Back to Journals » Journal of Asthma and Allergy » Volume 13
Therapeutic Potential of Lebrikizumab in the Treatment of Atopic Dermatitis
Authors Loh TY, Hsiao JL, Shi VY
Received 1 December 2019
Accepted for publication 29 January 2020
Published 11 February 2020 Volume 2020:13 Pages 109—114
DOI https://doi.org/10.2147/JAA.S211032
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Tiffany Y Loh,1 Jennifer L Hsiao,2 Vivian Y Shi1
1University of Arizona, Division of Dermatology, Tucson, AZ, USA; 2University of California Los Angeles, Division of Dermatology, Los Angeles, CA, USA
Correspondence: Vivian Y Shi
Division of Dermatology, University of Arizona, 7165 N Pima Canyon Drive, Tucson, AZ 85718, USA
Tel +1 520 694 2055
Fax +1 520 694 2005
Email [email protected]
Background: Atopic dermatitis (AD) is a chronic, relapsing skin condition with a wide disease spectrum. Moderate-to-severe cases often need systemic treatment. Conventional immunosuppressants have extensive side effect profiles and require close monitoring. In recent decades, there has been increasing interest in developing targeted systemic immunomodulators for AD, as they have been shown to have efficacy for AD as well as favorable safety profiles. Herein, we review the recent data on lebrikizumab, an interleukin (IL)-13 inhibitor, and its potential role in the treatment of AD.
Objective: Review the mechanism of action, and available data on the efficacy and safety of lebrikizumab for the treatment of AD.
Methods: PubMed, Google Scholar, and clinicaltrials.gov searches were performed with the following terms: “atopic dermatitis,” “dermatitis,” “eczema,” “lebrikizumab,” “IL-4,” and “IL-13.”
Results: Two Phase II randomized controlled clinical trials have been conducted to evaluate the use of lebrikizumab in a total of 289 patients with moderate-severe AD and inadequate response to topical corticosteroids. Patients treated with lebrikizumab experienced significantly more improvement in their AD compared to placebo, as measured by Eczema Area and Severity Index (EASI)-50 and EASI-75 scores, pruritus scores, and reduction in body surface area (BSA). Its clinical efficacy appears to be dose-dependent, and it has a favorable side effect profile and is generally well tolerated.
Conclusion: Lebrikizumab appears to be a promising emerging targeted biologic for the treatment of moderate-to-severe AD. Further Phase III studies investigating optimal dosing regimens and safety profile are needed.
Keywords: lebrikizumab, atopic dermatitis, eczema, dermatitis, IL-4, IL-13
Introduction
Atopic dermatitis (AD) is a chronic, inflammatory skin condition characterized by pruritus, impaired skin barrier function, and a relapsing course.1 AD affects a substantial portion of the population globally, with an estimated prevalence of up to 3% in adults and 20% in children.2 In the United States, approximately half of adult AD patients and one third of pediatric AD patients have moderate to severe disease.3
In mild AD cases with limited body surface area involvement, treatment with topical corticosteroids, topical calcineurin inhibitors, or phototherapy in conjunction with frequent moisturization and a gentle skin care routine may be adequate. However, in patients with moderate-to-severe disease, such treatments alone may be insufficient for controlling AD, and these patients often have significantly impaired quality of life. Increasing disease activity has been associated with greater quality of life impairment, and AD patients have been found to have poorer mental health scores in comparison to the general population.4
Conventional systemic agents for the treatment of moderate-to-severe AD include corticosteroids, methotrexate, mycophenolate mofetil, cyclosporine, and azathioprine.5,6 While these agents have shown efficacy, their extensive side effect profiles limit chronic use. Furthermore, none of these agents target any specific component of the AD disease pathway and instead, act as general immunosuppressants.
