Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Therapeutic Success of Tiotropium/Olodaterol, Measured Using the Clinical COPD Questionnaire (CCQ), in Routine Clinical Practice: A Multinational Non-Interventional Study
Authors Valipour A, Avdeev S , Barczyk A, Bayer V, Fridlender Z , Georgieva M, Kudela O, Medvedchikov A , Miron R, Sanzharovskaya M, Šileikienė V, Šorli J, Spielmanns M, Szalai Z
Received 13 November 2020
Accepted for publication 7 February 2021
Published 10 March 2021 Volume 2021:16 Pages 615—628
DOI https://doi.org/10.2147/COPD.S291920
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Arschang Valipour,1 Sergey Avdeev,2 Adam Barczyk,3 Valentina Bayer,4 Zvi Fridlender,5 Mariela Georgieva,6 Ondřej Kudela,7 Alexey Medvedchikov,8 Ramona Miron,9 Maria Sanzharovskaya,8 Virginija Šileikienė,10 Jurij Šorli,11 Marc Spielmanns,12 Zsuzsanna Szalai13
1Department of Respiratory and Critical Care Medicine, Karl-Landsteiner-Institute for Lung Research and Pulmonary Oncology, Vienna Health Care Group, Klinik Floridsdorf, Vienna, Austria; 2I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia; 3Wydział Nauk Medycznych Śląskiego Uniwersytetu Medycznego, Katowice, Poland; 4Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA; 5Hadassah-Hebrew University Medical Center, Jerusalem, Israel; 6Medical Center “Sv.ivan Rilski” OOD, Vidin, Bulgaria; 7Department of Pneumology, Faculty of Medicine in Hradec Kralove, University Hospital Hradec Kralove, Charles University in Prague, Hradec Kralove, Czech Republic; 8Boehringer Ingelheim RCV GmbH & Co. KG, Vienna, Austria; 9Clinical Pneumophtysiology Hospital Iasi, Iasi, Romania; 10Faculty of Medicine, Clinic of Chest Diseases, Immunology and Allergology, Institute of Clinical Medicine, Vilnius University, Vilnius, Lithuania; 11Bolnišnica Topolšica, Topolšica, Slovenia; 12Zürcher RehaZentrum Wald, Wald, Switzerland; 13Petz Aladar County Teaching Hospital, Gyor, Hungary
Correspondence: Arschang Valipour
Department of Respiratory and Critical Care Medicine, Karl-Landsteiner-Institute for Lung Research and Pulmonary Oncology, Vienna Health Care Group, Klinik Floridsdorf, Brünner Straße 68, 1210 Vienna, Austria
Tel +43 1 277 00-72201
Fax +43 1 277 00 99 2208
Email [email protected]
Background: The Clinical COPD Questionnaire (CCQ) is a simple patient-reported tool to measure clinical control of chronic obstructive pulmonary disease (COPD).
Objective: This open-label, single-arm, non-interventional study (NCT03663569) investigated changes in CCQ score during treatment with tiotropium/olodaterol in clinical practice.
Methods: Data were included from consenting COPD patients, enrolled in Bulgaria, Czech Republic, Hungary, Israel, Lithuania, Poland, Romania, Russia, Slovenia, Switzerland and Ukraine, who were receiving a new prescription for tiotropium/olodaterol according to the treating physician in a real-world environment. The primary endpoint was the occurrence of therapeutic success, defined as a 0.4-point decrease in CCQ score after treatment with tiotropium/olodaterol for approximately 6 weeks.
Results: Overall, 4819 patients were treated; baseline and Week 6 CCQ scores were available for 4700 patients, mostly classified as Global Initiative for Chronic Obstructive Lung Disease (GOLD) B (51.6%) or D (42.7%). After 6 weeks’ treatment, 81.4% (95% confidence interval [95% CI] 80.24– 82.49) of patients achieved therapeutic success; mean improvement in overall CCQ score was 1.02 points (95% CI 1.00– 1.05). Improved CCQ score was seen in 92.2% of patients (95% CI 91.43– 92.98), 2.5% had no change and 5.3% showed a worsening. When stratified by prior treatment, the greatest benefit was seen in treatment-naïve patients, with 85.7% achieving therapeutic success, compared with 79.5% of those pretreated with long-acting β2-agonist (LABA)/inhaled corticosteroid (ICS) and 74.2% of those pretreated with LABA or long-acting muscarinic antagonist (LAMA) monotherapy. Overall, rescue medication decreased by 1.25 puffs/day (95% CI 1.19– 1.31) versus baseline. In total, 29 patients (0.6%) reported drug-related adverse events and 7 patients reported serious adverse events (0.15%).
Conclusion: In 4700 COPD patients, 6 weeks’ treatment with tiotropium/olodaterol, as initial treatment or follow-up to LAMA or LABA monotherapy or LABA/ICS, improved CCQ and decreased rescue medication use. The adverse event profile was consistent with the known safety profile of tiotropium/olodaterol.
Keywords: tiotropium, olodaterol, COPD, CCQ, Clinical COPD Questionnaire, non-interventional study
Plain Language Summary
People with COPD may benefit from treatment with a combination of two inhaled medications, tiotropium and olodaterol, that help to open the airways. We carried out this study to find out if treatment with tiotropium/olodaterol for 6 weeks would improve disease control (“clinical control of COPD”) for people with COPD. We measured this using a questionnaire called the Clinical COPD Questionnaire (CCQ). The CCQ was filled in by 4700 patients with COPD from Bulgaria, Czech Republic, Hungary, Israel, Lithuania, Poland, Romania, Russia, Slovenia, Switzerland and Ukraine at the start and end of the study.
