Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 13
Therapeutic Potential of Cannabidiol (CBD) for Skin Health and Disorders
Authors Baswan SM , Klosner AE, Glynn K, Rajgopal A, Malik K, Yim S , Stern N
Received 14 October 2020
Accepted for publication 15 November 2020
Published 8 December 2020 Volume 2020:13 Pages 927—942
DOI https://doi.org/10.2147/CCID.S286411
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Sudhir M Baswan,1,* Allison E Klosner,2,* Kelly Glynn,1 Arun Rajgopal,1 Kausar Malik,1 Sunghan Yim,1 Nathan Stern1
1Innovation and Science, Amway Corporation, Ada, MI, 49355, USA; 2Innovation and Science, Nutrilite Health Institute, Amway Corporation, Buena Park, CA, 90621, USA
*These authors contributed equally to this work
Correspondence: Sudhir M Baswan Amway Corporation, 7575 Fulton St E, Ada, MI 49355, USA
Tel +1-616-787-0216
Email [email protected]
Abstract: Though there is limited research confirming the purported topical benefits of cannabinoids, it is certain that cutaneous biology is modulated by the human endocannabinoid system (ECS). Receptors from the ECS have been identified in the skin and systemic abuse of synthetic cannabinoids, and their analogs, have also been associated with the manifestation of dermatological disorders, indicating the effects of the ECS on cutaneous biology. In particular, cannabidiol (CBD), a non-psychoactive compound from the cannabis plant, has garnered significant attention in recent years for its anecdotal therapeutic potential for various pathologies, including skin and cosmetic disorders. Though a body of preclinical evidence suggests topical application of CBD may be efficacious for some skin disorders, such as eczema, psoriasis, pruritis, and inflammatory conditions, confirmed clinical efficacy and elucidation of underlying molecular mechanisms have yet to be fully identified. This article provides an update on the advances in CBD research to date and the potential areas of future exploration.
Keywords: cannabidiol, CBD, cannabinoids, endocannabinoids, endocannabinoid system, skin, CB1, CB2, FAAH, AEA
Introduction
The Endocannabinoid System in Skin
The ECS is an evolutionarily conserved network of molecular signaling that plays a role in bodily homeostasis.1–3 The ECS is made up of multiple components: (a) signaling molecules called endocannabinoids, (b) specific receptors, and (c) enzymes that synthesize and breakdown endocannabinoids and transporters of endocannabinoids. The most well-researched functions of the ECS are related to modulation of the central nervous system (CNS) and immune function in the body. Recent research has indicated the critical role of the ECS in maintaining skin homeostasis and barrier function, and its dysregulation has been implicated in various skin disorders like atopic dermatitis, itch, acne, hair growth/loss, and hyper/hypopigmentation.4–7
Endocannabinoids
The existence of an endogenous ECS ligand was first reported by Devane et al in 1988 when they showed that N-arachidonoylethanolamine glycerol (AEA/Anandamide) binds to the cannabinoid brain receptor in a murine model.8,9 Since then, detection of numerous endocannabinoids has also been reported in the human body including the peripheral organs like skin.10 Amongst all endocannabinoids present in skin, anandamide (N-arachidonoyl ethanolamide, AEA) and 2-arachidonoyl glycerol (2-AG) are the most widely studied.11,12 Anandamide and 2-AG were detected and quantified in the femtomolar range in both keratinocytes and fibroblast cells by Gegotek et al.13 The biosynthesis pathways and cellular uptake of these two lipid mediators are described in multiple review articles.2,14,15 Other less know endocannabinoids detected in skin by Kendall et al are N-palmitoyl ethanolamide (PEA), N-alpha-linolenoyl ethanolamide (ALEA) N-linoleoyl ethanolamide (LEA), N-oleoyl ethanolamide (OEA), N-stearoyl ethanolamide (SEA), N-eicosapentaenoyl ethanolamide (EPEA), and, N-docosahexaenoyl ethanolamide (DHEA).
Receptors
Cannabinoid (CB) 1 receptors are generally present in abundance in the central nervous system (brain and spinal cord) and CB2 receptors are present in the peripheral nervous system (nerves in extremities), the digestive system, and immune system. Research indicates that both CB1 and CB2 receptors are also found in epidermal keratinocytes, cutaneous nerve fibers, dermal cells, melanocytes, eccrine sweat glands and hair follicles.13,16,17,19–21,22 While cannabinoid receptors remain the primary targets for endocannabinoids, they have also been shown to bind to Transient Receptor Potential (TRP) receptors present in various types of skin cells (Figure 1) and are involved in different functions like formation and maintenance of the skin barrier, cell growth, cell differentiation, immunological and inflammatory processes.23
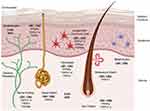 |
Figure 1 Schematic representation of the key components of the ECS in different cellular compartments of the skin. |
In addition, endocannabinoids also interact with peroxisome proliferator-activated receptors (PPAR) via direct (endocannabinoid) or indirect (secondary metabolite of endocannabinoids) signaling pathways. PPAR (α and γ) activation partially mediates major biological functions of endocannabinoids like neuroprotection, antiinflammation, and analgesic actions. The ECS and some other non-cannabinoid (indirect) targets influencing the ECS in different cellular compartments of the skin are also shown in Figure 1. A simplistic mechanism of action of endocannabinoids like AEA and 2-AG on CB1 and CB2 receptors in presynaptic neurons in the central and peripheral nervous systems is shown in Figure 2, which also shows the modulation of the ECS by phytocannabinoids (PCBs) by direct activation of CB1 (like THC). Indirect mechanisms of the ECS (not shown in the figure) include inhibition of enzymatic breakdown of endocannabinoids (ECBs) and/or receptor modulation.
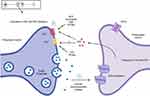 |
Figure 2 Modulation of the ECS by endocannabinoids and phytocannabinoids in presynaptic neurons in the central and peripheral nervous systems. |
Enzymes and Transporters
The synthesis of endocannabinoid AEA is mediated by Phospholipase D while diacylglycerol lipase (DAGL) regulates the synthesis of 2-AG.24,25 The degradation of AEA and 2-AG primarily is regulated by two enzymes, fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), respectively.2 The biological signaling of endocannabinoids via interaction with their receptors is inhibited by a two-step mechanism. The endocannabinoids are first removed from the intracellular space by a membrane transporter known as anandamide membrane transporter (AMT) and then, after reuptake, the endocannabinoids entering the cells are metabolized by enzymes like FAAH and MAGL.26
Types of Cannabinoids
Cannabinoids can be broken into three general categories based on where they are produced. Endocannabinoids (ECBs) are the cannabinoids compounds biosynthesized within the human body. Figure 3 represents the chemical structures of nine endocannabinoids found in human skin by Kendall et al.10 Phytocannabinoids (PCBs) are the cannabinoids obtained from plants while synthetic cannabinoids (SCs) are generated synthetically using various chemical processes (e.g., Dronabinol, Nabilone). Phytocannabinoids are found in abundance in the resin-producing trichomes of the Cannabis sativa L. plant (C. sativa). While there are many cultivars of C. sativa, regulatory bodies typically segment them into one of two different chemotypes. Industrial hemp is the chemotype with a minimal amount of tetrahydrocannabinol (THC) and higher levels of CBD, while the marijuana chemotype contains high levels of THC (e.g., above 0.3% w/w by dry weight). Figure 4 represents the most common phytocannabinoids found in hemp.
 |
Figure 3 Chemical Structures of 9 endocannabinoids found in human skin. |
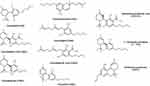 |
Figure 4 Chemical structures of the most common phytocannabinoids found in the hemp plant. |
Historically, hemp has been cultivated for its fiber, which can be used to produce paper or clothing, or for its nutritious seeds. More recently, hemp has gained popularity for its beneficial cannabinoid constituents including CBD. While the flowering tops and leaves of hemp have significant levels of CBD, the hemp stems/stalks and seeds have little to none. Hemp seeds contain nutritious omega-3 fatty acids and are high in protein, but only contain trace amounts of PCBs and no terpenoids.27 Steam distillation of hemp flowering tops and leaves is commonly used to produce an essential oil containing terpenoids such as myrcene, α-pinene, and β-caryophyllene.28 However, the volatile fraction produced from steam distillation will not contain appreciable amounts of PCBs.29 Ethanolic and supercritical CO2 extracts of whole hemp plant or flowering tops and leaves can have significant levels of CBD.30 These extracts have various commonly known nomenclatures like full spectrum hemp extract, broad spectrum hemp extract, hemp oil, and, phytocannabinoid-rich hemp oil/extract.