To date, the only FDA-approved targeted systemic therapy for AD is dupilumab, a monoclonal antibody that binds to the alpha subunit of the interleukin-4 receptor (IL-4Rα).7,8 IL-4Rα is expressed on mast cells, eosinophils, and macrophages, and activation leads to the release of inflammatory mediators such as histamine, eicosanoids, and leukotrienes.9,10 IL-4 and IL-13 share a common pathway in driving Th2-mediated inflammation.10,11 In addition, as increased levels of IL-13 mRNA have been found in lesional AD skin relative to IL-4 mRNA, IL-13 has been suggested to play an even more substantial role in AD pathogenesis.12 Lebrikizumab is a fully human monoclonal antibody targeting IL-13, thus inhibiting the IL-13 driven Th2 inflammatory response. Therefore, new therapies that selectively inhibit IL-13, such as lebrikizumab (DRM06), are of significant interest and may represent a promising alternative to immunosuppressants and dupilumab in AD treatment.
Methods
A literature search using PubMed, Google Scholar, and clinicaltrials.gov databases were performed using a combination of the following terms: “atopic dermatitis,” “dermatitis,” “eczema,” “lebrikizumab,” “IL-4,” and “IL-13.” Two Phase II randomized clinical trials (RCTs) on lebrikizumab in atopic dermatitis were identified. The results from both studies were accessible, although only one had a peer-reviewed publication available.
IL-13 in AD Pathogenesis
AD is a complex and multifactorial disease. Though the exact etiology has not been fully elucidated, known contributing factors include genetic predisposition, immune dysregulation, skin barrier dysfunction, cutaneous microbiome alteration, and an abnormal itch response.1,3
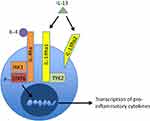
AD is characterized by aberrant Th2 cell activation and overexpression of associated Th2 cytokines, such as IL-4, IL-5, and IL-1313,14 (Figure 1). In patients with AD, inherent skin barrier dysfunction causes increased transepidermal water loss and facilitates the penetration of potential irritants, allergens and microbes.15,16 These antigens are taken up by cutaneous Langerhans cells, which then stimulate Th2 cells to release IL-4 and IL-13.17 In addition to promoting Th2 cell survival, IL-4 and IL-13 also induce IgE class switch.17 IgE binding and stimulation of mast cells and basophils induce histamine, leukotriene, and prostaglandin release, resulting in cutaneous vasodilation and itch.8
In addition to their effects on driving the Th2 inflammatory response, IL-4 and IL-13 appear to contribute to skin barrier dysfunction by downregulating structural proteins important for skin barrier integrity, such as loricrin, filaggrin, and involucrin.21,22 Similar to IL-4,23 the expression of IL-13 mRNA is increased by up to seven times in the lesional skin of AD patients when compared to healthy controls and correlates with disease severity.24
IL-4 and IL-13 are major mediators of the inflammatory response observed in AD through a shared heterodimeric receptor that consists of IL-4Rα and IL-13Rα110,11 (Figure 1). Binding of IL-4 and IL-13 to their respective receptor subunits leads to dimerization of the subunits and subsequent activation of the JAK-STAT pathway.10,11 In this downstream cascade, JAK1 phosphorylates STAT6, a transcription factor that promotes Th2 differentiation and IgE class switching.10,11,18 Additionally, STAT6 has been shown to suppress the activity of T regulatory cells, which are important for maintaining tolerance to self-antigens and preventing aberrant inflammation.19
Available evidence suggests that isolated overexpression of IL-13 in mouse models results in an inflammatory skin response that is phenotypically similar to AD.25 Recently, a study examining the relative importance of IL-4 and IL-13 in AD found that mRNA levels of IL-13 were significantly increased relative to IL-4 mRNA, suggesting that IL-13 may play an even more important role than IL-4 in AD pathogenesis.24
IL-13 is also capable of binding to IL-13Rα2, which does not have any known signaling motifs, and is thought to act as “decoy” receptor that internalizes excess IL-13. Lebrikizumab (previously known as TNX-650, Dermira, Inc. Menlo Park, California, United States) is a humanized IgG4κ monoclonal antibody that binds IL-13 with high affinity in a non-receptor binding domain. Although lebrikizumab’s effects in AD appears to be mediated through selective inhibition of the IL-4Rα/IL-13Rα1 signaling complex, it does not prevent IL-13 from binding to IL-13Rα2, thus leaving endogenous regulation of IL-13 levels through IL-13Rα2 intact.3,20
Given the crucial role of IL-13 in the Th2 inflammatory response, selectively inhibiting IL-13 should be helpful for treating AD. Lebrikizumab has previously been studied in adults with mild to moderate asthma with some efficacy.26,27 More recently, Phase II trials of lebrikizumab have demonstrated some efficacy for AD,28,29 and this new biologic may represent a promising alternative to existing systemic therapies. Herein, we review the current data available on lebrikizumab and its potential role in AD treatment.