After 6 weeks of treatment with tiotropium/olodaterol, over 80% of the patients we surveyed had better clinical control of COPD, defined as a decrease in CCQ score of 0.4 points, compared with at the start of the study. Patients in the study also needed less rescue medication (use of short-acting drugs such as salbutamol). Tiotropium/olodaterol had a good safety profile, similar to previous clinical studies.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common and serious condition, with an estimated global prevalence of almost 300 million in 2017.1 By 2030, it is predicted that COPD will be the fourth-leading cause of death worldwide.2 COPD is particularly prevalent in smokers and ex-smokers, people aged ≥40 years and men.3 Both preventable and treatable, COPD is characterized by persistent respiratory symptoms and airflow limitation, usually resulting from significant exposure to noxious particles or gases, such as tobacco smoke, air pollution or other environmental exposures.4 The global prevalence of COPD and its associated burden of morbidity and mortality is set to increase still further.4
According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2021 strategy report, long-acting bronchodilators such as long-acting muscarinic antagonists (LAMAs) and long-acting β2-agonists (LABAs) are the cornerstone of maintenance therapy for patients with moderate-to-very severe COPD.4 Dual bronchodilation with a LAMA plus a LABA provides additional benefits, including improvements in lung function and patient-reported outcomes, and, from an economic perspective, has been shown to be cost-effective, with clinical benefits and cost savings compared with monotherapy.4–8 For example, tiotropium, a well-established, inhaled, once-daily LAMA for the treatment of COPD,9–11 can be combined with olodaterol, a once-daily LABA that was developed as a complementary partner to tiotropium and has a fast onset of action.12,13 Clinical studies investigating treatment with tiotropium/olodaterol, administered using the Respimat® Soft MistTM inhaler (SMI), have shown significant improvements in lung function, symptoms, health-related quality of life and exercise capacity versus monocomponents or placebo in patients with COPD.13–16 Tiotropium/olodaterol was also shown to significantly reduce the risk of clinically important deterioration (as measured by a composite endpoint) in patients with COPD compared with tiotropium alone.17 However, data from various types of study, including real-world studies, are needed to fully evaluate the impact of pharmacotherapy on clinical control in COPD.18
Clinical control of COPD can be measured in clinical practice using patient-reported outcomes questionnaires such as the COPD Assessment Test (CAT) or Clinical COPD Questionnaire (CCQ), whereas its stability can be measured based on the presence or absence of exacerbations over time.19 Both the CAT and the CCQ have been recommended for the assessment of clinically relevant changes in health status in patients with COPD.20 The St. George’s Respiratory Questionnaire (SGRQ), though widely used in clinical trials and accepted as a well-validated and reliable questionnaire for assessing health-related quality of life in COPD, is time-consuming and complicated to use, and hence the CCQ provides an accepted practical alternative.21,22
The CCQ is a simple, 10-item, health-related quality of life questionnaire with good psychometric properties that was developed specifically for patients with COPD. It was validated in the Netherlands by van der Molen et al in 2003 and shows good correlation with the SGRQ.21,23,24 This questionnaire is easy to apply and takes less than 2 minutes to complete.21 The CCQ is responsive to intervention and has been validated in over 140 languages.
The aim of this non-interventional study (NCT03663569) was to prospectively investigate the potential changes in clinical control using the CCQ when patients with COPD (either treatment-naïve or those receiving LAMA or LABA monotherapy or LABA/inhaled corticosteroids [ICS] at baseline) were receiving treatment with tiotropium/olodaterol for 6 weeks in routine clinical practice.
Materials and Methods
Study Design
This open-label, single-arm, non-interventional observational study enrolled patients aged ≥40 years diagnosed with COPD who required a new prescription of tiotropium/olodaterol delivered via Respimat® SMI, based upon the investigator’s decision, and according to the approved summary of product characteristics (SmPC), GOLD COPD Strategy Document 2018 (GOLD COPD group B, C and D) and/or local COPD guidelines. The study was submitted to the ethics committee of participating countries according to national regulations (see Supplementary Text S1 for further details). Sites could only participate if independent ethics committee approval in their country was issued. Written informed consent was required prior to participation. Patients were enrolled from 11 countries (Bulgaria, Czech Republic, Hungary, Israel, Lithuania, Poland, Romania, Russia, Slovenia, Switzerland and Ukraine). Patients who met the entry criteria were enrolled consecutively and were followed over an observational period of approximately 6 weeks.
Patients were excluded if they had contraindications according to the tiotropium/olodaterol SmPC or if they fulfilled any of the following criteria: already on a LAMA/LABA combination (free or fixed dose) in the last 6 weeks before study entry; unwilling to stop LABA/ICS fixed-dose combination treatment after study enrollment; pregnant or lactating; or currently participating in any clinical trial or any other non-interventional study of a drug or device. During the study, patients could be prescribed ICS in a separate inhaler, in addition to tiotropium/olodaterol, if the physician decided that this was necessary.
Consecutive recruitment was employed to minimize selection bias. To prevent selection bias at the site level, participating centers were selected that had access to all available COPD treatment options approved for use in that country. The study was carried out in accordance with the principles of the Declaration of Helsinki.
Endpoints
The primary endpoint was the occurrence of therapeutic success, predefined as a 0.4-point decrease in the total CCQ score between Visit 1 (baseline visit) and Visit 2 (final visit, approximately 6 weeks after starting treatment; this correlates with the average time before the next pulmonologist consultation after an initial prescription of an inhaler therapy for COPD).
Secondary endpoints included absolute changes in total CCQ score and scores for the following CCQ domains between Visits 1 and 2: symptom domain (questions 1, 2, 5 and 6 of the CCQ); mental state domain (questions 3 and 4 of the CCQ); and functional state domain or CCQ-4 (questions 7, 8, 9 and 10 of the CCQ). General condition of the patient, evaluated using the Physician’s Global Evaluation (PGE) score, was assessed at Visits 1 and 2, and patient satisfaction and willingness to continue treatment with tiotropium/olodaterol after study end were assessed at Visit 2.
Use of rescue medication (eg, short-acting bronchodilators) was evaluated as an exploratory endpoint in the week prior to Visits 1 and 2.
Safety was monitored from Visit 1 following the signing of informed consent until the end of the study. Data on the safety of tiotropium/olodaterol, including adverse drug reactions and serious adverse events with fatal outcome, were considered treatment-emergent.
Assessments
All study assessments at Visits 1 and 2 are detailed in Supplementary Table S1.
For CCQ, each of the 10 questions was scored by the patient on a 7-point scale between 0 (never/not limited at all) and 6 (almost all the time/totally limited or unable to do) at baseline and at the end of the observation period (after approximately 6 weeks). The final score is the sum of all items divided by 10; separate scores for all three domains can be calculated. Higher scores indicate a worse health status. The minimal clinically important difference (MCID) of the CCQ total score is −0.4.23
Statistical Analysis
All analyses for the primary and secondary endpoints were descriptive and were performed on the full analysis set (FAS), which comprised patients with informed consent and at least one documented administration of tiotropium/olodaterol and available total CCQ score data at Visits 1 and 2. Safety and demographic/baseline data were analyzed on the treated set, comprising all patients with at least one documented administration of tiotropium/olodaterol.