Potential of Cannabidiol for Skin Health and Dermatological Conditions
Because the ECS plays an important regulatory function in the skin, it is plausible that treatment with topical cannabinoids could be efficacious for certain disorders or skin health in general. However, most of the clinical evidence to date has focused on the effects of CBD and other cannabinoids when consumed, inhaled, or injected. There is limited research investigating the therapeutic potential for topical applications. Yet, there is evidence to suggest applying cannabinoids, and specifically CBD, topically may be a viable route of administration for certain conditions. Although CBD has a reasonable molecular weight (314.46 Da), its high log P value (lipid/water partitioning) of ~6.3, poses unique challenges to its transdermal delivery.31 However, this challenge may be overcome if appropriate carrier systems are used, as seen with CBD being absorbed transcutaneously in preclinical models. In 2003, Lodzki et al reported successful transdermal delivery of CBD in a murine model by using ethosomal carriers.32 Similarly, Hammel et al investigated the efficacy of topically applied CBD (1–10%) in a gel format, specifically for reduction of inflammation-associated symptoms in a monoarthritic rat model, and found that it was well-absorbed, as the plasma concentration showed a linear relationship with the dose applied.33 In vitro diffusion studies using human tissue have demonstrated CBD’s permeation potential.34 However, at present no clinical trials investigating the topical absorptive capability in humans have been identified. Further work is warranted to better understand the appropriate doses and delivery methods for therapeutic CBD skin applications.
Skin Protection | Barrier Function
Skin serves as a protective barrier against environmental insults which can lead to the generation of reactive oxygen species (ROS).35,36 Oxidative stress induces cell damage and can result in chronic inflammation if left unchecked. It is also implicated in skin disorders and skin aging.36 Keratinocytes are the main cell type in the epidermis and are particularly sensitive to environmental stressors.37
The harmful accumulation of ROS is countered in healthy skin by activation of numerous defense mechanisms. Many of these systems are controlled by the master regulator of cellular antioxidant defense system, NRF2 (nuclear factor erythroid 2-like 2) and PPAR-γ.38 The stress-induced enzyme Hemeoxygenase1 (HMOX1) is one of the key NRF2 target genes and exhibits antioxidant and anti-inflammatory properties.39 In in vitro studies, CBD has demonstrated the ability to induce expression of HMOX1 and other NRF2-regulated genes.40,41 One study done in Normal Human Epidermal Keratinocytes (NHEK), reported that CBD induced the expression of several NRF2 target genes, with HMOX1 being the most upregulated by CBD.42 In the same study, increased levels of HMOX1 and expression of proliferation and wound repair keratins 16 and 17 were observed in mice epidermis after topical application of CBD. In another in vitro study using human keratinocytes, researchers showed that CBD was able to penetrate the cells and balance the oxidative stress response resulting from UVB irradiation and hydrogen peroxide. They also demonstrated that CBD had a protective effect against the peroxide-induced reduction of polyunsaturated fatty acids in the cell membrane, helping to protect membrane integrity.43 There is evidence to suggest CBD can activate PPAR-γ, as well. Treating 2D and 3D fibroblast cells with CBD resulted in activation of PPAR-γ with a corresponding decrease in levels of NF-kB.44 Since HMOX1 and PPAR-γ play strong cytoprotective roles with anti-inflammatory, antioxidant, and anti-apoptotic properties, treatments regulating their expression could be beneficial for skin conditions characterized by inflammation and keratin disorders, such as eczema or atopic dermatitis.
Pain and Muscle Relief
Tissue damage typically triggers an inflammatory response in the body which could result in irritation, ulcers, sensitization of peripheral tissues, neuropathies, and chronic wounds.45 If left unresolved, a chronic inflammatory state in the body leads to increased tissue damage and pain.46 Current therapies (cannabinoids, antidepressants, NSAIDs and anticonvulsants) for chronic pain management target the peripheral and central nervous system often producing undesirable side effects.47,48 Preclinical and clinical models have shown that targeting peripheral inflammation by topical therapy (e.g., clonidine49,50 capsaicin)51,52 is not only effective in reducing pain for specific conditions but also circumvents the CNS, thereby reducing the negative side effects, ie, respiratory depression, sedation, and tolerance. While preclinical models strongly indicate that ingestible cannabinoids may produce antinociceptive effects in neuropathic and inflammatory pain models53,54 and a moderate level of clinical evidence supports the use of ingestible cannabinoids for chronic pain (primarily THC and combination of THC+ CBD + lower levels of other cannabinoids)18,55,56 the clinical application of topically applied CBD for pain management has not yet been validated by robust scientific and clinical studies.
Eczema or Atopic Dermatitis
Atopic Dermatitis (AD) is a chronic inflammatory skin disorder associated with multifactorial causes like environmental triggers, damaged skin barrier function, microbiome imbalance, genetic predisposition, and an altered immune response.57 Phytocannabinoids have been shown to modulate inflammatory responses by regulating more than one underlying mechanism. Adelmidrol, a PEA derivative, has been shown to be effective in treating mild AD in a pediatric population.58 Though the efficacy of CBD is yet to be clinically validated, in a recent study by Petrosino et al, CBD was shown to exhibit anti-inflammatory properties in an experimental, allergic contact dermatitis model.59
The influence of microbiome imbalance, especially due to colonization and biofilm formation of Staphylococcus aureus (S. aureus), has also emerged as an influencing factor which can contribute towards the severity of dermatitis.60,61 The preliminary data indicating the antimicrobial and antibiofilm activity of hemp come from the essential oil (steam distillate) fraction of hemp which is composed mainly of terpenoids such as myrcene, α-pinene, β-caryophyllene and other terpenes, but no significant levels of CBD.28,62 Zengin et al evaluated the antimicrobial and antibiofilm efficacy of hemp essential oil (EO) against a reference strain (S. aureus American Type Culture Collection (ATCC) 29,213) and three clinical strains (S. aureus 101 TV, S. aureus 104, and S. aureus 105). The effective Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC), and the Minimum Biofilm Eradication Concentration (MBEC) hemp EO values against all S. aureus strain types were reported as 8, 16 and 24 mg/mL, respectively, which indicated the hemp EO may disrupt and eradicate a mature biofilm of S. aureus. Thus, the antimicrobial and antibiofilm activities of hemp EO against S. aureus suggest its therapeutic potential to prevent skin disorders like atopic dermatitis.63
Itch (Pruritis)
When it becomes chronic, itch or pruritis can severely affect one’s quality of life. The pathogenesis of pruritis is well researched and is described comprehensively in various recent review articles.64–66 Though most of the ECS research indicates that the itch response is primarily modulated through CB1 receptors in the CNS,67–69 some reports argue the involvement of peripheral CB1 receptors could also be a potent contributor to itch.70,71 The available data thus far for the involvement of peripheral CB2 receptors are conflicting and more research is needed to conclusively determine its role in pruritis.72,73 It has also been shown that all ionotropic cannabinoid responsive receptors (e.g., TRPV1−4, TRPA1 and TRPM8) play a vital role in the complex cutaneous communication between keratinocytes, immune (Mast) cells and the sensory nerves which leads to an itch sensation.74–78 Thus, inhibiting the activity of such ionotropic channels by selective PCBs may be helpful in alleviating pruritis.
