Back to Journals » Journal of Pain Research » Volume 7
Thoracic epidural infusion with chloroprocaine for postoperative analgesia following epicardial pacemaker placement in an infant
Authors Kamata M, Corridore M, Tobias J
Received 27 August 2014
Accepted for publication 16 September 2014
Published 23 October 2014 Volume 2014:7 Pages 609—613
DOI https://doi.org/10.2147/JPR.S73309
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Michael Schatman
Mineto Kamata,1 Marco Corridore,1,2 Joseph D Tobias1–3
1Department of Anesthesiology and Pain Medicine, Nationwide Children's Hospital, Columbus, OH, USA; 2Department of Anesthesiology and Pain Medicine, The Ohio State University College of Medicine, Columbus, OH, USA; 3Department of Pediatrics, The Ohio State University, Columbus, OH, USA
Abstract: In critically ill neonates and infants, major interventions, including thoracotomy, may result in significant postoperative respiratory insufficiency and pain leading to the need for postoperative mechanical ventilation. Although there are many potential options for providing postoperative analgesia, there continues to be expanding use of regional anesthesia in this population. One of the many reported advantages is the provision of postoperative analgesia while avoiding the deleterious effects on respiratory function that may be seen with systemic opioids. We report the use of thoracic epidural anesthesia using a continuous infusion of chloroprocaine to provide analgesia following thoracotomy and epicardial pacemaker placement in an infant. The perioperative plan was complicated by comorbid conditions including congenital complete heart block, recent rhinovirus infection with residual respiratory involvement, and prematurity.
Keywords: chloroprocaine, epidural anesthesia, thoracotomy, pacemaker
Introduction
Approximately 1% of all pacemakers are implanted in infants and children.1 The majority of these are required for complete heart block related to surgery for congenital heart disease or congenitally acquired problems.2,3 Although there has been recent progress suggesting that the transvenous route may be feasible, due to size constraints, the majority require an open thoracotomy and placement of epicardial leads.4–6 In critically ill neonates and infants, major interventions including thoracotomy may result in significant postoperative respiratory insufficiency and pain, leading to the need for postoperative mechanical ventilation. While various options are available for the provision of analgesia following major surgical procedures including systemic opioids and regional anesthetic techniques, intravenous opioids may result in sedation and respiratory depression. To avoid such issues, there has been increased use of regional anesthesia, even in the neonatal and infant population.7–11 We report the use of thoracic epidural anesthesia using a continuous infusion of chloroprocaine to provide analgesia following thoracotomy and epicardial pacemaker placement in an infant with comorbid conditions including congenital complete heart block, recent rhinovirus infection with residual respiratory involvement, and prematurity.
Case report
Institutional review board approval is not required for single-patient case reports at Nationwide Children’s Hospital. A 6-week-old female infant weighing 3.0 kg presented to the operating room for thoracotomy and pacemaker placement for complete heart block. She was born at 34 weeks, weighing 1.65 kg, via scheduled Cesarean-section secondary to a prenatal diagnosis of complete heart block with development of oligohydramnios. Her electrocardiogram at birth showed complete heart block and prolonged a QT interval. The initial heart rate (HR) was 51 bpm, with a QRS interval of 160 ms and a QTc of 558 ms. The maternal history was positive for systemic lupus erythematosus and diet-controlled gestational diabetes mellitus. Although the initial postpartum HR was 50 bpm, her hemodynamic status was stable, with no evidence of compromised end-organ perfusion. On day of life 17, her HR slowed to 30 bpm, and isoproterenol was started at 0.2 μg/kg/min. Although she was initially scheduled for pacemaker placement at 2–3 weeks of age, it was decided to postpone the procedure and wait for further weight gain. During the 4th week of life, she was diagnosed with a rhinovirus infection with an increase in nasal secretions and tachypnea. Over the ensuing days, there was progressive respiratory compromise requiring high-flow nasal cannula (HFNC) to treat respiratory