For the primary endpoint, the percentage of patients with therapeutic success is presented together with the 95% confidence interval (CI). For comparison of subgroups for the primary endpoint, χ2-Test (or Fisher’s exact test if χ2-Test was not valid) was used and p-values were interpreted nominally. 95% CIs were also calculated.
For absolute changes in CCQ score and CCQ-4 (functional state domain) score, summary statistics are provided. Additionally, for subgroups, changes from baseline in total CCQ and CCQ-4 score were compared by Wilcoxon rank-sum test (Mann–Whitney U-Test) or Kruskal–Wallis test; for change from baseline in CCQ and CCQ-4 score, the 95% CIs were also computed. In the context of CCQ, missing values were replaced according to the corresponding manual; no other missing data were imputed. For general condition of the patient (PGE) and patient’s satisfaction with tiotropium/olodaterol, the number and percentage of patients within each category are displayed. For comparison of subgroups for PGE and patient’s satisfaction, as well as patient’s willingness to continue the treatment, χ2-Test was used. If the χ2-Test was not valid, the comparison was done by Fisher’s exact test, or by treating the PGE and patient’s satisfaction as continuous outcomes and comparing them by Wilcoxon rank-sum test (Mann–Whitney U-Test) or Kruskal–Wallis test. For comparisons of subgroups, missing observations were not considered.
No formal hypothesis testing was performed due to the non-interventional nature of the study. Assessments were carried out using SAS® software version 9.4.
A number of subgroups were investigated, including treatment in the 6 weeks prior to initiation of tiotropium/olodaterol (ie, treatment-naïve patients versus patients already treated at baseline with long-acting bronchodilators [LAMA only or LABA only] or with LABA+ICS); GOLD classification (GOLD B versus C versus D); exacerbations (any) in the last 12 months (≤1 versus ≥2 exacerbations); and cardiac comorbidities (yes versus no). Treatment-naïve patients were defined as those who had not received pre-treatment with long-acting inhaled treatment such as bronchodilators or ICS in the 6 weeks prior to initiation of tiotropium/olodaterol; treatment with short-acting bronchodilators was permitted. Patients switching from pre-existing treatments to a combination of tiotropium/olodaterol together with ICS in a separate inhaler were excluded from subgroup analyses due to the low number of subjects. Patients on other prior therapies were also excluded.
Results
Patient Disposition and Baseline Characteristics
A total of 4825 patients were recruited; of these, 6 patients had no baseline examination and were excluded from the treated set (n=4819; Figure 1). There were 119 patients with missing CCQ scores, hence the FAS comprised 4700 patients. Overall, 3350 patients (69.4%) were recruited from outpatient clinics and 1475 (30.6%) were recruited from hospitals.
 |
Figure 1 Patient disposition. Abbreviation: CCQ, Clinical COPD Questionnaire. |
The mean age of the patients in the treated set was 65.4 years, and 55.0% of the patients were ≥65 years (Table 1). The majority of the patients (69.7%) were male. Almost half (48.1%) of patients were current smokers at the start of the study and 42.5% were ex-smokers.
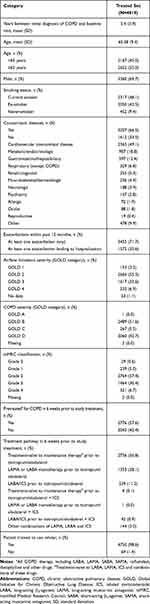 |
Table 1 Patient Demographics and Baseline Characteristics |
More than half of the patients were diagnosed with GOLD B COPD (51.6%), followed by GOLD D (42.7%), GOLD C (5.5%) and one patient with GOLD A.
Concomitant diseases were reported in 66.5% of patients, including 2365 (49.1%) patients with concomitant cardiovascular disease. Other comorbidities were recorded in <20% of patients (Table 1).
Of the patients in the treated set, 2736 (56.8%) were treatment-naïve at baseline, 1353 (28.1%) were on LAMA or LABA monotherapy, and 539 (11.2%) were on LABA/ICS (Table 1).
Median study duration for the FAS was 6 weeks (range: 0.71–29 weeks); mean study duration was 6.36 weeks. The vast majority of patients in the FAS (93.7%) were treated for 4–8 weeks, less than 1% of patients were treated for <4 weeks and 5.5% were treated for >8 weeks.
Efficacy
Primary Endpoint
Overall, 81.4% (95% CI 80.24–82.49) of the FAS achieved therapeutic success after 6 weeks of treatment (Figure 2). An improved CCQ score was seen in 92.2% of patients (95% CI 91.43–92.98), whereas 2.5% (95% CI 2.04–2.95) had no change and 5.3% (95% CI 4.67–5.98) showed a worsening in CCQ score.
 |
Figure 2 Proportion of patients achieving therapeutic success at Week 6, all patients and stratified by treatment pathway (full analysis set). Error bars are 95% CI. Therapeutic success was defined as a 0.4-point decrease in CCQ score between baseline and after 6 weeks of treatment. Based on prior findings,23 a change of 0.4 points was considered to be the minimum clinically important difference for the total CCQ score. Due to the low number of patients who switched from pre-existing treatments to a combination of tiotropium/olodaterol plus a separate ICS, this group was excluded from the subgroup analysis. Patients on other prior therapies were also excluded. Abbreviations: CCQ, Clinical COPD Questionnaire; CI, confidence interval; ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist. |
Regardless of treatment pathway, most patients achieved therapeutic success, but the greatest benefit was seen in treatment-naïve patients (85.7% therapeutic success, 95% CI 84.28–86.97), compared with 79.5% (95% CI 75.83–82.92) of those previously treated with LABA/ICS and 74.2% (95% CI 71.76–76.55) of those previously on LABA or LAMA only (nominal p-value <0.0001; Figure 2).
The proportion of patients achieving therapeutic success increased with GOLD spirometry severity (GOLD 1: 70.6%, 95% CI 62.69–77.67; GOLD 2: 81.0%, 95% CI 79.47–82.52; GOLD 3: 82.6%, 95% CI 80.59–84.40; GOLD 4: 85.5%, 95% CI 81.18–89.21).
In the GOLD D and GOLD B groups (ie, patients with more prominent symptoms), more patients achieved therapeutic success (GOLD D: 87.1%, 95% CI 85.54–88.53; GOLD B: 78.3%, 95% CI 76.61–79.92) than in the GOLD C group (66.5%, 95% CI 60.45–72.25) (nominal p-value <0.0001).