FAAH and MAGL inhibitors, which can increase the levels of endocannabinoids and modulate cannabinoid and non-cannabinoid receptor responses, were found to demonstrate anti-pruritic effects on murine models when administered via intraperitoneal and intrathecal routes.79–81 Though cannabinoids like THC and PEA have been shown to reduce itching in murine models,82 the human clinical data for testing the antipruritic potential of PEA have resulted in conflicting results.83,84 To add to the dilemma, a study by Spradley et al indicated that peripheral endocannabinoids have opposite effects on itching behavior in spinally versus trigeminally innervated skin of mice, and therapeutic treatment of itch might be more relevant for treating the lower body than itch arising from trigeminal innervated skin of the face or scalp.85 Since CBD is a FAAH inhibitor, a CB2 inverse agonist86 (antagonist of CB2 agonists) and TRPV1 agonist, it could potentially play a role in modulating itch response, but the scientific evidence remains scarce for this application to-date.
Wound Healing
Wound healing is an intricate process which includes three overlapping phases – inflammation, proliferation, and maturation/tissue remodeling.87–89 It is plausible that the complex process of wound healing is influenced by ECS signaling, as it modulates epidermal proliferation and differentiation, fibroblast functions, and cutaneous inflammation. CB1 and CB2 receptor involvement during the wound healing process in various immune and fibroblast cells are based on murine models.90–92 In these models, various cannabinoid analogs have generated a wound healing response possibly associated with activation of CB1 and/or CB2 receptors, upregulation of anti-inflammatory factors, indirect activation of TRPV1 and epidermal growth factor receptors, and inhibition of the FAAH enzyme.91,93,94 The evidence of the clinical application of PCBs, especially CBD, for wound healing is scarce. A single study reported three patients suffering from Epidermolysis bullosa (a rare skin disorder characterized by pain and blistering) had faster wound healing, less blistering and amelioration of pain with self-reported topical use of cannabidiol.95,96
Though there is a dearth of clinical evidence, the pre-clinical models indicate an optimistic outlook. A study by Sangiovanni et al reported the effects of CBD and Cannabis Sativa Extract (CSE, standardized to 5% CBD) on human keratinocytes (HaCaT cells) and human dermal fibroblast (HDF) cells.97 In keratinocytes, TNF-α (Tumor Necrosis Factor alpha) treatment resulted in upregulated expression of 26 genes involved in inflammatory pathways and included chemokines like CXCL8 and CXCL10, interleukins like IL−17C and IL−1B, and VEGF-A. Treatment with CSE downregulated all 26 inflammatory related genes, and CBD alone downregulated 15 genes. In HDF cells, TNF-α treatment upregulated 16 genes involved in the process of wound healing. While CSE was again able to downregulate all genes, CBD only downregulated 11 genes and did not exhibit any inhibitory effects on genes playing a role in inflammation and matrix remodeling, including IL−6 and MMP−9. These results indicate that the additional components within the complex cannabinoid extract, such as the other cannabinoids, flavonoids, and terpenes, may exert a synergistic anti-inflammatory effect greater than that of CBD alone. More robust preclinical and clinical studies are needed to draw a conclusion on the cutaneous wound healing response of the CBD and its related compounds.
Acne/Seborrhea
The major factors involved in acne onset are sebum overproduction, unwanted sebocyte proliferation, and inflammation. It is known that the ECS plays a key role in homeostasis of the skin, and specifically in lipogenesis. The endocannabinoid AEA, has been shown to stimulate lipid production in human sebocytes at low concentrations but induces apoptosis at higher levels.98 Although current research is limited, several in-vitro studies indicate that CBD could be a novel therapeutic in the management of acne by acting on pathways relating to sebum production, sebocyte proliferation, and inflammation. One notable study performed by Oláh et al addresses CBD’s potential effects on several of these outcomes. First, researchers investigated the effects of CBD on sebaceous gland function in human SZ95 cells. They found that a 24-hour treatment of CBD (1–10 μM) alone caused no changes in cellular lipid synthesis; however, when cells were first treated with AEA, CBD was able to quell the lipogenic actions in a dose-dependent manner. The researchers went on to test other lipogenic substances, including arachidonic acid and a mixture of linoleic acid and testosterone, and found that CBD was able to inhibit extraneous lipid synthesis induced by those compounds, as well. This finding suggests that CBD’s effect is a universal action and is not limited to direct ECS interaction.99 Also, it is important to note that CBD does not simply reduce lipid production but rather it is able to normalize lipogenesis in a state of imbalance. The same researchers went on to investigate the anti-proliferation abilities of CBD in-vitro. They found that CBD did not suppress cell counts beyond the starting number (did not reduce the number of viable cells) but did significantly reduce the overall proliferation of cells at 1−10 μM doses. Higher doses of CBD (50 μM) or elongated application (6 days) did result in apoptosis-driven cytotoxicity and overall viable cell count was reduced.
Finally, Oláh’s research group examined CBDs anti-inflammatory actions and found that it was able to prevent pro-acne mediators from elevating TNF-α mRNA expression. It also was able to normalize the LPS-induced expression of IL−1B and IL6. This data provided further evidence for CBD’s substantial anti-inflammatory actions. Notably, it is believed the control of sebocyte proliferation and lipid production was mediated through TRPV4 signaling, while the anti-inflammatory effects of CBD application were not.99
In addition to previously mentioned factors, imbalance in the skin microbiome may also contribute to the pathogenesis of acne. Specifically, Cutibacterium acnes (C. acnes) overgrowth has been linked to the establishment of acne for over 100 years.100 Therefore, the known anti-microbial effects of CBD may also prove effective in acne treatment. In an in-vitro study by Jin et al, a hemp seed hexane extract (HSHE) exhibited anti-microbial activity on C. acnes while inducing inflammation, and lipogenesis in sebocytes at the molecular and cellular level.101 With 20% HSHE treatment, complete inactivation of C. acnes was observed. In this study, the content of CBD in HSHE was not reported; hence, it is difficult to attribute the contribution of CBD alone towards inactivation of C. acnes. Similarly, in a small clinical study involving men with buccal facial acne, a 3% Cannabis seed extract containing cream led to decreased sebum content and erythema. As cannabis seed extract contains minimal CBD content, it limits our understanding of application of CBD for acne and seborrhea therapy.102 Likewise, hemp essential oil contains many terpenes which were shown to have anti-microbial effects against C. acnes (formerly known as Propionibacterium acnes).103,104
We speculate that Hemp seed extract or hemp EO could also have potential for treating acne vulgaris because of its anti-lipogenic, anti-proliferative, anti-inflammatory, and anti-microbial, properties, which may target similar or independent mechanisms than that of CBD. Unfortunately, no large-scale human trials have investigated the role of CBD for the management of acne. Larger studies will help to understand how CBD may impact acne at the clinical level.