Clinical Evidence
Although there have only been two Phase II studies performed to date on lebrikizumab in AD, the preliminary data demonstrate that lebrikizumab may be a promising targeted immunomodulator for patients with moderate to severe disease (Table 1).
 |
Table 1 Summary of Completed Phase II (TREBLE) and Phase IIb RCTs for Lebrikizumab in the Treatment of AD |
Simpson et al conducted a Phase II dose-ranging randomized clinical trial (TREBLE, NCT02340234) in 209 adult patients at 62 sites with moderate to severe AD over the course of 12 weeks.28 Patients between the ages of 18–75 with a diagnosis of moderate-to-severe AD and inadequate response to topical corticosteroids and regular emollients were included. In addition, patients were required to have an Eczema Area and Severity Index (EASI) ≥14 and Investigator Global Assessment (IGA) score ≥3 at screening and at the end of the run-in period, as well as ≥10% body surface area (BSA) involvement and Pruritus Visual Analog Scale (VAS) score ≥3 at screening. Patients were excluded if they used topical calcineurin inhibitors, had recent systemic immunosuppressants or phototherapy, or had evidence of other skin conditions such as T-cell lymphoma or allergic contact dermatitis. The patients were randomized 1:1:1:1 into four arms: Group 1 received lebrikizumab as a single dose subcutaneous injection of 125 mg; Group 2 received a single dose of 250 mg; Group 3 received 125 mg every 4 weeks with no loading dose; and Group 4 received placebo every 4 weeks. All patients were allowed to use concomitant topical corticosteroids as needed. The primary endpoint was to achieve a 50% reduction in Eczema Area and Severity Index (EASI) score from baseline (EASI-50) at week 12. Secondary endpoints included the percentage of patients achieving EASI-75, Investigator Global Assessment (IGA) score of 0 or 1, and SCORAD-50 at week 12.
In the TREBLE study, significantly more patients in group 3 achieved EASI-50 (82.4%, p=0.026) and EASI-75 (54.9%, p=0.036) compared to placebo (62.3% and 34.0%, respectively) at week 12. In addition, out of all the arms, group 3 also experienced the greatest reduction in BSA at week 12 (57.7% reduction). Significantly more patients in Groups 2 and 3 (51.0%, p=0.012 and 47.2%, p=0.030, respectively) achieved SCORAD-50 at week 12, compared to placebo (26.4%).
More recently, a double-blind randomized controlled Phase IIb trial was conducted to further evaluate the efficacy and safety and dose-dependent nature of lebrikizumab in AD (NCT03443024).29 Data currently available are topline results announced by Dermira, Inc. (Menlo Park, California, United States) in March 2019; peer-reviewed publications are not yet available.29 280 adult patients with moderate to severe AD from 57 US sites were evaluated over 16 weeks. Patients were included if they had chronic AD for at least 1 year, EASI score ≥16, IGA score of 3 or 4, and BSA ≥10% at screening and baseline. Subjects were divided into four arms in a 3:3:3:2 ratio: Group 1 received a 250 mg loading dose followed by 125 mg every 4 weeks; Group 2 received a 500 mg loading dose followed by 250 mg every 4 weeks; Group 3 received a 500 mg loading dose at baseline and at week 2 followed by 250 mg every 2 weeks; and Group 4 received placebo at baseline and every 2 weeks. Significantly more patients in Groups 2 and 3 achieved EASI-50, EASI-75, EASI-90, and IGA score of 0 or 1 compared to placebo at week 16, with responses observed as early as week 4 for all AD severity scores (Group 2: 77.0%, 56.1%, 36.1%, 33.7%; Group 3: 81.0%, 60.6%, 44.0%, 44.6%; placebo: 45.8%, 24.3%, 11.4%, 15.3%). In addition, all groups receiving lebrikizumab reported statistically significant improvements in pruritus Numeric Rating Scale (NRS) scores compared to placebo (Group 1: 36.9%, Group 2: 48.6%, Group 3: 61.8% improvement; mean worsening of 6.8% in placebo group).
