Back to Journals » Therapeutics and Clinical Risk Management » Volume 16
Therapeutic Potential of Dupilumab in the Treatment of Chronic Rhinosinusitis with Nasal Polyps: Evidence to Date
Authors Kim J , Naclerio R
Received 19 November 2019
Accepted for publication 21 December 2019
Published 23 January 2020 Volume 2020:16 Pages 31—37
DOI https://doi.org/10.2147/TCRM.S210648
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Garry Walsh
Video abstract presented by Jean Kim.
Views: 8288
Jean Kim,1,2 Robert Naclerio1
1Department of Otolaryngology-Head and Neck Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA; 2Department of Medicine: Allergy and Clinical Immunology, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Correspondence: Jean Kim
Johns Hopkins Bayview Medical Center, 4940 Eastern Ave, Suite A102B, Baltimore, MD 21224, USA
Tel +1 410-550-0460
Fax +1 410-550-2871
Email [email protected]
Abstract: Chronic rhinosinusitis with nasal polyposis (CRSwNP) is one of the most severe forms of chronic rhinosinusitis. CRSwNP is characterized by nasal and facial congestion, loss of sense of smell, rhinorrhea, and post-nasal drip. Treatments have been ineffective at controlling disease recurrence, despite multiple courses of medical and surgical therapies. Oral glucocorticoid therapy is often used to control exacerbations leaving the patient exposed to steroid-induced adverse effects. Thus, there is a clear unmet need for new treatments to achieve better control of the disease. Advances in understanding Type 2 inflammatory processes that occur in about 80% of the Western world patients with CRSwNP have resulted in new avenues for disease control. Biologics in the form of monoclonal antibodies, which target Type 2 inflammation, have helped control the severest forms of atopic dermatitis and asthma. Treatment regimes for CRSwNP now include biologics. In July 2019, dupilumab was the first monoclonal antibody to gain FDA approval for the treatment of CRSwNP. In this review, we summarize the proof of concept clinical trials and Phase 3 trials leading to approval of dupilumab, an anti-IL4 alpha receptor antagonist that blocks the actions of both IL4 and IL13. These studies show that dupilumab is a proven treatment option to control disease. Collective studies demonstrate a high safety profile. Questions arise as to the best use of dupilumab in the context of current treatment paradigms, and for which sub-population of the varied heterogeneous endotypes of CRSwNP patients. Recognizing the high cost of biologics forces the need for cost-effectiveness analysis.
Keywords: chronic rhinosinusitis, nasal polyps, Type 2 inflammation, dupilumab
Nasal Polyp Prevalence, Pathophysiology, Current Treatment
Chronic rhinosinusitis (CRS) is the second most common chronic condition in the United States.1 Chronic rhinosinusitis with nasal polyposis (CRSwNP), the most severe subtype of CRS, characterized by tissue and peripheral eosinophilia, with 4% prevalence or 13 million individuals in the USA, incurs the majority of the health-care cost.1 In CRSwNP, there are frequent recurrences after medical and surgical treatment.2 Medical management of chronic rhinosinusitis with nasal polyposis addresses the underlying inflammation, mucous production, nasal airway obstruction and reduced sense of smell. Treatments include topical intranasal corticosteroids, nasal saline irrigation, antibiotics to address acute bacterial exacerbations, and/or short-course oral steroids.3 Sinus surgery is an option for those patients whose symptoms persist despite appropriate medical treatment. Sinus surgery is followed by medical therapy primarily in the form of topical corticosteroids. Disease recurrence after surgery in CRSwNP patients can be as high as 50% when followed over a 3-year period, even after multimodal medical treatment approaches have been tried.4
Dupilumab Mechanism of Action
The rational for biologic drug development derives from recent advances in the understanding of the pathogenesis of CRSwNP and it’s related lower airway disease, asthma. CRSwNP is characterized by defective barrier function of epithelium and Type 2 pattern of inflammation that is also observed with asthma.5 Epithelial activation by microbes and T cells are thought to result in epithelial-derived cytokines secretion, including interleukin IL25, IL33 and thymic stromal lymphopoietin (TSLP) (see Figure 1 of the original study by Hulse).6 These cytokines activate type 2 innate lymphoid cells (ILCs), adaptive T helper cells, dendritic cells and mast cells in this tissue to promote Type 2 pattern of inflammation. Subsequent Type 2 immune responses are typified by the production of IL4, IL5, IL13 from ILC2, Tc2 (CD8+ T cells that express prostaglandin DP2 receptor CRTH2) and Th2- T cells. These responses are thought to recruit eosinophils, promote IgE production and goblet hyperplasia. The increase in tissue T cells, B cells and plasma cells are thought to explain the high levels of mucosal IgE, which further perpetuates the inflammatory response by activating mast cells and eosinophils. Elevated levels of IL4 and IL13 observed proximally, and IL5 and eosinophilia observed distally in the inflammatory cascade have become the hallmarks of the Type 2 inflammation seen in polyp tissue. Thus these key cytokines have become the drug targets for the biologics. The efficacy of dupilumab in Type 2 disease was first tested in moderate to severe atopic dermatitis, and later in moderate to severe asthmatic adult patients.7–10 Recent approval for these two indications resulted in amassing safety data demonstrating low adverse effects .