Modulation of Hair Growth
The human hair follicle is an immune-privileged miniaturized organ consisting of epithelial and mesenchymal tissue. As part of the pilosebaceous complex, the hair follicle is extensively regulated, the extent of which is still not completely understood. Human scalp hair growth is a complex and dynamic process including a period of keratinocyte proliferation and hair fiber growth (anagen), followed by a stage of apoptotic follicle regression (catagen) and a semi-quiescent stage (telogen).105 Hair growth abnormalities include lack of hair growth (alopecia), and excessive hair growth (hirsutism and hypertrichosis). Given the success of topically applied compounds to treat hair loss106 coupled with the detection of major cannabinoid compounds in hair fibers, including CBD, following cannabis consumption107 and topical application of hemp oil108 further understanding of how cannabinoid compounds can potentially benefit hair-related issues is needed.109–111
Immunohistochemical analysis of human skin revealed differential expression of CB1 and CB2 receptors within the hair follicle. CB1 was detected in portions of the infundibulum and the inner root sheath, but absent from the outer root sheath, the bugle, hair bulb, and arrector pili muscle. CB2 was present in the outer root sheath and hair bulb, but absent in the inner root sheath, bulge, and arrector pili muscle.19 Surgically isolated facial hair follicle cultures showed production of ECBs - AEA and 2-AG. Intriguingly, AEA, and ∆9-THC suppressed hair follicle growth and induced the catagen cycle. The effects of these endo-exo cannabinoids were ameliorated by the addition of a CB1 antagonist. 2-AG treatment, however, resulted in comparable follicle growth compared to vehicle control-treated follicles.112
An orally administered synthetic antagonist of CB1 promoted hair growth stimulation in obese mice but had no effect when applied topically. Whether oral administration of the CB1 antagonist specifically targeted CB1 to induce hair growth was not discussed by Srivastava et al.113 Bodo et al identified the expression of TRPV1 in human hair follicles and outer root sheath keratinocytes.114 Activation of TRPV1 in follicle organ cultures leads to inhibition of cell proliferation, while inducing apoptosis and catagen entry. Hair growth activators, HGF, IGF1, and SCF, were also suppressed with TRPV1 stimulation. TRPV3 and TRPV4 were also detected in human hair follicles and outer root sheath keratinocytes, and receptor activation, though not exclusively by cannabinoids, resulted in suppression of hair follicle elongation.115,116 A metabolite of the endocannabinoid anandamide, bimatoprost, is recognized as a topical prostamide treatment for eyebrow hypotrichosis.117 Khidhir et al also showed human scalp hair follicles possess select prostamide receptors within the dermal papilla. Working with human scalp, organ-cultured hair follicles, bimatoprost treatment resulted in follicle growth and it stimulated hair regrowth when applied to mouse skin.118 A limited clinical study showed bimatoprost application also accelerated hair regrowth in alopecia areata patients to a greater extent than a topical steroid treatment.119 Szabo et al in a pilot study using ex vivo human hair follicles and primary outer root sheath keratinocytes found systemic-like application of CBD had dose-dependent opposing effects on hair growth dynamics.116 At the 0.1 µM and 1.0 µM doses, the hair shafts grew similar to controls, while at the 10 µM dose, growth was significantly suppressed and follicle catagen was induced. As CBD dosage increased, keratinocyte proliferation decreased. The researchers proposed that CBD concentration may lead to differential receptor activation, with low doses favorably affecting hair growth pathways and higher doses activating suppression targets such as TRPV4.
As the hair follicle contains ECS and cannabinoid-responsive receptors, coupled with the evidence of cannabinoid deposition within the fiber followed by cannabis consumption and topical application, there may be potential to use compounds like CBD to treat certain hair disorders. However, given the complexity of hair growth dynamics, research conducted employing hair follicle organ culture and systemic-like treatment, and the potential of pleiotropic effects of certain cannabinoids seen to date, additional research is needed, including clinical trials, to determine if phytocannabinoids like CBD can be effective topical interventions to treat hair loss or excessive hair growth conditions.
Skin and Hair Pigmentation
The pigmentation of human skin is the manifestation of synthesis of dark pigment, melanin, which is regulated by a melanogenesis process in melanocytes.120,121 Melanogenesis is a complex process regulated by more than 250 genes.122 Microphthalmia Transcription Factor (MITF) acts as a master regulator of melanogenesis, directly controlling the transcription of key genes involved in pigmentation such as tyrosinase (TYR), tyrosinase-related protein (TYRP)-1, and TYRP-2.123
Due to a limited number of studies, the involvement of the ECS in the cascades of the melanogenesis process is not clear. In 2012, Pucci et al showed that a fully functional ECS was present in normal human primary epidermal melanocytes. Lower concentrations of AEA, as well as other endocannabinoids like Arachidonoyl-2′-chloroethylamide (ACEA), and 2-AG, demonstrated induction of melanogenesis in a dose-dependent manner via the CB1 receptor.21 However, other studies demonstrated contrasting results with CB1 agonism inhibiting melanogenesis or having no influence. Zhou et al demonstrated that OEA acts as an inhibitor of melanin synthesis and MITF production in α-MSH stimulated B16 cells via activation of ERK, Akt, and p38 pathways and inhibition of the CREB pathway.124 Another study by Magina et al demonstrated that CB1 agonism, under UVB exposure in a co-culture model using HaCat and SK-mel-1 cells, inhibited basal melanogenesis, whereas the inhibition was reversed when a CB1 antagonist was introduced.125 Kim et al demonstrated that a major metabolite from JWH-073, a synthetic cannabinoid, had no significant effect on hair pigmentation.126 Unfortunately, there are limited studies regarding the effect of phytocannabinoids, such as CBD, on melanogenesis. Hwang and colleagues demonstrated that CBD stimulated both melanin content and tyrosinase activity, mediated by the CB1 receptors in human epidermal melanocytes. The melanogenic effects of CBD occur primarily through MITF upregulation, which is mediated by the activation of p42/44 MAPK and p38 MAPK signaling.127
Involvement of ECS pathways in melanocytes is very complex and unclear, thus requiring additional research with various in vitro and in vivo models. Although endocannabinoids are potential mediators for healthy and diseased skin, it is believed to be premature to target pigmentation disorders using cannabinoids.124
Potential Applications in Oral Care
There is little published about the use of CBD in oral care. In the 1950s, the topical preparations from C. sativa were found to contain antiseptic properties against several oral cavities and skin lesions.128,129 In 2012, Ali et al studied the effect of C. sativa seed oil, and petroleum ether and methanol extracts of the whole plant, on two Gram-positive bacteria (B. subtilis, S. aureus), two Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa) and two fungi (Aspergillus and Candida albicans). The seed oil exhibited pronounced antibacterial activity against the Gram-positive bacteria, moderate to high activity against Gram-negative bacteria, but was ineffective against the fungi.130 Rashid et al tested ethanol and methanol extracts of cannabis leaves and stem against different microorganisms. A significant inhibitory effect was observed in the ethanol leaf extracts of C. sativa, with 13.8 and 21.33 mm zones of inhibition against S. aureus and K. pneumoniae, respectively.131 At 4 µg/mL and 10 µg/mL concentrations, the aqueous and acetone extracts of C. sativa cannabinoids demonstrated a high antimicrobial activity by showing clear zones of inhibition against the bacteria Pseudomonas aeruginosa (3–8 mm), and Vibrio cholerae (4–10 mm) and the fungi Cryptococcus neoformans (4–10 mm), and Candida albicans (4–12 mm).132 It must be noted that hemp seeds and stalks barely contain CBD content, whereas other parts of the hemp plant contain significant amounts of CBD and other cannabinoids.
