT. While adequate correlations were found between these outcomes,14,30 physical performance measures might not be useful as stand-alone tests to assess fall risk among older adults.31 For example, it was shown that short walk tests do not exhibit a high enough degree of concurrent validity with the 10MWT to be used interchangeably for assessing gait speed among older adults.32 The results obtained from the different outcome measures seem to indicate a positive influence of the intervention on different aspects of balance and gait.
Among the elderly, age-related degeneration of joints and ligaments may lead to stiffness.33 Improvements in the participants’ performance could be explained by a general decrease in stiffness in the pelvis and trunk. In addition, fear of falling could also lead to active stiffening due to increases in background muscle activity.34 The thoracopelvic assisted movement training might help increase joint range of motion and normalization of muscle activity, as documented with other passive motion devices.12 Increased pelvic and trunk motion may enhance stability, improve gait pattern, and decrease expenditure of muscle energy.5,6
Another explanation for the improvements seen could be related to the sensory feedback gained from the rotational movement. Decreased locomotion function in the elderly might be due to age-related deterioration of sensory feedback systems.5 Dynamic control of movement depends on sensory feedback from mechanosensory afferents,35 and sensory activity could contribute to a preprogrammed motoneuronal drive, such as coordination of the human walking pattern.36 Thus, some of the positive outcomes achieved through the training could be related to this mechanism.
This case series has several limitations, including the lack of control group and the relatively small number of participants who resided in an assisted living home.
Although beneficial effects on gait and balance were demonstrated in most participants, the degree of the effect differed across subjects. While 68% improved their 10MW speed >MDC after 12 sessions, only 53% improved their TUG >MDC during the same period. Furthermore, 4 subjects (21%) improved >MDC in all 3 outcomes; however, one subject (5%) did not show any improvement at the 6-week (Mid) measurement. Thus, the beneficial results cannot be generalized to a broader population without further research examining characteristics of older adults that may lead to improvements in mobility and balance from this type of training. These investigations should be undertaken with larger samples including community-dwelling adults, with appropriate control groups. Kinematic and myoelectrical studies are required to understand the biomechanical effects of the thoracopelvic assisted movement device and to clarify the relation between these effects to changes in gait. Furthermore, increased axial stiffness has also been implicated in movement disorders found in patients with neurological problems, such as hemiplegia and Parkinson’s disease.37 Thus, it is suggested that future investigations include these patient populations. Larger studies might enable a more comprehensive analysis as well. Yet, it should be noted that descriptive data are indispensable, and if they are of good quality, valid and important conclusions can be drawn.38 Finally, future research should also evaluate outcomes related to community ambulation, participation, and quality of life. The promising results of the present investigation suggest that such studies are warranted.
Conclusion
In summary, this case series suggests that thoracopelvic assisted movement training that mimics normal walking patterns could have clinical implications, by facilitating skills that enhance balance and gait. This finding should encourage further research aimed at testing the utility of this form of training in treating individuals with gait difficulties. Additional clinical investigations, including randomized controlled studies with larger cohorts and variety of subjects, are required to accurately evaluate the clinical effect of this concept.
Disclosure
Shmuel Springer, Itamar Friedman, and Avi Ohry are consultants for Mopair Technologies, the company that developed the Balanseat. The authors report no other conflicts of interest in this work.
References
Rubenstein LZ, Josephson KR. Falls and their prevention in elderly people: what does the evidence show? Med Clin North Am. 2006;90(5):807–824. | ||
Wood B, Bilclough J, Bowron A, Walker R. Incidence and prediction of falls in Parkinson’s disease: a prospective multidisciplinary study. J Neurol Neurosurg Psychiatry. 2002;72(6):721–725. | ||
Kannus P, Sievänen H, Palvanen M, Järvinen T, Parkkari J. Prevention of falls and consequent injuries in elderly people. Lancet. 2005;366(9500):1885–1893. | ||
Cavanaugh JT, Shinberg M, Ray L, Shipp KM, Kuchibhatla M, Schenkman M. Kinematic characterization of standing reach: comparison of younger vs. older subjects. Clin Biomech. 1999;14(4):271–279. | ||
McGibbon CA, Krebs DE. Age-related changes in lower trunk coordination and energy transfer during gait. J Neurophysiol. 2001;85(5):1923–1931. | ||
Sung PS, Lee KJ, Park WH. Coordination of trunk and pelvis in young and elderly individuals during axial trunk rotation. Gait Posture. 2012;36(2):330–331. | ||