At the time of the preparation of this article, a Phase 3 randomized double-blind, placebo-controlled, parallel group trial on lebrikizumab in AD is actively recruiting patients (ADvocate1) (NCT04146363).30 The study will investigate the safety and efficacy of lebrikizumab as monotherapy for moderate-to-severe AD and will include a 16-week induction period followed by a 36-week maintenance period. During the induction period, patients will be randomized to receive either placebo or two loading doses of lebrikizumab at baseline and at week 2, followed by a single injection every 2 weeks from weeks 4 to 14. For the maintenance period (weeks 16 to 52), patients will be re-randomized to receive one injection every 2 weeks or one injection every 4 weeks. The co-primary endpoints are the percentage of participants with IGA score 0 or 1 and a reduction of 2 or more points at week 16 from baseline. Secondary endpoints include the percentage of patients achieving EASI-75, EASI-90, change in NRS score, and change in sleep-loss score from baseline to week 16.
Adverse Effects
Thus far, data suggest that lebrikizumab is well tolerated in AD patients. The most common adverse events include upper respiratory tract infection (2.7–11.3%), nasopharyngitis (2.5–12.0%), headache (1.3–5.3%), injection site pain (0.0–5.3%),29 and injection site reaction (1.3%) 3.28 Although there have been reports of conjunctivitis occurring in patients receiving lebrikizumab for AD, the rates of conjunctivitis were 9.6% in the Phase II TREBLE trial and 2.7%, 3.8%, 1.4%, and 0.0% for groups 1, 2, 3, and 4 in the Phase IIb trial); these rates are lower than those in Phase II and Phase III dupilumab trials (8.6–22.1%).31 Conjunctival goblet cells have been demonstrated to be IL-13 responsive and are important for maintaining the epithelial barrier and stimulating mucin production.32 It is possible that conjunctivitis seen with lebrikizumab and dupilumab treatment are due to similar processes. However, further studies are needed to better elucidate this relationship. Lastly, there have been prior reports of peripheral eosinophilia (up to an absolute eosinophil count of 6.0–7.0 x 108/L, normal range up to 5.0 x 108/L)33,34 with lebrikizumab in asthma trials, which is thought to occur via blockage of IL-13 mediated eosinophil chemotaxis.35,36 Peripheral eosinophilia has also been seen in dupilumab trials.37 However, treatment-emergent eosinophilia appears to be transient and is infrequently associated with clinically significant signs or symptoms.28
Conclusions
Due to the identification of key immune mediators implicated in the pathogenesis of AD, there has been a surge of novel immunomodulators targeting these specific etiologic factors.38 IL-13 is one such mediator that appears to play a central role in AD pathogenesis. Lebrikizumab has been studied in asthma with promising results, and based on recent available data, selective inhibition of the IL-13 pathway with lebrikizumab appears to be effective for the treatment of moderate-to-severe AD as well, although optimal dosing regimens have yet to be determined. In addition, lebrikizumab appears to have a relatively favorable side effect profile, with the majority of patients experiencing no significant side effects. However, notably, lebrikizumab appears to have lower rates of ocular complications as dupilumab. IL-4 and IL-13 are both known to stimulate goblet cell secretion,39 and further studies may be needed to elucidate the relative impact of these cytokines in the development of ocular complications.
Given these observations, lebrikizumab may represent a promising targeted immunomodulator for patients with moderate-to-severe AD. The upcoming Phase III clinical trial represents an exciting new phase within the realm of emerging pipeline immunomodulators, and head-to-head studies against dupilumab and other targeted therapies may be warranted in the future.
Disclosure
VYS is a stock shareholder of Learn Health and has served as an advisory board member, investigator, and/or received research funding from Sanofi Genzyme, Regeneron, AbbVie, Eli Lilly, Novartis, Dermira, SUN Pharma, LEO Pharma, Pfizer, Galderma, Menlo Therapeutics, Burt’s Bees, GpSkin, the National Eczema Association, Global Parents for Eczema Research, the Foundation for Atopic Dermatitis and Skin Actives Scientific.