Dental plaque is associated with several dental diseases and should be regularly removed using mechanical (toothbrushes, floss) and chemical (mouthwashes) oral regimens. Dental plaque is the complex biofilm that acts as a reservoir of several microbes adhering to the tooth surface and gum line. An antimicrobial treatment can be used as an effective aid for plaque control and to improve the inflamed tissues of gums and bones.130 Microorganisms forming the biofilm of the dental plaque are Gram-positive bacteria, such as Streptococcus mutans, Gram-negative bacteria, and several other anaerobes such as Fusobacterium and Actinobacteria. In 2019, Stahl et al assessed the efficacy of cannabinoids (BGA, cannabigerolic acid; CBN, cannabinol; CBG, cannabigerol; CBD; and CBC, cannabichromene) in comparison to commercial oral care products. Cannabinoids were more effective in reducing the bacterial content of the dental plaque compared to the commercially available synthetic oral care products. Therefore, natural cannabinoids may have the potential to be used as an effective treatment to remove dental plaque associated oral bacteria and provide a safer alternative to synthetic antibiotics.129
Other Skin Disorders
Skin Infections
CBD and CBG have been reported to have potent activity against a variety of Gram-positive Methicillin-resistant Staphylococcus aureus (MRSA) strains.133 Likewise, an antimicrobial effect of CBD against Listeria monocytogenes, Enterococcus faecalis and Methicillin-resistant Staphylococcus epidermidis (MRSE) was reported with Minimum Inhibitory Concentration (MIC) values of 4 µg/mL for MRSA and L. monocytogenes and 8 µg/mL for E. faecalis and MRSE.134 This study also characterized CBD as a helper compound that potentiates the effect of Bacitracin (BAC), a skin antibiotic. The MIC value of BAC was remarkably reduced to at least a 64-fold reduction for MRSA, MRSE and E. faecalis, when combined with ½ MIC of CBD as compared to MIC of BAC alone.
Furthermore, to assess the potentiating and synergistic effect against MRSA, growth curve and time kill assay results showed the combined activity of CBD and BAC reduced bacterial viability by 6-log10 cfu/mL as compared to CBD or BAC alone. Interestingly, CBD was able to potentiate the effects of BAC against MRSA (S. aureus USA300) and other Gram-positive bacteria. The spectrum of use of CBD and BAC on growth of Gram-negative bacteria, including Pseudomonas aeruginosa, Salmonella typhimurium, Klebsiella pneumoniae, and Escherichia coli, was also measured. The results obtained from the combined effect of CBD and BAC against these Gram-negative bacteria concluded that the combined activity of CBD and BAC was considered ineffective against Gram-negative bacteria. Due to potent antibacterial properties against Gram-positive bacteria, cannabinoids can be used as an effective helper compound when combined with known antimicrobial actives to fight antibiotic resistant Gram-positive bacteria which cause skin disorders and other infections.134
Psoriatic Plaques
Some anecdotal information suggests the use of CBD for treating psoriatic plaques which are characterized by keratinocyte hyperproliferation and chronic inflammation. NF-kB plays a significant role in skin inflammatory conditions like psoriasis, and its expression is strongly induced by TNF-α.135 Sangiovanni et al demonstrated that CBD and C. sativa extract (CSE, standardized to 5% CBD) inhibited TNF-α induced NF-kB transcription in a dose-dependent manner in HaCaT cells.97 However, in HDF cells, only CSE exhibited NF-kB inhibitory effects. In other cell types, CBD has been reported to have the ability to impair the NF-kB pathway both in-vitro and in-vivo.136,137 Though CBD has shown to have anti-inflammatory properties, its role in keratinocyte differentiation and proliferation is not clear. Some in vitro studies have shown that CBD inhibits differentiation in immortalized HaCaT cells138 and also exerts antiproliferative actions on transformed human keratinocytes (HPV16).139 On the contrary, a study by Casares et al indicated that the role of CBD in treating psoriasis should be approached with caution due to its proliferative effects for keratins 16 and 17.42 Thus, more robust experimentation is needed to determine the use of treatment for psoriatic lesions.
Cutaneous Malignancies
The therapeutic potential of targeting the ECS for cutaneous malignancies such as melanoma and non-melanoma skin tumors is described at length in an excellent review article by Toth et al and is beyond the scope of this article.4 Cannabinoids such as ∆9-THC and AEA have better scientific research for this application. Though some preclinical studies have shown that CBD inhibits transporter proteins involved in breast cancer140 the application of CBD for treating cutaneous malignancies has yet to be explored in detail.
Open Questions and Future Research
The significance of the ECS in maintaining skin homeostasis and the resulting dermatological conditions from its imbalance has garnered scientific attention. Despite promising research on the topical therapeutic potential targeting the ECS, much remains unknown on the complexity of interactions of cannabinoids with other systems of the human body. A good example of this is the unintended interaction of BIA 10–2474 (a FAAH inhibitor intended for treating pain and anxiety) with the lipid network in human cortical neurons resulting in metabolic dysregulation of the nervous system.141,142 Though the outcomes of this study are stemming from oral treatment at higher doses, it does have sound advice to treat any topical application of cannabinoids with due diligence. While many topical CBD products appear to be relatively well tolerated, topical safety studies are underway, and the evidence is still emerging.
The authors conclude that while the therapeutic potential of CBD for acne, seborrhea, eczema/dermatitis, and skin barrier function is promising, more robust studies are needed to fully validate its efficacy. The therapeutic potential of CBD should also be balanced with largely unknown/contrasting early studies in modulating pigmentation and hair growth. Thus, there is an underlying need for intense fundamental scientific research as any speculative science could lead to unwanted effects like hair growth/loss or hyper/hypopigmentation issues. Looking beyond the horizon of the buzzword CBD, the therapeutic benefits of hemp phytocannabinoids and other botanicals with phytocannabinoid-like activity (Table 1), will most likely be the focus of future research.
 |
Table 1 Examples of Phytochemicals Targeting the ECS with Phytocannabinoid-Like Activity |
Disclosure
All the authors are employees of Amway Corporation which has commercial offerings in the wellness space. The authors report no other potential conflicts of interest for this work.
References
1. Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur J Pharmacol. 1998;359(1):1–18. doi:10.1016/S0014-2999(98)00649-9
2. Di Marzo V. Endocannabinoids: synthesis and degradation. Rev Physiol Biochem Pharmacol. 2008;160:1–24. doi:10.1007/112_0505
3. Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol. 2006;147(S1):S163–S171.
4. Tóth KF, Ádám D, Bíró T, Oláh A. Cannabinoid signaling in the skin: therapeutic potential of the “C (ut) annabinoid” system. Molecules. 2019;24(5):918.
5. Tüting T, Gaffal E. Chapter 57 - regulatory role of cannabinoids for skin barrier functions and cutaneous inflammation. In: Preedy VR, editor. Handbook of Cannabis and Related Pathologies. San Diego: Academic Press; 2017:543–549.
6. Mounessa JS, Siegel JA, Dunnick CA, Dellavalle RP. The role of cannabinoids in dermatology. J Am Acad Dermatol. 2017;77(1):188–190.
7. Sugawara K, Biro T, Tsuruta D, et al. Endocannabinoids limit excessive mast cell maturation and activation in human skin. J Allergy Clin Immunol. 2012;129(3):726–738.e728.
8. Devane WA, Dysarz F, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34(5):605–613.
9. Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949.