van der Burg J, Pijnappels M, van Dieen JH. The influence of artificially increased trunk stiffness on the balance recovery after a trip. Gait Posture. 2007;26(2):272–278. | ||
Wang Y. Angular movements of the trunk and pelvis when stepping over obstacles of different heights. Res Sports Med. 2003;11(4):219–234. | ||
Li F, Harmer P, Fitzgerald K. Implementing an evidence-based fall prevention intervention in community senior centers. Am J Public Health. 2016;106(11):2026–2031. | ||
Hamm J, Money AG, Atwal A, Paraskevopoulos I. Fall prevention intervention technologies: a conceptual framework and survey of the state of the art. J Biomed Inform. 2016;59:319–345. | ||
Montero-Odasso M, Schapira M, Soriano ER, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2005;60(10):1304–1309. | ||
O’Driscoll SW, Giori NJ. Continuous passive motion (CPM): theory and principles of clincial application. J Rehabil Res Dev. 2000;37(2):179–188. | ||
Nielsen JB, Sinkjaer T. Afferent feedback in the control of human gait. J Electromyogr Kinesiol. 2002;12(3):213–217. | ||
Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. | ||
Thomas JI, Lane JV. A pilot study to explore the predictive validity of 4 measures of falls risk in frail elderly patients. Arch Phys Med Rehabil. 2005;86(8):1636–1640. | ||
Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. J Aging Phys Act. 2015;23(2):314–322. | ||
Haley SM, Fragala-Pinkham MA. Interpreting change scores of tests and measures used in physical therapy. Phys Ther. 2006;86(5):735–743. | ||
Ries JD, Echternach JL, Nof L, Gagnon Blodgett M. Test-retest reliability and minimal detectable change scores for the timed “up & go” test, the six-minute walk test, and gait speed in people with Alzheimer disease. Phys Ther. 2009;89(6):569–579. | ||
Huang SL, Hsieh CL, Wu RM, Tai CH, Lin CH, Lu WS. Minimal detectable change of the timed “up & go” test and the dynamic gait index in people with Parkinson disease. Phys Ther. 2011;91(1):114–121. | ||
Rose DJ, Jones CJ, Lucchese N. Predicting the probability of falls in community-residing older adults using the 8-foot up-and-go: a new measure of functional mobility. J Aging Phys Act. 2002;10(4):466–475. | ||
Smithson F, Morris ME, Iansek R. Performance on clinical tests of balance in Parkinson’s disease. Phys Ther. 1998;78(6):577–592. | ||
Bohannon RW, Glenney SS. Minimal clinically important difference for change in comfortable gait speed of adults with pathology: a systematic review. J Eval Clin Pract. 2014;20(4):295–300. | ||
Palombaro KM, Craik RL, Mangione KK, Tomlinson JD. Determining meaningful changes in gait speed after hip fracture. Phys Ther. 2006;86(6):809–816. | ||
Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26(6):982–989. | ||
Pai YC, Bhatt T, Wang E, Espy D, Pavol MJ. Inoculation against falls: rapid adaptation by young and older adults to slips during daily activities. Arch Phys Med Rehabil. 2010;91(3):452–459. | ||
Mirelman A, Rochester L, Maidan I, et al. Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (V-TIME): a randomised controlled trial. Lancet. 2016;388(10050):1170–1182. | ||
Smith C, Walton A, Loveland A, Umberger G, Kryscio R, Gash D. Memories that last in old age: motor skill learning and memory preservation. Neurobiol Aging. 2005;26(6):883–890. | ||
Bhatt T, Pai YC. Long-term retention of gait stability improvements. J Neurophysiol. 2005;94(3):1971–1979. | ||
Anthony K, Robinson K, Logan P, Gordon AL, Harwood RH, Masud T. Chair-based exercises for frail older people: a systematic review. Biomed Res Int. 2013;2013:309506. | ||
Brooks D, Davis AM, Naglie G. Validity of 3 physical performance measures in inpatient geriatric rehabilitation. Arch Phys Med Rehabil. 2006;87(1):105–110. | ||
Singh DK, Pillai SG, Tan ST, Tai CC, Shahar S. Association between physiological falls risk and physical performance tests among community-dwelling older adults. Clin Interv Aging. 2015;10:1319–1326. | ||
Peters DM, Fritz SL, Krotish DE. Assessing the reliability and validity of a shorter walk test compared with the 10-Meter Walk Test for measurements of gait speed in healthy, older adults. J Geriatr Phys Ther. 2013;36(1):24–30. | ||
Grimby G. Muscle performance and structure in the elderly as studied cross-sectionally and longitudinally. J Gerontol A Biol Sci Med Sci. 1995;50:17–22. | ||
Reelick MF, van Iersel MB, Kessels RP, Rikkert MGO. The influence of fear of falling on gait and balance in older people. Age Ageing. 2009;38(4):435–440. | ||
Koch SC, Del Barrio MG, Dalet A, et al. RORβ spinal interneurons gate sensory transmission during locomotion to secure a fluid walking gait. Neuron. 2017;96(6):1419–1431.e5. | ||
Nielsen JB, Sinkjær T. Afferent feedback in the control of human gait. J Electromyogr Kinesiol. 2002;12(3):213–217. | ||
Ferrarin M, Lopiano L, Rizzone M, et al. Quantitative analysis of gait in Parkinson’s disease: a pilot study on the effects of bilateral sub-thalamic stimulation. Gait Posture. 2002;16(2):135–148. | ||
Spriestersbach A, Röhrig B, du Prel JB, Gerhold-Ay A, Blettner M. Descriptive statistics: the specification of statistical measures and their presentation in tables and graphs. Part 7 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2009;106(36):578–583. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