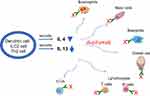 |
Figure 1 Dupilumab mechanism of action in Type 2 inflammation of chronic rhinosinusitis with nasal polyposis.Notes: X=dupilumab. Y= IL4Rα. |
Dupilumab is a fully humanized monoclonal IgG4 antibody that inhibits interleukin-4 (IL4) and interleukin-13 (IL13) signaling by specifically binding to the IL4Rα subunit, shared by the IL4 and IL13 receptor complexes: Type 1 receptor complex for IL4 and Type II receptor complex for IL13 and IL4.11 Blockade of the receptor complex results in inhibition of downstream STAT6 pathway signaling. This results in inhibition of cytokine-induced responses, chemokines and IgE production. Peak serum concentration of dupilumab occurs at 1 week after initiation of subcutaneous administration. Median times to non-detectable serum levels of dupilumab occur at 10–13 weeks.11 A paradoxical transient elevation of serum IL4 and IL13 cytokines following dupilumab initial exposure is observed, the mechanism of which is not clear. In addition, a parallel transient increase in serum eosinophils is consistently observed across all clinical trials, hypothesized to represent inhibition of eotaxin-3 by dupilumab, resulting in lack of recruitment of eosinophils from peripheral blood to into polyp tissue.10
Dupilumab in Chronic Rhinosinusitis with Nasal Polyposis
The efficacy of dupilumab as add on therapy to intranasal steroids in adult chronic rhinosinusitis with nasal polyposis patients was first studied in a randomized double-blinded, placebo-controlled, proof-of-concept Phase II trial by Bachert and colleagues.12 After 16 weeks of treatment with mometasone nasal spray and 300 mg weekly subcutaneous dose (with 600 mg loading dose), the addition of dupilumab significantly reduced endoscopically graded nasal polyp score and nasal congestion scores as compared to treatment with mometasone alone. Secondary endpoints including Lund-Mackay sinus CT scores, quality of life assessments (22 item SNOT questionnaire score), and smell function (assessed by UPSIT score) significantly improved. In the subset of CRSwNP patients with asthma (57%), dupilumab plus mometasone furoate nasal spray improved asthma control and lung function as assessed by the FEV1 percent predicted.
The inflammatory response was studied by following Type 2 biomarkers.12 Total serum IgE, serum TARC (thymus and serum activated cytokine), and plasma eotaxin-3 decreased. Serum IgE progressively decreased throughout the 16-week treatment period. Both eotaxin-3 and TARC precipitously dropped 2 weeks into dupilumab treatment and remained low throughout the 16-week period. However, eosinophil levels transiently increased at 4 weeks, but soon recovered to pretreatment levels, with no effect on baseline eosinophilia. The authors hypothesize that the transient eosinophilia represented a reduction in eotaxin-3 resulting in inability for eosinophils to migrate from serum to tissue.
In a follow up analysis of the same study cohort, the effect of dupilumab add-on treatment on clinical outcomes was analyzed.13 The authors reported improved self-reported quality of life (sinonasal outcome test-22 [SNOT-22]), 36-item short-form health survey [SF-36] and 5-dimension EuroQoL [ED-5D] visual analog scale [VAS] scores of health related quality of life, fewer missed days of work, and improve sense of smell and taste.