insufficiency. HFNC at 6–10 L/min was required for approximately 2 weeks. As the acute rhinovirus infection resolved, the HFNC was weaned back to standard nasal cannula with a flow rate of 0.2–0.5 L/min. At 6 weeks of life with a weight of 3.0 kg, it was decided to proceed with pacemaker placement. Echocardiogram 2 days prior to surgery showed a small patent foramen ovale, mild tricuspid and aortic valve regurgitation, mild enlargement of all cardiac chambers, and mildly depressed ventricular systolic function. Her medication regimen included furosemide twice a day and an isoproterenol infusion at 0.46 μg/kg/min. Laboratory evaluation 3 days prior to the procedure showed a hemoglobin of 12.3 g/dL, a hematocrit of 38.2%, and a platelet count of 222,000/mm3. Coagulation function, including prothrombin time and partial thromboplastin time, were normal for age. The patient was transported to the operating room, and routine American Society of Anesthesiologists’ monitors were placed. Baseline vital signs revealed an HR of 63 bpm, blood pressure of 86/62 mmHg, respiratory rate of 60 breaths/minute, with an oxygen saturation of 91% on 0.2 lpm of oxygen via nasal cannula. Following pre-oxygenation with 100% oxygen, anesthesia was induced with sevoflurane (inspired concentration 4%), fentanyl (3.3 μg/kg), and rocuronium (1 mg/kg). After a 3.0 mm cuffed endotracheal tube was placed, a 24-gauge intravenous cannula and a 24-gauge radial arterial cannula were placed. Following anesthetic induction and tracheal intubation, the caudal epidural space was accessed through the sacrococcygeal ligament with an 18-gauge, 4.45 cm (1¾ inch) Crawford needle, and a 20-gauge, single orifice epidural catheter was threaded to T5 level. After negative aspiration for blood, the epidural catheter was dosed with 0.1 mL/kg of 0.2% ropivacaine with 1:200,000 epinephrine. When there was no response noted, the bolus dose of 3 mL was administered in increments. There was no change in the hemodynamic parameters during anesthetic induction, epidural catheter insertion, and subsequent dosing. Anesthesia was maintained with isoflurane (end-tidal concentration 0.3%–0.8%). There was no change in the hemodynamic parameters with skin incision for thoracotomy. No additional opioids were administered intraoperatively. A left thoracotomy was performed for epicardial pacemaker lead placement as well as the abdominal incision for the pocket for a generator. After pacemaker placement, pacing was initiated at setting of VVI with a rate of 120 bpm, and the isoproterenol infusion was discontinued. The surgical duration was 93 minutes. The patient was turned to the supine position, and her trachea was extubated in the operating room. She was transported to the cardiothoracic intensive care unit. Postoperative analgesia was provided by a continuous epidural anesthesia of 1.5% chloroprocaine at 3 mL/hour (1 mL/kg/hour). Other adjunctive pain medication included intravenous acetaminophen for every 6 hours for 72 hours and as-needed doses of intravenous fentanyl (0.5 μg/kg) and oral oxycodone. Postoperative pain scores assessed using the FLACC (face, legs, activity, cry, consolability) scoring system were 0–2 during the first 48 postoperative hours. During this time, two doses of intravenous fentanyl and one dose of oral oxycodone were administered. The epidural infusion was discontinued, and the catheter removed on postoperative day (POD) 3. The patient was transferred to the inpatient floor on POD 4 and discharged to home on POD 8. Her postoperative course was uncomplicated. There were no major issues and complications.
Discussion
Neonates and infants who undergo major abdominal and thoracic surgery with general anesthesia often require a period of postoperative ventilation. The combination of low birth weight, prematurity, and extensive upper abdominal and thoracic surgery places the neonate at risk for needing prolonged mechanical ventilation. These issues are further compounded by the respiratory effect of pain related to upper abdominal or thoracic procedures. In our patient, several comorbid factors were present including the congenital heart block which resulted in low cardiac output at baseline with a resting HR of 50–60 bpm, preterm birth, and recent rhinovirus infection with an ongoing oxygen requirement. Perioperative respiratory function was further impaired by the combination of a thoracotomy with a second upper abdominal incision with tissue dissection to create a pocket for the pacemaker placement.