A greater proportion of patients with ≥2 exacerbations in the previous 12 months achieved therapeutic success (85.5%, 95% CI 83.60–87.18) compared with those with 0–1 prior exacerbation (79.4%, 95% CI 77.93–80.78; nominal p-value <0.0001). Similar findings were found when patients were stratified by hospitalization due to exacerbations, with 86.1% (95% CI 84.21–87.75) of patients hospitalized ≥1 time achieving therapeutic success versus 79.1% (95% CI 77.68–80.54) of participants who had not been hospitalized (nominal p-value <0.0001).
When the data were stratified by cardiac comorbidities, therapeutic success was 82.7% (95% CI 81.14–84.21) for patients without and 80.0% (95% CI 78.32–81.62) for patients with cardiac comorbidities. Results were not significantly different as observed from the 95% CI overlap (although nominal p-value = 0.0173).
Secondary Endpoints
Mean reductions in CCQ total and domain scores between Visit 1 (baseline) and Visit 2 (6 weeks) are shown in Figure 3. When comparing absolute changes in CCQ domain scores by prior treatment, improvements were seen in all prior treatment groups, with the greatest reduction in score seen in patients who were treatment-naïve (Supplementary Table S2).
An overall improvement in PGE scores was seen in the FAS between baseline and Week 6 (Figure 4). At baseline, 55.4% of patients had a PGE score of 3–4 (satisfactory) and 30.4% had a PGE score of 5–6 (good). At Week 6, the proportion of patients with PGE score 3–4 reduced to 18.6% while the proportion of patients with PGE score 5–6 increased to 57.3%. Following stratification by treatment pathway, an improvement in PGE score was observed for all treatment groups (Supplementary Table S3), with a similar pattern to that of the overall group.
In terms of patient satisfaction, 75.1% of the FAS were satisfied or very satisfied with their treatment, 78.7% were satisfied or very satisfied with inhaling from the Respimat®, and 77.4% were satisfied or very satisfied with handling of the Respimat® (Supplementary Figure S1). Overall, patient satisfaction was greater among treatment-naïve patients versus pretreated patients (nominal p-value = 0.002); the same was true for handling of the Respimat® (nominal p-value = 0.0002) and for inhaling from the Respimat® (nominal p-value = 0.0021; Supplementary Figure S1). Overall, 96.6% of the patients were willing to continue treatment with tiotropium/olodaterol after end of study participation.
The mean daily number of puffs of COPD rescue medication was 1.82 puffs (95% CI 1.76–1.89) during the week before Visit 1, compared with 0.57 puffs (95% CI 0.54–0.60) in the week before Visit 2, representing an overall decrease of 1.25 puffs per day (95% CI 1.19–1.31; Supplementary Table S4). When the data were stratified by treatment pathway, the reduction in use of rescue medication in puffs per day was 1.38 (95% CI 1.29–1.47) in treatment-naïve patients, 1.01 (95% CI 0.92–1.11) in those previously on LABA or LAMA only, and 1.17 (95% CI 1.01–1.33) in those previously treated with LABA/ICS (Supplementary Table S4).
Safety
In total, investigator-defined drug-related adverse events occurred in 29 patients (0.6%; Supplementary Table S5).
Serious adverse events that resulted in the death of the patient occurred in 7 patients (0.15%, Supplementary Table S5). None of the serious adverse events were considered related to study treatment.
Discussion
This non-interventional study evaluated clinical control, assessed using the CCQ, following approximately 6 weeks of treatment with tiotropium/olodaterol in patients with COPD in routine clinical practice. In this study, over 80% of patients achieved therapeutic success, with an improved CCQ score seen in more than 90% of patients taking tiotropium/olodaterol. Indeed, the 0.4-point threshold in CCQ score that was used to define therapeutic success was far exceeded, with a mean change of 1.02 points overall.
Clinical control of COPD is an important concept because it helps to differentiate between patients with similar clinical characteristics and degree of airflow obstruction but with a different disease trajectory, which may require different approaches to treatment.25 Hence, clinical control is dynamic in that it takes into account both the current impact of the condition and its stability over time (ie, whether certain aspects such as symptoms, exacerbation history or airflow limitation are worsening or improving).25 Control of COPD, which can be evaluated over time using validated questionnaires such as the CCQ or CAT, provides an additional dimension in the management of the condition and should be considered alongside the assessment of symptoms, comorbidities and the degree of severity of the disease in order to individualize treatment to the patient.25 Recent studies have shown the potential for using clinical control as a sensitive marker of health status and exacerbation risk that can easily be used in clinical practice at each clinic visit.26,27 The magnitude of CCQ change observed with LAMA/LABA therapy in the present report (1.0-point reduction in total CCQ score) is within the range of well-established non-pharmacologic treatment options in COPD, such as pulmonary rehabilitation (0.2–1.3-point reduction in CCQ score) and smoking cessation (0.4–0.5-point reduction in CCQ score), and reflects the mean improvement of CCQ score observed after recovery from COPD exacerbation.28
In this study, there was a high proportion of treatment-naïve patients (57%), with a further 28% previously treated with monotherapy only. According to the GOLD 2021 report, bronchodilation is the basis of maintenance therapy for many patients with COPD, and monotherapy with long-acting bronchodilators such as a LAMA is the preferred first step.4 Dual bronchodilation with a LAMA plus a LABA is recommended as initial therapy only for highly symptomatic GOLD D patients or for those whose disease is not adequately controlled by monotherapy.4 Consistent with this recommendation, a previous real-world study retrospectively surveying patients from a large US claims database found that patients treated with LAMA/LABA combination therapy had a lower CCQ symptom score in comparison with patients receiving monotherapy.29 Previous clinical studies have also shown improvements in health status, as measured using the SGRQ, with tiotropium/olodaterol compared with monotherapy in patients with COPD.14 The present report confirms the benefit of dual bronchodilator therapy, demonstrating a 74% therapeutic success rate for the combination therapy in those patients previously receiving monotherapy and a reduction in CCQ score of 0.78 in these patients.