Phase 3 Trials of Dupilumab Add-on Therapy for Chronic Rhinosinusitis with Nasal Polyposis
The positive Phase 2 results were followed by Phase 3 clinical trials. Bachert and colleagues proceeded with two parallel multicentered, multinational, randomized double-blinded, placebo-controlled studies of dupilumab add-on therapy for CRSwNP (Sinus 24 and Sinus 52).14 Both studies had a four week run in period with mometasone alone. Sinus 24 study was a two-armed study (1:1, n=(133:143)) comparing dupilumab vs placebo group, given on a background of mometasone for 24 weeks duration. This was followed by 28 additional weeks of mometasone only treatment. Outcomes were assessed at week 24 while on dupilumab and at 52 weeks while off dupilumab from week 25–52. The Sinus 52 study was a three armed study (1:1:1, n=(153:145:150)) performed with background mometasone therapy while comparing placebo vs dupilumab 300 mg/2 weeks for 52 weeks vs dupilumab 300 mg/2 weeks for 24 weeks, then reducing the dose to 300 mg/4 weeks from week 25–52. Co-primary outcomes measured were endoscopic nasal polyp scores, and nasal congestion scores. Results demonstrate that add-on dupilumab treatment in both Sinus 24 and Sinus 52 studies significantly reduced both co-primary outcomes: nasal polyp score and nasal congestion.14 In the Sinus 52 week study, both treatment arms of dupilumab (300mg/2 week for 52 weeks and 300mg/2 week for 24 weeks followed by 300 mg/4 week for 28 weeks) showed similar results. When dupilumab was stopped at 24 weeks in the Sinus 24 study, both nasal polyp scores and nasal congestion symptoms worsen by week 52 (see Figure 2 from original publication of Bachert et al. 14). This finding attests to the role of dupilumab as a viable treatment option, but not as a disease modifier.
In both the Sinus 24 and Sinus 52 studies, the following secondary outcome measures improved after treatment with dupilumab at 24 weeks: sinus CT scores, SNOT-22 scores, UPSIT smell test, rhinosinusitis disease severity assessed with visual analog scale, peak nasal inspiratory flow, and rhinorrhea daily symptom score. Patients with comorbid asthma, NSAID-exacerbated respiratory disease, or previous surgery all showed similar improvements. Analysis of CRSwNP patients with comorbid asthma at week 24, dupilumab significantly improved lung function (assessed with FEV1) and asthma control (assessed with ACQ-6) compared with placebo. The improvement in these outcomes occurred independent of patient stratification by serum eosinophil counts.
Analyses of Type 2 biomarkers was performed only in the SINUS-52 study. Treatment with dupilumab resulted in decrease in concentrations of total serum IgE, periostin, TARC, and plasma eotaxin-3 at weeks 24 and 52 and in concentrations of ECP, total IgE, eotaxin-3, and IL5 in nasal secretions at week 24.14 Since disease recurred after 28 weeks of cessation of dupilumab in the Sinus 24 study, it would have been interesting to know whether these markers would have reversed back to pretreatment levels after dupilumab cessation, suggesting to their possible usefulness as biomarkers of disease. In both studies, and consistent with previous dupilumab studies, a transient, increase in blood eosinophil counts in patients treated with dupilumab was reported, which returned to baseline levels by the end of the 52-week treatment period. Additionally, the placebo group consistently displayed greater tendency for requiring treatment with systemic corticosteroids and sinonasal surgery throughout the entire duration of the study.14 Sixty-two to 65% had improvement in baseline nasal polyp score by at least 1 grade.
Adverse Effects and Safety of Dupilumab
Safety of dupilumab has been well-established due to the collective acquisition of safety data in multiple phase 3 clinical trials for not only CRSwNP, but also for asthma and atopic dermatitis, and in post-marketing surveillance. In general, dupilumab was well tolerated with no serious drug-related adverse effects.11 Vital signs, physical examination, laboratory testing, or electrocardiogram were monitored without evidence of adverse effect with dupilumab and placebo during all trials. In the CRSwNP studies, the most commonly reported adverse events were nasopharyngitis, injection site erythema, conjunctivitis and keratitis, cough, bronchitis and arthralgia.14 Progression of nasal polyps, need for nasal polyp surgery or systemic corticosteroids, or both, headache, worsening of asthma, and epistaxis, were more frequent with placebo than with dupilumab.14
Hypersensitivity, generalized urtircaria, serum sickness, and serum sickness-like reactions were reported in less than 1% of subjects who received dupilumab in atopic dermatitis clinical trials.11 Two subjects experienced serum sickness or serum sickness-like reactions that were associated with high titers of auto-antibodies to dupilumab. Of the subjects who developed antibodies to dupilumab in the clinical trials (7%), approximately 30% (2% of all subjects receiving dupilumab) developed neutralizing antibodies.