The authors report no other conflicts of interest in this work.
References
1. McPherson T. Current understanding in pathogenesis of atopic dermatitis. Indian J Dermatol. 2016;61:649–655. doi:10.4103/0019-5154.193674
2. Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(Suppl 1):8–16. doi:10.1159/000370220
3. Moyle M, Cevikbas F, Harden JL, Guttman-Yassky E. Understanding the immune landscape in atopic dermatitis: the era of biologics and emerging therapeutic approaches. Exp Dermatol. 2019;28:1–13.
4. Maksimovic N, Jankovic S, Marinkovic J, Sekulovic LK, Zivkovic Z, Spiric VT. Health-related quality of life in patients with atopic dermatitis. J Dermatol. 2012;39:42–47. doi:10.1111/j.1346-8138.2011.01295.x
5. Megna M, Napolitano M, Patruno C, et al. Systemic treatment of adult atopic dermatitis: a review. Dermatol Ther (Heidelb). 2017;7:1–23. doi:10.1007/s13555-016-0170-1
6. Giavina-Bianchi M, Giavina-Bianchi P. Systemic treatment for severe atopic dermatitis. Arch Immunol Ther Exp (Warsz). 2019;67:69–78. doi:10.1007/s00005-018-0521-y
7. Seegraber M, Srour J, Walter A, Knop M, Wollenberg A. Dupilumab for treatment of atopic dermatitis. Expert Rev Clin Pharmacol. 2018;11:467–474. doi:10.1080/17512433.2018.1449642
8. Frampton JE, Blair HA. Dupiluma: a review in moderate-to-severe atopic dermatitis. Am J Clin Dermatol. 2018;19:617–624. doi:10.1007/s40257-018-0370-9
9. Gadani SP, Cronk JK, Norris GT, Kipnis J. Interleukin-4: a cytokine to remember. J Immunol. 2012;189:4213–4219.
10. Silverberg JK, Kantor R. The role of interleukins 4 and/or 13 in the pathophysiology and treatment of atopic dermatitis. Dermatol Clin. 2017;35:327–334. doi:10.1016/j.det.2017.02.005
11. McCormick SM, Heller NM. Commentary: IL-4 and IL-13 receptors and signaling. Cytokine. 2015;75:38–50. doi:10.1016/j.cyto.2015.05.023
12. Bieber T. Interleukin-13: targeting an underestimated cytokine in atopic dermatitis. Allergy. 2019;75:1–9.
13. Li R, Hadi S, Guttman-Yassky E. Current and emerging biologic and small molecule therapies for atopic dermatitis. Expert Opin Biol Ther. 2019;19(4):367–380.
14. Brandt EB, Sivaprasad U. Th2 cytokines and atopic dermatitis. J Clin Cell Immunol. 2011;2:110. doi:10.4049/jimmunol.1202246
15. Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. doi:10.1056/NEJMra074081
16. Bonness S, Bieber T. Molecular basis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2007;7:382–386. doi:10.1097/ACI.0b013e3282a643c3
17. Guttman-Yassky E, Dhingra N, Leung DY. New era of biologic therapeutics in atopic dermatitis. Expert Opin Biol Ther. 2013;13:549–561. doi:10.1517/14712598.2013.758708
18. Goenka S, Kaplan MH. Transcriptional regulation by STAT6. Immunol Res. 2011;50:87–96. doi:10.1007/s12026-011-8205-2
19. Dorsey NJ, Chapoval SP, Smith EP, Skupsky J, Scott DW, Keegan AD. STAT6 controls the number of regulatory T cells in vivo, thereby regulating allergic lung inflammation. J Immunol. 2013;191:1517–1528. doi:10.4049/jimmunol.1300486
20. Ultsch M, Bevers J, Nakamura G, et al. Structural basis of signaling blockade by anti-IL-13 antibody lebrikizumab. J Mol Biol. 2013;425:1330–1339. doi:10.1016/j.jmb.2013.01.024
21. Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008;126:332–337. doi:10.1016/j.clim.2007.11.006
22. Howell MD, Kim BE, Gao P, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2009;124(3 Suppl 2):R7–R12. doi:10.1016/j.jaci.2009.07.012