An even higher therapeutic success rate was observed in treatment-naïve patients, who were treated in line with national guidelines and real-world prescribing practice (LAMA/LABA as initial therapy), rather than the recommendations in the GOLD strategy report. This is consistent with previous findings from the OTIVACTO study, which demonstrated the benefits of tiotropium/olodaterol in the vast majority of maintenance-naïve patients with COPD.30 Several other studies have also shown the benefits of tiotropium/olodaterol, as well as alternative LAMA/LABA combinations, including umeclidinium/vilanterol and indacaterol/glycopyrronium, compared with LAMA monotherapy in maintenance-naïve patients.17,31–34 Together, these results suggest that earlier introduction of dual therapy than currently recommended in the GOLD strategy report may be beneficial. For example, there is a strong recommendation in the American Thoracic Society guidelines for dual bronchodilation over monotherapy in patients with COPD and dyspnea or exercise intolerance.35
Therapeutic success was also high in previously treated patients, including those pretreated with LABA/ICS. Most patients in the latter group were responders, with only a small proportion having worsening CCQ scores. This is supported by previous randomized clinical trials of patients with infrequent exacerbations which suggest that switching treatment from LABA/ICS to LAMA/LABA improves lung function and symptom severity.36,37 In the CRYSTAL study, which evaluated a direct switch from LABA/ICS to indacaterol/glycopyrronium in patients with moderate COPD and a history of ≤1 moderate exacerbation and no severe exacerbation in the previous year, LAMA/LABA was found to be superior to LABA/ICS in terms of improvement in trough forced expiratory volume in 1 second (FEV1) and transition dyspnea index.36 Improvements in health status and lower rescue medication use were also observed with indacaterol/glycopyrronium.36 The FLASH study, which assessed a direct switch from LABA/ICS to indacaterol/glycopyrronium in patients with moderate-to-severe COPD and ≤1 exacerbation in the previous year, demonstrated significant improvement in pre-dose FEV1 and forced vital capacity with the LAMA/LABA compared with LABA/ICS, as well as a numerical improvement in transition dyspnea index.37 The potential benefits of ICS withdrawal coupled with optimization of bronchodilation with LAMA/LABA, including improvements in clinical status and reductions in pneumonia, are discussed in more detail in a recent review by Avdeev et al, which also provides a simple algorithm for withdrawal of ICS therapy in circumstances where ICS use is not warranted.38 This algorithm is further supported by recent European Respiratory Society guidelines on ICS withdrawal.39
As mentioned previously, an algorithm-based approach to COPD treatment is recommended according to the GOLD strategy report, national guidelines and key COPD experts.4,40–44 In Russia, for example, LAMA/LABA treatment is recommended as initial treatment for highly symptomatic patients (modified Medical Research Council score ≥2 or CAT score >10) to relieve dyspnea and improve exercise tolerance.40 As reflected in these algorithms,4,40–44 although highly symptomatic patients may have greater benefits from dual bronchodilation therapy versus monotherapy, patients with frequent exacerbations should receive phenotype-driven therapy on top of LAMA/LABA therapy.43,45,46 In the current report, the majority of GOLD D patients (ie, frequent exacerbators) achieved therapeutic success, as did GOLD B patients. These results confirm findings from OTIVACTO, in which the highest therapeutic success rates, defined as a ≥10-point increase in the 10-question Physical Functioning Questionnaire score after approximately 6 weeks, were found in GOLD D and B patients.30 This supports the hypothesis that more symptomatic patients get the most benefit from dual therapy, although less symptomatic patients may also benefit30 (66.5% of GOLD C patients achieved therapeutic success according to CCQ; data not shown).
Patients with cardiovascular comorbidities are frequently excluded from randomized clinical trials. In this real-world study, almost 50% of patients had concomitant cardiovascular disease, which reflects routine clinical practice. Patients with cardiovascular disease may have other reasons for dyspnea, which should be thoroughly investigated if a patient does not respond to inhaler therapy.47 Although more patients without cardiac comorbidities achieved therapeutic success compared with those with comorbidities, the changes were not statistically significant.
Notably, the use of COPD rescue medicine (short-acting β2-agonist) decreased by 1.3 puffs per day, averaged over a 1-week period before Visit 1 and Visit 2. The observed reductions in rescue medication in our report correspond with previous findings and can be considered of clinical relevance.48 The greatest reduction was seen in treatment-naïve patients, followed by those previously treated with LABA/ICS. The magnitude of the decrease compares favorably with previous studies comparing tiotropium/olodaterol with tiotropium or olodaterol monotherapy13 or switching from LABA/ICS to indacaterol/glycopyrronium.36 Evidence from systematic reviews and meta-analyses also supports a reduction in rescue medication usage with LAMA/LABA combination therapy compared with LAMA monotherapy or LABA/ICS.49,50 Declining rescue medication use is important as it reflects better clinical disease control48,51 and is likely to have other important consequences, such as reduced side effects.
Improvements in patients’ general condition were also observed throughout the study, as evidenced by the change in PGE scores from baseline to Week 6. Furthermore, the majority of patients were satisfied or very satisfied with their treatment, and with inhalation from/handling of the device, translating to over 95% of patients being willing to continue tiotropium/olodaterol treatment after the study. Similar results in terms of PGE and treatment satisfaction have previously been reported with tiotropium/olodaterol in real-life studies.30,52 Studies in patients with COPD have also previously shown that the Respimat® device is easy to use, reliable and durable, with high reported levels of satisfaction with the device.52–56 In the current study, satisfaction with device handling was slightly higher for younger patients, but still had good results in older patients (80% of those ≤65 years were satisfied or very satisfied, versus 75% for those >65 years).
The number of patients reporting adverse events in this study was low and consistent with the known safety profile of tiotropium/olodaterol, with serious adverse events (measured here as those leading to a fatal outcome) reported by considerably fewer patients in this real-world study compared with randomized controlled trials.57 Exacerbation rate data were not captured, but analyses of adverse drug reactions and serious adverse events leading to death did not show any strong signals regarding exacerbations. Although seven deaths (0.15% of patients) were reported during the study, none were considered to be related to study medication.