10. Kendall AC, Pilkington SM, Massey KA, Sassano G, Rhodes LE, Nicolaou A. Distribution of bioactive lipid mediators in human skin. J Invest Dermatol. 2015;135(6):1510–1520. doi:10.1038/jid.2015.41
11. Sugiura T, Kondo S, Sukagawa A, et al. 2-Arachidonoylgylcerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215(1):89–97. doi:10.1006/bbrc.1995.2437
12. Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83–90. doi:10.1016/0006-2952(95)00109-D
13. Gegotek A, Biernacki M, Ambrozewicz E, Surazynski A, Wronski A, Skrzydlewska E. The cross-talk between electrophiles, antioxidant defence and the endocannabinoid system in fibroblasts and keratinocytes after UVA and UVB irradiation. J Dermatol Sci. 2016;81(2):107–117. doi:10.1016/j.jdermsci.2015.11.005
14. Ahn K, McKinney MK, Cravatt BF. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev. 2008;108(5):1687–1707. doi:10.1021/cr0782067
15. Liu J, Wang L, Harvey-White J, et al. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology. 2008;54(1):1–7. doi:10.1016/j.neuropharm.2007.05.020
16. Maccarrone M, Di Rienzo M, Battista N, et al. The endocannabinoid system in human keratinocytes. Evidence that anandamide inhibits epidermal differentiation through CB1 receptor-dependent inhibition of protein kinase C, activation protein-1, and transglutaminase. J Biol Chem. 2003;278(36):33896–33903. doi:10.1074/jbc.M303994200
17. Paradisi A, Pasquariello N, Barcaroli D, Maccarrone M. Anandamide regulates keratinocyte differentiation by inducing DNA methylation in a CB1 receptor-dependent manner. J Biological Chemistry. 2008;283(10):6005–6012. doi:10.1074/jbc.M707964200
18. Lynch ME, Ware MA. Cannabinoids for the treatment of chronic non-cancer pain: an updated systematic review of randomized controlled trials. J Neuroimmune Pharmacol. 2015;10(2):293–301.
19. Sonja Ständer S, Schmelz M, Metze D, Luger T, Rukwied R. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. 2005;38(3):177–188. doi:10.1016/j.jdermsci.2005.01.007
20. Tóth BI, Dobrosi N, Dajnoki A, et al. Endocannabinoids modulate human epidermal keratinocyte proliferation and survival via the sequential engagement of cannabinoid receptor-1 and transient receptor potential vanilloid-1. J Investigative Dermatol. 2011;131(5):1095–1104.
21. Pucci M, Pasquariello N, Battista N, et al. Endocannabinoids stimulate human melanogenesis via type-1 cannabinoid receptor. J Biol Chem. 2012;287(19):15466–15478.
22. Czifra G, Szollosi AG, Toth BI, et al. Endocannabinoids regulate growth and survival of human eccrine sweat gland-derived epithelial cells. J Invest Dermatol. 2012;132(8):1967–1976.
23. Rio CD, Millan E, Garcia V, Appendino G, DeMesa J, Munoz E. The endocannabinoid system of the skin. A potential approach for the treatment of skin disorders. Biochem Pharmacol. 2018;157:122–133.
24. Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biological Chemistry. 2004;279(7):5298–5305.
25. Bisogno T, Melck D, Bobrov MY, et al. N-acyl-dopamines: novel synthetic CB1 cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochemical J. 2000;351(3):817–824.
26. Petrocellis LD, Cascio MG, Marzo VD. The endocannabinoid system: a general view and latest additions. Br J Pharmacol. 2004;141(5):765–774.
27. Callaway J. Hempseed as a nutritional resource: an overview. Euphytica. 2004;140(1–2):65–72.
28. Brighenti V, Pellati F, Steinbach M, Maran D, Benvenuti S. Development of a new extraction technique and HPLC method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L. (hemp). J Pharm Biomed Anal. 2017;143:228–236.
29. Vuerich M, Ferfuia C, Zuliani F, Piani B, Sepulcri A. Yield and quality of essential oils in hemp varieties in different environments. Agronomy. 2019;9(7):356.
30. Grijó DR, Osorio IAV, Cardozo-Filho L. Supercritical extraction strategies using CO2 and ethanol to obtain cannabinoid compounds from Cannabis hybrid flowers. J CO2 Utilization. 2019;30:241–248.
31. Chemspider. Available from: http://www.chemspider.com/Chemical-Structure.454786.html?rid=6132fbe7-8093-4cec-abb1-bd866deb4232&page_num=0.
32. Lodzki M, Godin B, Rakou L, Mechoulam R, Gallily R, Touitou E. Cannabidiol—transdermal delivery and anti-inflammatory effect in a murine model. J Controlled Release. 2003;93(3):377–387.
33. Hammell D, Zhang L, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain‐related behaviours in a rat model of arthritis. European j Pain. 2016;20(6):936–948.
34. Stinchcomb AL, Valiveti S, Hammell DC, Ramsey DR. Human skin permeation of Δ8‐tetrahydrocannabinol, cannabidiol and cannabinol. J Pharmacy Pharmacology. 2004;56(3):291–297.
35. Yim S, Lee J, Jo H, et al. Chrysanthemum morifolium extract and ascorbic acid-2-glucoside (AA2G) blend inhibits UVA-induced delayed cyclobutane pyrimidine dimer (CPD) production in melanocytes. Clin Cosmet Investig Dermatol. 2019;12:823–832.
36. Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006;126(12):2565–2575.
37. Dalmau N, Andrieu-Abadie N, Tauler R, Bedia C. Phenotypic and lipidomic characterization of primary human epidermal keratinocytes exposed to simulated solar UV radiation. J Dermatol Sci. 2018;92(1):97–105.
38. Lee C. Collaborative power of nrf2 and pparγ activators against metabolic and drug-induced oxidative injury. Oxid Med Cell Longev. 2017;2017:1378175.
39. Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354.
40. Juknat A, Pietr M, Kozela E, et al. Differential transcriptional profiles mediated by exposure to the cannabinoids cannabidiol and Delta9-tetrahydrocannabinol in BV-2 microglial cells. Br J Pharmacol. 2012;165(8):2512–2528.
41. Juknat A, Pietr M, Kozela E, et al. Microarray and pathway analysis reveal distinct mechanisms underlying cannabinoid-mediated modulation of LPS-induced activation of BV-2 microglial cells. PLoS One. 2013;8(4):e61462.
42. Casares L, García V, Garrido-Rodríguez M, et al. Cannabidiol induces antioxidant pathways in keratinocytes by targeting BACH1. Redox Biol. 2020;28:101321.
43. Atalay S, Jarocka-Karpowicz I, Skrzydlewska E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants. 2020;9(1):21.
44. Gęgotek A, Atalay S, Domingues P, Skrzydlewska E. The differences in the proteome profile of cannabidiol-treated skin fibroblasts following uva or uvb irradiation in 2d and 3d cell cultures. Cells. 2019;8(9):995.
45. Amaya F, Izumi Y, Matsuda M, Sasaki M. Tissue injury and related mediators of pain exacerbation. Curr Neuropharmacol. 2013;11(6):592–597.
46. Stockbridge EL, Suzuki S, Pagán JA. Chronic pain and health care spending: an analysis of longitudinal data from the medical expenditure panel survey. Health Serv Res. 2015;50(3):847–870.
47. Chan HN, Fam J, Ng B-Y. Use of antidepressants in the treatment of chronic pain. Annals Acad Med Singapore. 2009;38(11):974.
48. Ryder S-A, Stannard CF. Treatment of chronic pain: antidepressant, antiepileptic and antiarrhythmic drugs. Continuing Education Anaesthesia, Critical Care Pain. 2005;5(1):18–21.
49. Romero-Sandoval A, Bynum T, Eisenach JC. Analgesia induced by perineural clonidine is enhanced in persistent neuritis. Neuroreport. 2007;18(1):67–71.
50. Campbell CM, Kipnes MS, Stouch BC, et al. Randomized control trial of topical clonidine for treatment of painful diabetic neuropathy. PAIN®. 2012;153(9):1815–1823.