A drug interaction warning for patients receiving concomitant cytochrome P 450 substrates is given. Inflammatory cytokines (e.g., IL1, IL4, IL6, IL10, IL13, TNFα, and IFN) are known to alter CYP450 enzymes. Therefore, upon initiation or discontinuation of dupilumab in patients who are receiving concomitant drugs which are CYP450 substrates, such as warfarin, monitoring of drug concentration is recommended.11
Dosing of Dupilumab for Chronic Rhinosinusitis with Nasal Polyposis
Dupilumab is given subcutaneously and thus it can be self-administered by the patient by 300 mg every other week. It can be used with or without topical nasal steroid spray. Steady-state concentrations occur by 16 weeks. The bioavailability of 64% is achieved using the subcutaneous route of administration. Current dosing recommendations suggest that if a dose is missed for less than 7 days, it can be administered late. However if a dose is missed longer than 1 week, it is advised that the dose should be skipped until the next-scheduled dosing interval.11 The question of patient adherence to the regimen will need to be studied over time. The Sinus 52 data on dosing suggests that spreading the interval to once every 4 weeks maintains efficacy in most parameters.14
Dosing regimens need to be established. For CRSwNP, efficacy required that the drug be actively administered every 2 weeks for 24 weeks before switching to 4-week interval dosing. However, after dupilumab is withdrawn at 24 weeks, polyposis recurred, mimicking responses observed using oral steroids as primary treatment.14 This strongly suggests that the natural history of the CRSwNP is not altered by dupilumab, indicating that prolonged continuous treatment is needed to control the disease.
Role of Dupilumab in Current Clinical Practice for Treatment of Chronic Rhinosinusitis with Nasal Polyposis
The question of where dupilumab (and other biologics) fits into the established standard of care for CRSwNP needs to be determined. The current management of severe CRSwNP includes sinus surgery plus medical management. The surgery aims to establish physiologic anatomy, a necessary, but not always sufficient condition for restoration of sinus health. It also includes multitude of FDA approved and unapproved medical therapies including antibiotics to treat bacterial infections or to function presumably as an anti-inflammatory (doxycycline),15 topical steroids nasal sprays, corticosteroid irrigations, bursts of systemic steroids and ASA desensitization in aspirin exacerbated respiratory disease patients (AERD).16 The most effective of conventional medical treatments is systemic oral steroids followed by topical steroids.17 Interestingly, systemic steroids, with known adverse effects with prolonged usage or after multiple oral steroid bursts, does not have formal FDA indication for the treatment of nasal polyps. But it has been used in clinical practice as the most effective medical option, prior to the advent of biologics.
The idea of using topical application of high dose steroids directly into the nasal and sinus cavity is not a new one. For more than 15 years, patients have been using off label high dose steroids in large volumes of saline as a vehicle for delivery to control disease recurrence postoperatively. In a pilot study of eight CRSwNP patients by Steinke et al., 3 months of budesonide suspension in nasal saline irrigation treatment resulted in significantly improved CT scan score, visual analog scale of symptoms, and subjective sense of smell.18 A small clinical trial (n=12 post-operative patients) by Kang et al. has demonstrated proof of concept efficacy with improved SNOT-22, nasal endoscopic polyp score, and decreased need for oral and inhaled steroid use for asthma, for up to 6 months.19 Sustained topical delivery of steroids into the site of disease deeply seated against the skull base has been a challenge. More recently, high dose delivery topical steroid sprays by an exhalational deep delivery system of fluticasone (EDS Xhance, Optinose) has demonstrated efficacy by reducing nasal polyp score, improving nasal congestion, and SNOT-22 scores.20 Han et al. demonstrated postoperative control of polyps by sustained mometasone delivery using bioabsorbable steroid-eluting stents with reduced nasal polyp score and ethmoid polyp burden.21,22 However, like dupilumab, it is unclear where these high dose, deeper delivery topical steroid options fit into a treatment approach for CRSwNP patients. The advantage of dupilumab is effective delivery to the sinus mucosa by the systemic route, without adverse side effects observed with oral steroids and without the difficulty in delivery that some forms of topical medication use poses.