The study had some strengths and limitations. To the best of our knowledge, this was the largest prospective real-world study to assess clinical control with a fixed-dose combination of tiotropium/olodaterol. Evaluation of treatment success using the CCQ benefited from simplicity for the patient, as well as a good correlation with the SGRQ.24 The CCQ is also preferred over the CAT by patients with COPD, as it reflects their health status better by providing more details on breathing problems.58
Non-interventional studies also have their own inherent weaknesses. Firstly, the real-world observational nature of the current study is a limitation compared with a randomized controlled clinical trial, with no control group with which to compare the intervention. However, the differential responses observed in different subgroups, such as the differences between GOLD subgroups, are suggestive of treatment benefit rather than a mere placebo effect. In addition, the observation period was short in this study, although this was intentional in order to reduce the risk of recall bias. An average 6 weeks’ follow-up window, however, did not allow time to collect additional information, such as exacerbation data. There were also several other potential sources of bias in this study, including recruitment bias and potential bias towards the Respimat® device in the patient satisfaction data. The proportion of patients on ICS-containing regimens was also lower than in other studies, which may reflect recruitment bias or the reluctance in clinical practice to step up patients from LABA/ICS to LAMA/LABA (making them eligible for this study) despite clinical recommendations, although the numbers were sufficient to make a valid comparison with other treatment pathways. In general, assessment of patient satisfaction has inherent weaknesses and is very subjective, which may also be viewed as a study limitation. Another potential weakness is suggested by a recent study, which found that MCID estimates of health status questionnaires can vary significantly depending on baseline patient characteristics/disease severity.59
Conclusions
Over 6 weeks, treatment with tiotropium/olodaterol improved clinical control, assessed using the CCQ, in a large multinational population of patients with COPD. Tiotropium/olodaterol also improved the general condition of the patient and reduced the use of rescue medication, with 75% of patients either satisfied or very satisfied with their treatment. Tiotropium/olodaterol was well tolerated, with a low incidence of drug-related adverse events in these typical COPD patients.
Key data from this study, which evaluated treatment success with tiotropium/olodaterol in patients with COPD in a real-world setting, confirm findings from the tiotropium/olodaterol clinical trial program.
Abbreviations
CAT, COPD Assessment Test; CCQ, Clinical COPD Questionnaire; CI, confidence interval; COPD, chronic obstructive pulmonary disease; FAS, full analysis set; FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; MCID, minimal clinically important difference; PGE, Physician’s Global Evaluation; SGRQ, St. George’s Respiratory Questionnaire; SMI, Soft MistTM inhaler; SmPC, summary of product characteristics.
Data Sharing Statement
Data used in this analysis are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study was submitted to the ethics committee of participating countries according to national regulations. Sites could only participate if independent ethics committee approval in their country was issued. Written informed consent was required by all study subjects prior to participation. Approvals were as follows: Bulgaria (Republic of Bulgaria Ministry of Health Ethical Commission for Clinical Trials; positive vote on 20 March 2019), Czech Republic (no approval needed), Hungary (Medical Research Council Scientific and Research Ethics Commission; positive vote on 29 August 2018), Israel (Meir Medical Center Helsinki Committee; positive vote separately for each site; first approval on 06 November 2018), Lithuania (Lithuanian Bioethics Committee; positive vote on 07 August 2018), Poland (no approval needed, acknowledgement of notification on 10 July 2018), Romania (National Commission for Bioethics of Medicines and Medical Devices; positive vote on 19 January 2016), Russia (Independent Interdisciplinary Committee on Ethical Review of Clinical Trials; positive vote of the main ethics committee on 24 August 2018), Slovenia (Commission of the Republic of Slovenia for Medical Ethics; positive vote on 23 July 2018), Switzerland (Cantonal Ethics Committee of Zurich; positive vote on 27 November 2018) and Ukraine (Ethics Commission at the Lviv Municipal Institution regional council; positive vote separately for each site; first approval on 24 September 2018).
Acknowledgments
Medical writing assistance was provided by Cindy Macpherson, PhD, of MediTech Media and was funded by Boehringer Ingelheim International GmbH.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Boehringer Ingelheim.
Disclosure
Arschang Valipour has received speaker/consultancy fees from Boehringer Ingelheim during the conduct of the study; and speaker/consultancy fees from Boehringer Ingelheim, Chiesi, Novartis, Menarini and AstraZeneca outside the submitted work. Sergey Avdeev has nothing to disclose. Adam Barczyk has received personal fees from Boehringer Ingelheim. Valentina Bayer was an employee of Boehringer Ingelheim during the conduct of the study. Zvi Fridlender received institutional support for conducting the study from Boehringer Ingelheim. Dr Fridlender received personal fees for lectures/conference travel/advice from GlaxoSmithKline, Novartis, AstraZeneca, Boehringer Ingelheim, Teva and Rafa outside the submitted work. In addition, Dr. Fridlender has a patent for a neutrophil-specific drug delivery pending. Mariela Georgieva received trial sponsorship fees from Chiesi and AstraZeneca, grants for travel and lectures from Boehringer Ingelheim, grants for lectures from Mundipharma, and participated in lectures for Novartis outside the submitted work. Ondřej Kudela reports personal fees from the Charles University in Prague and Boehringer Ingelheim outside the submitted work. Alexey Medvedchikov was an employee of Boehringer Ingelheim during the conduct of the study. Ramona Miron has nothing to disclose. Maria Sanzharovskaya was an employee of Boehringer Ingelheim during the conduct of the study. Virginija Šileikienė has received advisory fees from AstraZeneca and GlaxoSmithKline, speaker fees from AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, GlaxoSmithKline and Novartis, and has been involved with clinical trials for Boehringer Ingelheim, MSD and Novartis outside the submitted work. Jurij Šorli has nothing to disclose. Marc Spielmanns reports personal fees from Boehringer Ingelheim during the conduct of the study. Zsuzsanna Szalai has nothing to disclose. The authors report no other conflicts of interest in this work.