Given these concerns and the need to avoid the potential deleterious effects of opioids on respiratory function, we chose to use regional anesthesia (thoracic epidural anesthesia). As there remains a concern regarding the potential for spinal cord damage with direct thoracic placement of an epidural catheter, various investigators have reported techniques to advance a catheter from the caudal level to the thoracic dermatomes.7,8 In the original study by Bösenberg et al,8 an examination of human cadaver specimens suggested the feasibility of the technique which was followed by demonstration of its efficacy in piglets. The technique was then applied to 20 infants ranging in age from 4 weeks to 5 months and in weight from 2.7 to 6.5 kg for biliary surgery. In 19 of 20 patients, the catheter tip was within one vertebra of the goal of T7. In the remaining patient, the tip was at T12. In 14 of 20 patients, slight resistance was noted, which was dealt with by gentle flexion and extension of the back with subsequent catheter insertion. Subsequently, as others evaluated the technique, modifications were suggested including use of nerve stimulation or fluoroscopy during placement or the injection of radio-opaque dye to demonstrate catheter location after placement to ensure its correct location at the desired dermatomes.12–14 Our current clinical practice is the use of a radio-opaque catheter (Flextip Plus® Catheter for Caudal Placement; Arrow International, Reading, PA, USA). Its appearance on a plain radiograph is demonstrated in Figure 1. Given the potential for the catheter to loop back caudally during blind placement, we have also found ultrasound useful during placement to ensure continued cephalad movement as the catheter is advanced.
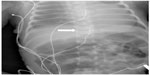  | Figure 1 Postoperative radiograph demonstrating the radio-opaque epidural catheter (white arrow) used in our patient. |
Despite its efficacy, concerns remain regarding the potential morbidity related to systemic toxicity following the initial bolus dose of subsequent infusion of amide-type local anesthetic agents used. Infants may have greater risks from local anesthetic toxicity compared with adults. They generally require a larger volume of the local anesthetic agent to achieve epidural anesthesia than adults, and given their cognitive state are unable to report the initial symptoms of local anesthetic toxicity. Besides, most regional anesthetic procedures in infants are performed during general anesthesia, thereby eliminating the ability to detect increasing plasma concentrations. Agitation as a prodromal symptom of local anesthetic toxicity may be misinterpreted as pain, leading to the administration of additional bolus doses or an increase in the infusion rate.
These factors are compounded by the fact that the metabolism and elimination of amide-type local anesthetic agents are delayed in neonates given the immaturity of their hepatic microsomal enzymes. Infants have a decreased plasma concentration of α1-acid glycoprotein, the principal binding protein for local anesthetic agents, thereby resulting in an increased concentration of the unbound (free) amide local anesthetic agents. The plasma concentration of α1-acid glycoprotein levels in neonates and infants younger than 6 months can be less than 50% of that in children and adults.15 Furthermore, the limited data from epidural anesthesia in infants and children demonstrate a risk of bupivacaine accumulation during prolonged infusions.16 In a cohort of eight infants, ranging in age from 3 to 12 months, a continuous infusion of bupivacaine infused at an average rate of 0.38 mg/kg/hour resulted in a concentration of 2.02 μg/mL (reported toxic level in adults ≥2 μg/mL) and evidence of accumulation, with increasing concentrations in three of the eight patients at the time the infusion was discontinued after 32 hours of use. Our patient also had an underlying prolonged QT interval, which may predispose to local anesthetic toxicity from the effects of local anesthetic agents on the cardiac conduction system.
Given these concerns, others have suggested that ropivacaine or lidocaine may be a more appropriate alternative.17,18 It has been demonstrated that ropivacaine concentrations do not show the same pattern of potential accumulation as bupivacaine, while lidocaine has been anecdotally reported with its suggested advantages being the ease of obtaining plasma concentrations in most laboratories and a decreased risk of cardiovascular toxicity when compared with bupivacaine.
Chloroprocaine is an ester local anesthetic which provides a rapid onset of anesthesia with a potency and duration of action comparable to procaine.19,20 Chloroprocaine has a very short duration of action with a plasma half-life of 20–25 seconds in adults and 40 seconds in neonates.21 As it is metabolized by plasma esterases, there is no clinically significant difference in metabolism based on chronologic or gestational age. Given the concerns regarding accumulation and the potential for local anesthetic toxicity, our current practice includes the routine use of chloroprocaine for epidural anesthesia and analgesia in neonates and young infants.