51. Jones VM, Moore KA, Peterson DM. Capsaicin 8% topical patch (Qutenza)—a review of the evidence. J Pain Palliat Care Pharmacother. 2011;25(1):32–41.
52. Peppin JF, Pappagallo M. Capsaicinoids in the treatment of neuropathic pain: a review. Ther Adv Neurol Disord. 2014;7(1):22–32.
53. Donvito G, Nass SR, Wilkerson JL, et al. The endogenous cannabinoid system: a budding source of targets for treating inflammatory and neuropathic pain. Neuropsychopharmacology. 2018;43(1):52–79.
54. Bridges D, Ahmad K, Rice AS. The synthetic cannabinoid WIN55, 212‐2 attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Br J Pharmacol. 2001;133(4):586–594.
55. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456–2473.
56. Russo EB, Guy GW, Robson PJ. Cannabis, pain, and sleep: lessons from therapeutic clinical trials of Sativex, a cannabis-based medicine. Chem Biodivers. 2007;4(8):1729–1743.
57. Leung DY, Soter NA. Cellular and immunologic mechanisms in atopic dermatitis. J Am Acad Dermatol. 2001;44(1 Suppl):S1–s12.
58. Pulvirenti N, Nasca MR, Micali G. Topical adelmidrol 2% emulsion, a novel aliamide, in the treatment of mild atopic dermatitis in pediatric subjects: a pilot study. Acta Dermatovenerologica Croatica. 2007;15(2).
59. Petrosino S, Verde R, Vaia M, Allarà M, Iuvone T, Di Marzo V. Anti-inflammatory properties of cannabidiol, a nonpsychotropic cannabinoid, in experimental allergic contact dermatitis. J Pharmacology Experimental Therapeutics. 2018;365(3):652–663.
60. Dimitriu PA, Iker B, Malik K, Leung H, Mohn WW, Hillebrand GG. New insights into the intrinsic and extrinsic factors that shape the human skin microbiome. mBio. 2019;10(4):e00839–00819.
61. Nakagawa S, Hillebrand GG, Nunez G. Extracts containing carnosic acid and carnosol are potent quorum sensing inhibitors of staphylococcus aureus virulence. Antibiotics. 2020;9(4):149.
62. Mediavilla V, Steinemann S. Essential oil of Cannabis sativa L. strains. J Int Hemp Assoc. 1997;4:80–82.
63. Zengin G, Menghini L, Di Sotto A, et al. Chromatographic analyses, in vitro biological activities, and cytotoxicity of cannabis sativa l. Essential oil: A multidisciplinary study. Molecules. 2018;23(12):3266.
64. Gupta K, Harvima IT. Mast cell‐neural interactions contribute to pain and itch. Immunol Rev. 2018;282(1):168–187.
65. Yosipovitch G, Rosen JD, Hashimoto T. Itch: from mechanism to (novel) therapeutic approaches. J Allergy Clinical Immunology. 2018;142(5):1375–1390.
66. Meng J, Steinhoff M. Molecular mechanisms of pruritus. Current Res Translational Med. 2016;64(4):203–206.
67. Ward SJ, Lefever TW, Rawls SM, Whiteside GT, Walker EA. Age-dependent effects of the cannabinoid CB1 antagonist SR141716A on food intake, body weight change, and pruritus in rats. Psychopharmacology. 2009;206(1):155–165.
68. Bilir K, Anli G, Ozkan E, Gunduz O, Ulugol A. Involvement of spinal cannabinoid receptors in the antipruritic effects of WIN 55,212‐2, a cannabinoid receptor agonist. Clin Exp Dermatol. 2018;43(5):553–558.
69. Pavon FJ, Bilbao A, Hernández-Folgado L, et al. Antiobesity effects of the novel in vivo neutral cannabinoid receptor antagonist 5-(4-chlorophenyl)-1-(2, 4-dichlorophenyl)-3-hexyl-1H-1, 2, 4-triazole–LH 21. Neuropharmacology. 2006;51(2):358–366.
70. Nattkemper LA, Tey HL, Valdes-Rodriguez R, et al. The genetics of chronic itch: gene expression in the skin of patients with atopic dermatitis and psoriasis with severe itch. J Investigative Dermatol. 2018;138(6):1311–1317.
71. Dvorak M, Watkinson A, McGlone F, Rukwied R. Histamine induced responses are attenuated by a cannabinoid receptor agonist in human skin. Inflammation Research. 2003;52(6):238–245.
72. Haruna T, Soga M, Morioka Y, et al. S-777469, a novel cannabinoid type 2 receptor agonist, suppresses itch-associated scratching behavior in rodents through inhibition of itch signal transmission. Pharmacology. 2015;95(1–2):95–103.
73. Maekawa T, Nojima H, Kuraishi Y, Aisaka K. The cannabinoid CB2 receptor inverse agonist JTE-907 suppresses spontaneous itch-associated responses of NC mice, a model of atopic dermatitis. Eur J Pharmacol. 2006;542(1–3):179–183.
74. Caterina MJ, Pang Z. TRP channels in skin biology and pathophysiology. Pharmaceuticals. 2016;9(4):77.
75. Tóth BI, Oláh A, Szöllősi AG, Bíró T. TRP channels in the skin. Br J Pharmacol. 2014;171(10):2568–2581.
76. Tóth BI, Szallasi A, Bíró T. Transient Receptor Potential Channels and Itch: How Deep Should We Scratch? In: Pharmacology of Itch. Springer; 2015:89–133.
77. Moore C, Gupta R, Jordt S-E, Chen Y, Liedtke WB. Regulation of pain and itch by trp channels. Neurosci Bull. 2018;34(1):120–142.
78. Xie Z, Hu H. TRP Channels as Drug Targets to Relieve Itch. Pharmaceuticals. 2018;11:4.
79. Yesilyurt O, Cayirli M, Sakin YS, Seyrek M, Akar A, Dogrul A. Systemic and spinal administration of FAAH, MAGL inhibitors and dual FAAH/MAGL inhibitors produce antipruritic effect in mice. Arch Dermatol Res. 2016;308(5):335–345.
80. Tosun NC, Gunduz O, Ulugol A. Attenuation of serotonin-induced itch responses by inhibition of endocannabinoid degradative enzymes, fatty acid amide hydrolase and monoacylglycerol lipase. J Neural Transm. 2015;122(3):363–367.
81. Schlosburg JE, Boger DL, Cravatt BF, Lichtman AH. Endocannabinoid modulation of scratching response in an acute allergenic model: a new prospective neural therapeutic target for pruritus. J Pharmacology Experimental Therapeutics. 2009;329(1):314–323.
82. Vaia M, Petrosino S, De Filippis D, et al. Palmitoylethanolamide reduces inflammation and itch in a mouse model of contact allergic dermatitis. Eur J Pharmacol. 2016;791:669–674.
83. Stander S, Reinhardt HW, Luger TA. [Topical cannabinoid agonists. An effective new possibility for treating chronic pruritus]. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und verwandte Gebiete. 2006;57(9):801–807. German.
84. Visse K, Blome C, Phan NQ, Augustin M, Ständer S. Efficacy of body lotion containing N-palmitoylethanolamine in subjects with chronic pruritus due to dry skin: a dermatocosmetic study. Acta Derm Venereol. 2017;97(5):639–641.
85. Spradley JM, Davoodi A, Gee LB, Carstens MI, Carstens E. Differences in peripheral endocannabinoid modulation of scratching behavior in facial vs. spinally-innervated skin. Neuropharmacology. 2012;63(4):743–749.
86. Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150(5):613–623.