References
1. GBD Disease Injury Incidence Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1789–1858.
2. Mathers CD, Loncar D, Samet J. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi:10.1371/journal.pmed.0030442
3. Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523–532. doi:10.1183/09031936.06.00124605
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2020[
5. Wilson MR, Patel JG, Coleman A, McDade CL, Stanford RH, Earnshaw SR. Cost-effectiveness analysis of umeclidinium/vilanterol for the management of patients with moderate to very severe COPD using an economic model. Int J Chron Obstruct Pulmon Dis. 2017;12:997–1008. doi:10.2147/COPD.S124420
6. Miravitlles M, Galdiz JB, Huerta A, Villacampa A, Carcedo D, Garcia-Rio F. Cost-effectiveness of combination therapy umeclidinium/vilanterol versus tiotropium in symptomatic COPD Spanish patients. Int J Chron Obstruct Pulmon Dis. 2016;11:123–132. doi:10.2147/COPD.S94006
7. Plunkett T, Carty P, O’Neill M, Harrington P, Smith SM, Ryan M. VP19 cost-effectiveness of combination inhaled long-acting bronchodilators. Int J Technol Assess Health Care. 2019;35(S1):80. doi:10.1017/S0266462319002952
8. Hoogendoorn M, Corro Ramos I, Soulard S, et al. PRS30 cost-effectiveness of the fixed-dose combination tiotropium+olodaterol versus tiotropium and Laba/Ics in Finland, Sweden and the Netherlands. Value Health. 2020;23:S722. doi:10.1016/j.jval.2020.08.1910
9. Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi:10.1056/NEJMoa0805800
10. Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364(12):1093–1103. doi:10.1056/NEJMoa1008378
11. Wise RA, Anzueto A, Cotton D, et al. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med. 2013;369(16):1491–1501. doi:10.1056/NEJMoa1303342
12. Ferguson GT, Feldman GJ, Hofbauer P, et al. Efficacy and safety of olodaterol once daily delivered via Respimat® in patients with GOLD 2–4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:629–645. doi:10.2147/COPD.S61717
13. Buhl R, Maltais F, Abrahams R, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2–4). Eur Respir J. 2015;45(4):969–979. doi:10.1183/09031936.00136014
14. Singh D, Ferguson GT, Bolitschek J, et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respir Med. 2015;109(10):1312–1319. doi:10.1016/j.rmed.2015.08.002
15. Maltais F, Aumann JL, Kirsten A-M, et al. Dual bronchodilation with tiotropium/olodaterol further reduces activity-related breathlessness versus tiotropium alone in COPD. Eur Respir J. 2019;53(3):1802049. doi:10.1183/13993003.02049-2018
16. Troosters T, Maltais F, Leidy N, et al. Effect of bronchodilation, exercise training, and behavior modification on symptoms and physical activity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(8):1021–1032. doi:10.1164/rccm.201706-1288OC
17. Rabe KF, Chalmers JD, Miravitlles M, et al. Tiotropium/olodaterol delays clinically important deterioration compared with tiotropium monotherapy in patients with early COPD: a post hoc analysis of the TONADO® trials. Adv Ther. 2021;38(1):579–593. doi:10.1007/s12325-020-01528-2
18. Tashkin DP, Amin AN, Kerwin EM. Comparing randomized controlled trials and real-world studies in chronic obstructive pulmonary disease pharmacotherapy. Int J Chron Obstruct Pulmon Dis. 2020;15:1225–1243. doi:10.2147/COPD.S244942
19. Soler-Cataluna JJ, Alcazar-Navarrete B, Miravitlles M. The concept of control of COPD in clinical practice. Int J Chron Obstruct Pulmon Dis. 2014;9:1397–1405. doi:10.2147/COPD.S71370
20. Alma H, de Jong C, Tsiligianni I, Sanderman R, Kocks J, van der Molen T. Clinically relevant differences in COPD health status: systematic review and triangulation. Eur Respir J. 2018;52(3):1800412. doi:10.1183/13993003.00412-2018
21. Kon SS, Dilaver D, Mittal M, et al. The clinical COPD questionnaire: response to pulmonary rehabilitation and minimal clinically important difference. Thorax. 2014;69(9):793–798. doi:10.1136/thoraxjnl-2013-204119
22. Jones PW, Quirk FH, Baveystock CM. The St George’s respiratory questionnaire. Respir Med. 1991;85(Suppl B):25–31. doi:10.1016/S0954-6111(06)80166-6
23. Kocks JW, Tuinenga MG, Uil SM, van den Berg JW, Stahl E, van der Molen T. Health status measurement in COPD: the minimal clinically important difference of the clinical COPD questionnaire. Respir Res. 2006;7(1):62. doi:10.1186/1465-9921-7-62
24. van der Molen T, Willemse BW, Schokker S, Ten Hacken NH, Postma DS, Juniper EF. Development, validity and responsiveness of the clinical COPD questionnaire. Health Qual Life Outcomes. 2003;1(1):13. doi:10.1186/1477-7525-1-13
25. Soler-Cataluña JJ, Alcázar-Navarrete B, Miravitlles M. The concept of control in COPD: a new proposal for optimising therapy. Eur Respir J. 2014;44(4):1072–1075. doi:10.1183/09031936.00064414
26. Miravitlles M, Sliwinski P, Rhee CK, et al. Predictive value of control of COPD for risk of exacerbations: an international, prospective study. Respirology. 2020;25(11):1136–1143. doi:10.1111/resp.13811
27. Soler X. Evaluation of changes in control status in COPD: an opportunity for early intervention. Chest. 2020;157(5):1064–1066. doi:10.1016/j.chest.2020.03.017
28. Zhou Z, Zhou A, Zhao Y, Chen P. Evaluating the clinical COPD questionnaire: a systematic review. Respirology. 2017;22(2):251–262. doi:10.1111/resp.12970
29. Strange C, Walker V, DePietro M, et al. Patient-reported outcomes of dual bronchodilator fixed-dose combination versus bronchodilator monotherapy in individuals with COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:1377–1388. doi:10.2147/COPD.S194856
30. Valipour A, Tamm M, Kociánová J, et al. Improvement of self-reported physical functioning with tiotropium/olodaterol in central and Eastern European COPD patients. Int J Chron Obstruct Pulmon Dis. 2019;14:2343–2354. doi:10.2147/COPD.S204388
31. Muro SYH, Kostikas K, Olsson P, Gupta P, Wedzicha JA. Indacaterol/glycopyrronium versus tiotropium or glycopyrronium in long‐acting bronchodilator‐naïve COPD patients: a pooled analysis. Respirology. 2020;25(4):393–400. doi:10.1111/resp.13651.
32. Maleki-Yazdi MR, Singh D, Anzueto A, Tombs L, Fahy WA, Naya I. Assessing short-term deterioration in maintenance-naive patients with COPD receiving umeclidinium/vilanterol and tiotropium: a pooled analysis of three randomized trials. Adv Ther. 2016;33(12):2188–2199. doi:10.1007/s12325-016-0430-6
33. Bjermer LH, Kerwin E, Maltais F, et al. Comparative efficacy and safety of umeclidinium/vilanterol, umeclidinium and salmeterol in symptomatic maintenance-naïve and maintenance-treated chronic obstructive pulmonary disease: a pre-specified secondary analysis of the EMAX trial. Am J Respir Crit Care Med. 2019;199:A3317.