To date, there are a limited number of reports regarding the use of chloroprocaine for epidural anesthesia in neonates.9,22,23 As a means of avoiding the need for general anesthesia, Henderson et al22 reported the use of caudal epidural anesthesia with chloroprocaine as a bolus followed by an infusion to provide surgical anesthesia during sub-umbilical surgery in a cohort of ten former preterm infants, who were 35 to 49.5 weeks postconceptional age at the time of surgery. Given the prolonged duration of the surgical procedure, the authors felt that the dosing requirements for bupivacaine would exceed the upper limits of safety. Plasma 2-chloroprocaine concentrations were measured in five infants and found to be zero or well below the toxic level. A similar technique has been combined with general anesthesia to allow for early tracheal extubation following intra-abdominal procedures in neonates.9 Anecdotal experience has also demonstrated the utility of using chloroprocaine for providing postoperative epidural analgesia.24,25 Given the limited clinical experience with the use of chloroprocaine for postoperative analgesia, we have extrapolated postoperative dosing from intraoperative administration and infusion rates. In the two largest series in neonates and infants, effective analgesia/anesthesia was obtained with a loading dose of 1.0–1.5 mL/kg followed by a continuous infusion of 1.0–1.5 mL/kg/hour.9,22
Previously, concerns had been expressed regarding the potential for neurotoxicity related to the low pH of the solution and the preservative, sodium metabisulfite, which was in the commercially available chloroprocaine solutions.19,26 This was subsequently replaced with a solution containing ethylenediaminetetraacetic acid (EDTA) as a preservative. The use of this solution has been reported to cause transient back pain following epidural anesthesia.27 These effects are thought to result from local binding of calcium by EDTA with resultant tetany of the paraspinus muscles. Currently, the chloroprocaine in use in most countries is preservative-free.
In summary, we present anecdotal experience with thoracic epidural analgesia following thoracotomy and pacemaker placement in a high-risk infant. Our patient had several comorbid features which placed her at respiratory for perioperative respiratory insufficiency, including a combined abdominal and thoracic incision, recent rhinovirus infection with residual respiratory involvement, a prolonged QT interval, and prematurity. In order to avoid the deleterious effects of opioids on respiratory function and the need for postoperative mechanical ventilation, postoperative analgesia was provided by a continuous thoracic epidural anesthesia. Considering the patient’s prematurity and chronological age, chloroprocaine was used to minimize the potential for local anesthetic systemic toxicity. Given its rapid metabolism, the plasma half-life of chloroprocaine is approximately 40 seconds, even in neonates. The utility of such a practice is demonstrated by a recent case report reporting a transient wide-complex bradycardia (HR 30 beats/minute) with spontaneous resolution in 30 seconds following the inadvertent intravenous injection of chloroprocaine in a 2-month-old infant during regional anesthesia.28 Despite its limited use in current clinical practice, we suggest that chloroprocaine should be considered for regional anesthesia in neonates and infants when a continuous infusion is in use. Future studies are needed to more fully evaluate its efficacy and adverse profile.
Disclosure
The authors report no conflicts of interest in this work.