Cost Considerations of Dupilumab
The estimated cost of this treatment at the current regimen is $43,000/year.23–25 The high cost of a treatment that is effective, but that does not modify the disease will most certainly need to be considered. The health utility of dupilumab will need to be assessed and compared to current treatment regimens that consider the cost of surgery and medications, the cost of managing the adverse effects of oral steroids, and the cost of direct and indirect patient management.
Summary and Future Direction
Dupilumab represents a major advance in the treatment of CRSwNP. It demonstrated efficacy in controlling diseases characterized by Type 2 inflammation with minimal adverse effects. The results were consistent over a wide range of outcome measures. Its safety data in clinical trials is support by the safety data generated with the same biologic in the management of atopic dermatitis and asthma.
Dupilumab as an alternative treatment option for CRSwNP was sorely needed in this disease because it avoids the adverse effects of systemic steroids. However, the potential role of dupilumab preoperatively, postoperatively, as an alternative to surgery, for prevention of recurrence, as rescue therapy after failed conventional treatments, or as use in conjunction with other modalities are all questions that need to be studied. Presently, there is no evidence to suggest that dupilumab may replace any or all modalities of treatments currently in practice. The additional question of identifying which patients who may benefit from this treatment needs to be determined. The identification and characterization of CRSwNP responders or non-responders needs to be addressed. To date, biomarkers of Type 2 inflammation have not been helpful in predicting individual patient responses, a problem shared with asthma and atopic dermatitis. This emphasizes the need for future studies to aggressively analyze for biomarkers and the need to execute clinical trials with nested molecular studies to identify meaningful biomarkers. In this manner, treatment regimens in the future may have the advantage of a “personalized medicine” approach for this heterogenous disease. Therefore, in the absence of clinically useful disease identifying biomarkers, the question of therapeutic trials using clinically meaningful parameters with high fidelity to disease, such as sense of smell, should be considered as a more utilitarian endpoint in the immediate future.
The study cohort of CRSwNP subjects in Sinus 24 and Sinus 52 clinical trials of dupilumab were a heterogenous group of patients, with variable treatment histories of multiple medical regimens and surgeries, but with the most severe form of the disease. Now the questions remain as to how to use this new biologic in the context of current therapy for recalcitrant disease in order to best serve our patients and the public. This issue will become even more pressing as additional biologics become approved for the treatment of CRSwNP, thereby exponentially increasing the complexity of defining optimal treatment for patients.
Author Contributions
Jean Kim, MD PhD drafted the manuscript; analyzed and interpreted the data and references as the basis for this manuscript; and co-revised the approved final version for submission. Robert Naclerio, MD reviewed the manuscript for critical content and co-revised the approved final version for submission. All authors contributed to data analysis and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosures
Jean Kim, MD PhD reports no conflicts of interest in this work. RobertNaclerio, MD is speaker for Sanofi and Optinose and is part of the Advisory Board for Lyra, AstraZeneca, Sanofi, Regeneron, American Chemistry Council, and Celgene. He also reports personal fees from AstraZeneca, Celgene, American Chemistry Council, Sanofi, and Optinose, outside the submitted work.
References
1. Chaaban MR, Walsh EM, Woodworth BA. Epidemiology and differential diagnosis of nasal polyps. Am J Rhinol Allergy. 2013;27(6):473–478. doi:10.2500/ajra.2013.27.3981
2. Wynn R, Har-El G. Recurrence rates after endoscopic sinus surgery for massive sinus polyposis. Laryngoscope. 2004;114(5):811–813. doi:10.1097/00005537-200405000-00004
3. Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: european position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50(1):1–12. doi:10.4193/Rhin
4. Vlaminck S, Vauterin T, Hellings PW, et al. The importance of local eosinophilia in the surgical outcome of chronic rhinosinusitis: a 3-year prospective observational study. Am J Rhinol Allergy. 2014;28(3):260–264. doi:10.2500/ajra.2014.28.4024
5. Schleimer RP. Immunopathogenesis of chronic rhinosinusitis and nasal polyposis. Annu Rev Pathol. 2017;12:331–357. doi:10.1146/annurev-pathol-052016-100401
6. Hulse KE. Immune mechanisms of chronic rhinosinusitis. Curr Allergy Asthma Rep. 2015;16(1):1–8. doi:10.1007/s11882-015-0579-0
7. Blauvelt A, de Bruin-weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–2303. doi:10.1016/S0140-6736(17)31191-1
8. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496.