23. Sehra S, Yaho Y, Howell MD, et al. IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J Immunol. 2010;184:3186–3190. doi:10.4049/jimmunol.0901860
24. Tazawa T, Sugiura H, Sugiura Y, Uehara M. Relative importance of IL-4 and IL-13 in lesional skin of atopic dermatitis. Arch Dermatol Res. 2004;295:459–464. doi:10.1007/s00403-004-0455-6
25. Zheng T, Oh MH, Oh SY, Schroeder JT, Glick AB, Zhu Z. Transgenic expression of interleukin-13 in the skin induces a pruritic dermatitis and skin remodeling. J Invest Dermatol. 2009;129:742–751. doi:10.1038/jid.2008.295
26. Korenblat P, Kerwin E, Leshchenko I, et al. Efficacy and safety of lebrikizumab in adult patients with mild-to-moderate asthma not receiving inhaled corticosteroids. Respir Med. 2018;134:143–149. doi:10.1016/j.rmed.2017.12.006
27. Bujarski S, Parulekar AD, Hanania NA. Lebrikizumab in the treatment of asthma. Expert Opin Biol Ther. 2016;16:847–852. doi:10.1080/14712598.2016.1182152
28. Simpson EL, Flohr C, Eichenfield LF, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, Placebo-controlled Phase II trial (TREBLE). J Am Acad Dermatol. 2018;78(5):863–871.e11.
29. Dermira presents data from phase 2b study of lebrikizumab in patients with atopic dermatitis at fall clinical dermatology conference. Dermira, Inc.; October 10, 2019. Available from: https://investor.dermira.com/news-releases/press-release-details/2019/Dermira-Presents-Data-From-Phase-2b-Study-of-Lebrikizumab-in-Patients-With-Atopic-Dermatitis-at-Fall-Clinical-Dermatology-Conference/default.aspx.
30. Evaluation of the efficacy and safety of lebrikizumab in moderate to severe atopic dermatitis (ADvocate1). ClinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT04146363?term=lebrikizumab&phase=2&draw=2&rank=4.
31. Akinlade B, Guttman-Yassky E, de Bruin-weller M, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol. 2019;181:459–473. doi:10.1111/bjd.17869
32. Henriksson JT, Coursey TG, Corry DB, De Paiva CS, Pflugfelder SC. IL-13 stimulates proliferation and expression of mucin and immunomodulatory genes in cultured conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2015;56:4187–4197.
33. Hanania NA, Noonan M, Corren J, et al. Lebrikizumab in moderate-tot-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax. 2015;70:748–756. doi:10.1136/thoraxjnl-2014-206719
34. Doran E, Cai F, Holweg CTJ, Wong K, Brumm J, Arron JR. Interleukin-13 in asthma and other eosinophilic disorders. Front Med (Lausanne). 2017;4:139. doi:10.3389/fmed.2017.00139
35. Corren J, Lemanske RF, Hanania NA, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi:10.1056/NEJMoa1106469
36. Hanania NA, Korenblat P, Chapman KR, et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomized, double-blind, placebo-controlled trials. Lancet Respir Med. 2016;4:781–796. doi:10.1016/S2213-2600(16)30265-X
37. Faiz S, Giovannelli J, Celine Podevin C, et al. Effectiveness and safety of dupilumab for the treatment of atopic dermatitis in a real-life FRe ch multicenter adult cohort. J Am Acad Dermatol. 2019;81:143–151. doi:10.1016/j.jaad.2019.02.053
38. Lee DE, Clark AK, Tran KA, Shi VY. New and emerging targeted systemic therapies: a new era for atopic dermatitis. J Dermatolog Treat. 2018;29:364–374. doi:10.1080/09546634.2017.1373736
39. Garcia-Posada L, Hodges RR, Diebold Y, Dartt DA. Context-dependent regulation of conjunctival goblet cell unction by allergic mediators. Sci Rep. 2018;8:12162. doi:10.1038/s41598-018-30002-x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.