34. Buhl R, de la Hoz A, Xue W, Singh D, Ferguson GT. Efficacy of tiotropium/olodaterol compared with tiotropium as a first-line maintenance treatment in patients with COPD who are naïve to LAMA, LABA and ICS: pooled analysis of four clinical trials. Adv Ther. 2020;37(10):4175–4189. doi:10.1007/s12325-020-01411-0
35. Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56–e69. doi:10.1164/rccm.202003-0625ST
36. Vogelmeier CF, Gaga M, Aalamian-Mattheis M, et al. Efficacy and safety of direct switch to indacaterol/glycopyrronium in patients with moderate COPD: the CRYSTAL open-label randomised trial. Respir Res. 2017;18(1):140. doi:10.1186/s12931-017-0622-x
37. Frith PA, Ashmawi S, Krishnamurthy S, et al. Efficacy and safety of the direct switch to indacaterol/glycopyrronium from salmeterol/fluticasone in non‐frequently exacerbating COPD patients: the FLASH randomized controlled trial. Respirology. 2018;23(12):1152–1159. doi:10.1111/resp.13374
38. Avdeev S, Aisanov Z, Arkhipov V, et al. Withdrawal of inhaled corticosteroids in COPD patients: rationale and algorithms. Int J Chron Obstruct Pulmon Dis. 2019;14:1267–1280. doi:10.2147/COPD.S207775
39. Chalmers JD, Laska IF, Franssen FME, et al. Withdrawal of inhaled corticosteroids in COPD: a European Respiratory Society guideline. Eur Respir J. 2020;55(6):2000351. doi:10.1183/13993003.00351-2020
40. Aisanov Z, Avdeev S, Arkhipov V, et al. Russian guidelines for the management of COPD: algorithm of pharmacologic treatment. Int J Chron Obstruct Pulmon Dis. 2018;13:183–187. doi:10.2147/COPD.S153770
41. Vukoja M, Kopitovic I, Lazic Z, et al. Diagnosis and management of chronic obstructive pulmonary disease in Serbia: an expert group position statement. Int J Chron Obstruct Pulmon Dis. 2019;14:1993–2002. doi:10.2147/COPD.S214690
42. Ulmeanu R, Fildan AP, Oancea C, Mihaltan F. Recomandări De Diagnostic Și Tratament În Bronhopneumopatia Obstructivă Cronică. Bucharest: MEDICALA; 2019.
43. Miravitlles M, Anzueto A. A new two-step algorithm for the treatment of COPD. Eur Respir J. 2017;49(2):1602200. doi:10.1183/13993003.02200-2016
44. Matsunaga K, Oishi K, Miravitlles M, Anzueto A. Time to revise COPD treatment algorithm. Int J Chron Obstruct Pulmon Dis. 2019;14:2229–2234. doi:10.2147/COPD.S219051
45. Ariel A, Altraja A, Belevskiy A, et al. Inhaled therapies in patients with moderate COPD in clinical practice: current thinking. Int J Chron Obstruct Pulmon Dis. 2017;13:45–56. doi:10.2147/COPD.S145573
46. Tudoric N, Koblizek V, Miravitlles M, et al. GOLD 2017 on the way to a phenotypic approach? Analysis from the Phenotypes of COPD in Central and Eastern Europe (POPE) cohort. Eur Respir J. 2017;49(4):1602158. doi:10.1183/13993003.02518-2016
47. Trinkmann F, Saur J, Borggrefe M, Akin I. Cardiovascular comorbidities in chronic obstructive pulmonary disease (COPD)—current considerations for clinical practice. J Clin Med. 2019;8(1):69. doi:10.3390/jcm8010069
48. Punekar YS, Sharma S, Pahwa A, Takyar J, Naya I, Jones PW. Rescue medication use as a patient-reported outcome in COPD: a systematic review and regression analysis. Respir Res. 2017;18(1):86. doi:10.1186/s12931-017-0566-1
49. Rogliani P, Calzetta L, Braido F, et al. LABA/LAMA fixed-dose combinations in patients with COPD: a systematic review. Int J Chron Obstruct Pulmon Dis. 2018;13:3115–3130. doi:10.2147/COPD.S170606
50. Rodrigo GJ, Price D, Anzueto A, et al. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;12:907–922. doi:10.2147/COPD.S130482
51. Sharafkhaneh A, Altan AE, Colice GL, et al. A simple rule to identify patients with chronic obstructive pulmonary disease who may need treatment reevaluation. Respir Med. 2014;108(9):1310–1320. doi:10.1016/j.rmed.2014.07.002
52. Steinmetz KO, Abenhardt B, Pabst S, et al. Assessment of physical functioning and handling of tiotropium/olodaterol Respimat® in patients with COPD in a real-world clinical setting. Int J Chron Obstruct Pulmon Dis. 2019;14:1441–1453. doi:10.2147/COPD.S195852
53. Taube C, Bayer V, Zehendner CM, Valipour A. Assessment of patient experiences with Respimat® in everyday clinical practice. Pulm Ther. 2020;6(2):371–380. doi:10.1007/s41030-020-00127-4
54. Davis KH, Su J, Gonzalez JM, et al. Quantifying the importance of inhaler attributes corresponding to items in the patient satisfaction and preference questionnaire in patients using Combivent Respimat. Health Qual Life Outcomes. 2017;15(1):201. doi:10.1186/s12955-017-0780-z
55. Dekhuijzen PN, Lavorini F, Usmani OS. Patients’ perspectives and preferences in the choice of inhalers: the case for Respimat® or HandiHaler®. Patient Prefer Adherence. 2016;10:1561–1572. doi:10.2147/PPA.S82857
56. Schurmann W, Schmidtmann S, Moroni P, Massey D, Qidan M. Respimat® Soft Mist™ inhaler versus hydrofluoroalkane metered dose inhaler: patient preference and satisfaction. Treat Respir Med. 2005;4(1):53–61. doi:10.2165/00151829-200504010-00006
57. Ferguson GT, Buhl R, Bothner U, et al. Safety of tiotropium/olodaterol in chronic obstructive pulmonary disease: pooled analysis of three large, 52-week, randomized clinical trials. Respir Med. 2018;143:67–73. doi:10.1016/j.rmed.2018.08.012
58. Tsiligianni IG, van der Molen T, Moraitaki D, et al. Assessing health status in COPD. A head-to-head comparison between the COPD assessment test (CAT) and the clinical COPD questionnaire (CCQ). BMC Pulm Med. 2012;12(1):20. doi:10.1186/1471-2466-12-20
59. Alma H, de Jong C, Jelusic D, et al. Baseline health status and setting impacted minimal clinically important differences in COPD: an exploratory study. J Clin Epidemiol. 2019;116:49–61. doi:10.1016/j.jclinepi.2019.07.015
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