9. Busse WW, Maspero JF, Rabe KF, et al. Liberty asthma QUEST: phase 3 randomized, double-blind, placebo-controlled, parallel-group study to evaluate Dupilumab efficacy/safety in patients with uncontrolled, moderate-to-severe asthma. Adv Ther. 2018;35(5):737–748. doi:10.1007/s12325-018-0702-4
10. Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378(26):2475–2485. doi:10.1056/NEJMoa1804093
11. FDA. Dupixent® (Dupilumab) Injection, for Subcutaneous Use: US Prescribing Information. 2017. US Food and Drug Administration.
12. Bachert C, Mannent L, Naclerio RM, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016;315(5):469–479. doi:10.1001/jama.2015.19330
13. Bachert C, Hellings PW, Mullol J, et al. Dupilumab improves health-related quality of life in patients with chronic rhinosinusitis with nasal polyposis. Allergy. 2019. doi:10.1111/all.13984
14. Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394:1638–1650. doi:10.1016/S0140-6736(19)31881-1
15. Van Zele T, Gevaert P, Holtappels G, et al. Oral steroids and doxycycline: two different approaches to treat nasal polyps. J Allergy Clin Immunol. 2010;125(5):1069–1076. doi:10.1016/j.jaci.2010.02.020
16. Kariyawasam HH, Scadding GK. Chronic rhinosinusitis: therapeutic efficacy of anti-inflammatory and antibiotic approaches. Allergy Asthma Immunol Res. 2011;3(4):226–235. doi:10.4168/aair.2011.3.4.226
17. Benítez P, Alobid I, de Haro J, et al. A short course of oral prednisone followed by intranasal budesonide is an effective treatment of severe nasal polyps. Laryngoscope. 2006;116(5):770–775. doi:10.1097/01.mlg.0000205218.37514.0f
18. Steinke JW, Payne SC, Tessier ME, et al. Pilot study of budesonide inhalant suspension irrigations for chronic eosinophilic sinusitis. J Allergy Clin Immunol. 2009;124(6):1352–1354. doi:10.1016/j.jaci.2009.09.018
19. Kang TW, Chung JH, Cho SH, et al. The effectiveness of budesonide nasal irrigation after endoscopic sinus surgery in chronic rhinosinusitis with asthma. Clin Exp Otorhinolaryngol. 2017;10(1):91–96. doi:10.21053/ceo.2016.00220
20. Leopold DA, Elkayam D, Messina JC, et al. NAVIGATE II: randomized, double-blind trial of the exhalation delivery system with fluticasone for nasal polyposis. J Allergy Clin Immunol. 2019;143(1):126–134.e5. doi:10.1016/j.jaci.2018.06.010
21. Han JK, Forwith KD, Smith TL, et al. RESOLVE: a randomized, controlled, blinded study of bioabsorbable steroid-eluting sinus implants for in-office treatment of recurrent sinonasal polyposis. Int Forum Allergy Rhinol. 2014;4(11):861–870. doi:10.1002/alr.2014.4.issue-11
22. Shen J, Welch K, Kern R. Mometasone furoate sinus implant – a new targeted approach to treating recurrent nasal polyp disease. Expert Rev Clin Pharmacol. 2018;11(12):1163–1170. doi:10.1080/17512433.2018.1549485
23. Amrol DJ. New agent for chronic sinusitis. In: Amrol DJ, editor. NEJM Journal Watch. 2019. Available from: https://www.jwatch.org/na50008/2019/10/08/new-agent-chronic-sinusitis.Accessed January 02, 2020.
24. Kuznik A, Bégo-Le-Bagousse G, Eckert L, et al. Economic evaluation of dupilumab for the treatment of moderate-to-severe atopic dermatitis in adults. Dermatol Ther (Heidelb). 2017;7(4):493–505. doi:10.1007/s13555-017-0201-6
25. Health, C.A.f.D.a.T.i. Pharmacoeconomic review report Dupilumab. In: CADTH Common Drug Review. Canadian Agency for Drugs and Technologies in Health. 2018. Available from: https://www.cadth.ca/dupilumab-0. Accessed January 02, 2020.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