References
Antretter H, Colvin J, Schweigmann U, et al. Special problems of pacing in children. Indian Pacing Electrophysiol J. 2003;3:23–33. | |
Cohen MI, Bush DM, Vetter VL, et al. Permanent epicardial pacing in pediatric patients. Seventeen years of experience and 1200 outpatient visits. Circulation. 2001;103:2585–2590. | |
Martelli H, Bonnet D, Iserin L, Butera G, Kachaner J. Characteristics and results of epicardial pacing in neonates and infants. Pacing Clin Electrophysiol. 2000;23:2052–2056. | |
Benrey J, Gillette PC, Nasrallah AT, Hallman GL. Permanent pacemaker implantation in infants, children and adolescents. Circulation. 1976;53:245–248. | |
Sachweh JS, Vazquez-Jiminez JF, Schondube FA, et al. Twenty years’ experience with pediatric pacing: epimyocardial and transvenous stimulation. Eur J Cardiothorac Surg. 2000;17:455–461. | |
Robledo-Nolasco R, Ortiz-Avalos M, Rodriguez-Diez G, et al. Transvenous pacing in children weighing less than 10 kilograms. Pacing Clin Electrophysiol. 2009;32(Suppl 1):S177–S181. | |
Bösenberg AT. Epidural analgesia for major neonatal surgery. Paediatr Anaesth. 1998;8:479–483. | |
Bösenberg AT, Bland BA, Schulte-Steinberg O, Downing JW. Thoracic epidural anesthesia via caudal route in infants. Anesthesiology. 1988;69:265–269. | |
Tobias JD, Rasmussen GE, Holcomb GW 3rd, Brock JW 3rd, Morgan WM 3rd. Continuous caudal anaesthesia with chloroprocaine as an adjunct to general anaesthesia in neonates. Can J Anaesth. 1996;43;69–72. | |
Williams RK, McBride WJ, Abajian JC. Combined spinal and epidural anaesthesia for major abdominal surgery in infants. Can J Anaesth. 1997;44:511–514. | |
Di Pede A, Morini F, Lombardi MH, et al. Comparison of regional vs systemic analgesia for post-thoracotomy care in infants. Paediatr Anaesth. 2014;24:569–573. | |
Seefelder C. The caudal catheter in neonates: what are the restrictions? Curr Opin Anesthesiol. 2002;15:343–348. | |
van Niekerk J, Bax-Vermeire BM, Geurts JW, Kramer PP. Epidurography in premature infants. Anaesthesia. 1990;45:722–725. | |
Tsui BC, Wagner A, Cave D, Kearney R. Thoracic and lumbar epidural analgesia via the caudal approach using electrical stimulation guidance in pediatric patients: a review of 289 patients. Anesthesiology. 2004;100:683–689. | |
Philip AGS, Hewitt JR. Alpha 1-acid glycoprotein in the neonate with and without infection. Biol Neonate. 1983;43:118–124. | |
Peutrell JM, Holder K, Gregory M. Plasma bupivacaine concentrations associated with continuous extradural infusions in babies. Br J Anaesth. 1997;78:160–162. | |
Kost-Byerly S, Jackson EV, Yaster M, Kozlowski LJ, Mathews RI, Gearhart JP. Perioperative anesthetic and analgesic management of newborn bladder exstrophy repair. J Pediatr Urol. 2008;4:280–285. | |
Bösenberg AT, Thomas J, Cronje L, et al. Pharmacokinetics and efficacy of ropivacaine for continuous epidural infusion in neonates and infants. Paediatr Anaesth. 2005;15:739–749. | |
Cox B, Durieux ME, Marcus MA. Toxicity of local anaesthetics. Best Pract Res Clin Anaesthesiol. 2003;17:111–136. | |
Gunter JB. Benefit and risks of local anesthetics in infants and children. Paediatr Drugs. 2002;4:649–672. | |
Finster M. Toxicity of local anesthetics in the fetus and newborn. Bull NY Acad Med. 1976;52:222–225. | |
Henderson K, Sethna NF, Berde CB. Continuous caudal anesthesia for inguinal hernia repair in former preterm infants. J Clin Anesth. 1993;5(2):129–133. | |
Tobias JD, Lowe S, O’Dell N, Pietsch JB, Neblett WW 3rd. Continuous regional anaesthesia in infants. Can J Anaesth. 1993;40:1065–1068. | |
Tobias JD, O’Dell N. Chloroprocaine for epidural anesthesia in infants and children. AANA J. 1995;63:131–135. | |
Tobias JD. Anaesthetic management of the child with myotonic dystrophy: epidural anaesthesia as an alternative to general anaesthesia. Paediatr Anaesth. 1995;5:335–338. | |
Moore DC, Spierdijk J, vanKleef JD, Coleman RL, Love GF. Chloroprocaine neurotoxicity: four additional cases. Anesth Analg. 1982;61:155–159. | |
Stevens RA, Urmey WF, Urquhart BL, Kao TC. Back pain after epidural anesthesia with chloroprocaine. Anesthesiology. 1993;78:492–497. | |
Cladis FP, Litman RS. Transient cardiovascular toxicity with unintentional intravascular injection of 3% 2-chloroprocaine in a 2-month-old infant. Anesthesiology. 2004;100:181–183. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