87. Broughton GI, Janis JE, Attinger CE. The Basic Science of Wound Healing. Plast Reconstr Surg. 2006;117(7S):12S–34S.
88. Ellis S, Lin EJ, Tartar D. Immunology of wound healing. Curr Dermatol Rep. 2018;7(4):350–358.
89. Johnson TR, Gómez BI, McIntyre MK, et al. The cutaneous microbiome and wounds: new molecular targets to promote wound healing. Int J Mol Sci. 2018;19(9):2699.
90. Zhao Z, Guan D, Liu W, et al. Expression of cannabinoid receptor I during mice skin incised wound healing course. Fa Yi Xue Za Zhi. 2010;26(4):241–245.
91. Zheng J-L, Yu T-S, Li X-N, et al. Cannabinoid receptor type 2 is time-dependently expressed during skin wound healing in mice. Int J Legal Med. 2012;126(5):807–814.
92. Wang LL, Zhao R, Li JY, et al. Pharmacological activation of cannabinoid 2 receptor attenuates inflammation, fibrogenesis, and promotes re-epithelialization during skin wound healing. Eur J Pharmacol. 2016;786:128–136.
93. Bort A, Alvarado-Vazquez PA, Moracho-Vilrriales C, et al. Effects of JWH015 in cytokine secretion in primary human keratinocytes and fibroblasts and its suitability for topical/transdermal delivery. Mol Pain. 2017;13:1744806916688220.
94. Del Río C, Navarrete C, Collado JA, et al. The cannabinoid quinol VCE-004.8 alleviates bleomycin-induced scleroderma and exerts potent antifibrotic effects through peroxisome proliferator-activated receptor-γ and CB2 pathways. Sci Rep. 2016;6(1):21703.
95. Ramot Y, Oláh A. Cover Image: neuroendocrine treatment of inherited keratin disorders by cannabinoids? British J Dermatol. 2018;178(6):1469.
96. Chelliah MP, Zinn Z, Khuu P, Teng JMC. Self-initiated use of topical cannabidiol oil for epidermolysis bullosa. Pediatr Dermatol. 2018;35(4):e224–e227.
97. Sangiovanni E, Fumagalli M, Pacchetti B, et al. Cannabis sativa L. Extract and Cannabidiol Inhibit in vitro Mediators of Skin Inflammation and Wound Injury. 2019;33(8):2083–2093.
98. Dobrosi N, Tóth BI, Nagy G, et al. Endocannabinoids enhance lipid synthesis and apoptosis of human sebocytes via cannabinoid receptor-2-mediated signaling. FASEB J. 2008;22(10):3685–3695.
99. Oláh A, Tóth BI, Borbíró I, et al. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J Clin Invest. 2014;124(9):3713–3724.
100. Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Research. 2018;7.
101. Jin S, Lee MY. The ameliorative effect of hemp seed hexane extracts on the Propionibacterium acnes-induced inflammation and lipogenesis in sebocytes. PLoS One. 2018;13(8):e0202933. doi:10.1371/journal.pone.0202933
102. Ali A, Akhtar N. The safety and efficacy of 3% Cannabis seeds extract cream for reduction of human cheek skin sebum and erythema content. Pak J Pharm Sci. 2015;28(4):1389–1395.
103. Kim -S-S, Baik JS, Oh T-H, Yoon W-J, Lee NH, Hyun C-G. Biological activities of Korean citrus obovoides and citrus natsudaidai essential oils against acne-inducing bacteria. Biosci Biotechnol Biochem. 2008;72(10):2507–2513. doi:10.1271/bbb.70388
104. Raman A, Weir U, Bloomfield S. Antimicrobial effects of tea‐tree oil and its major components on Staphylococcus aureus, Staph. epidermidis and Propionibacterium acnes. Lett Appl Microbiol. 1995;21(4):242–245. doi:10.1111/j.1472-765X.1995.tb01051.x
105. Krause K, Foitzik K Biology of the hair follicle: the basics. Paper presented at: Seminars in cutaneous medicine and surgery 2006.
106. Ramezani V, Honarvar M, Seyedabadi M, Karimollah A, Ranjbar AM, Hashemi M. Formulation and optimization of transfersome containing minoxidil and caffeine. J Drug Deliv Sci Technol. 2018;44:129–135. doi:10.1016/j.jddst.2017.12.003
107. Skopp G, Strohbeck-Kuehner P, Mann K, Hermann D. Deposition of cannabinoids in hair after long-term use of cannabis. Forensic Sci Int. 2007;170(1):46–50. doi:10.1016/j.forsciint.2006.09.003
108. Paul R, Williams R, Hodson V, Peake C. Detection of cannabinoids in hair after cosmetic application of hemp oil. Sci Rep. 2019;9(1):2582.
109. Pratt CH, King LE, Messenger AG, Christiano AM, Sundberg JP. Alopecia areata. Nature Reviews Disease Primers. 2017;3(1):1–17. doi:10.1038/nrdp.2017.11
110. Brodell LA, Mercurio MG. Hirsutism: diagnosis and management. Gend Med. 2010;7(2):79–87. doi:10.1016/j.genm.2010.04.002
111. Wendelin DS, Pope DN, Mallory SB. Hypertrichosis. J Am Acad Dermatol. 2003;48(2):161–182. doi:10.1067/mjd.2003.100
112. Telek A, Bíró T, Bodó E, et al. Inhibition of human hair follicle growth by endo- and exocannabinoids. FASEB J. 2007;21(13):3534–3541. doi:10.1096/fj.06-7689com
113. Srivastava BK, Soni R, Patel JZ, et al. Hair growth stimulator property of thienyl substituted pyrazole carboxamide derivatives as a CB1 receptor antagonist with in vivo antiobesity effect. Bioorg Med Chem Lett. 2009;19(9):2546–2550. doi:10.1016/j.bmcl.2009.03.046
114. Bodó E, Bíró T, Telek A, et al. A hot new twist to hair biology: involvement of vanilloid receptor-1 (VR1/TRPV1) signaling in human hair growth control. Am J Pathol. 2005;166(4):985–998. doi:10.1016/S0002-9440(10)62320-6
115. Borbíró I, Lisztes E, Tóth BI, et al. Activation of transient receptor potential vanilloid-3 inhibits human hair growth. J Investigative Dermatol. 2011;131(8):1605–1614. doi:10.1038/jid.2011.122
116. Szabó IL, Herczeg-Lisztes E, Szegedi A, et al. Transient receptor potential vanilloid 4 is expressed in human hair follicles and inhibits hair growth in vitro. J Investig Dermatol. 2018.
117. Chanasumon N, Sriphojanart T, Suchonwanit P. Therapeutic potential of bimatoprost for the treatment of eyebrow hypotrichosis. Drug Des Devel Ther. 2018;12:365. doi:10.2147/DDDT.S156467
118. Khidhir KG, Woodward DF, Farjo NP, et al. The prostamide-related glaucoma therapy, bimatoprost, offers a novel approach for treating scalp alopecias. FASEB J. 2013;27(2):557–567. doi:10.1096/fj.12-218156
119. Zaher H, Gawdat HI, Hegazy RA, Hassan M. Bimatoprost versus mometasone furoate in the treatment of scalp alopecia areata: a pilot study. Dermatology. 2015;230(4):308–313. doi:10.1159/000371416
120. Costin G-E, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007;21(4):976–994.
121. Baswan SM, Yim S, Leverett J, Scholten J, Pawelek J. Cytidine decreases melanin content in a reconstituted three-dimensional human epidermal model. Arch Dermatol Res. 2019;311(3):249–250. doi:10.1007/s00403-019-01897-x
122. Hearing VJ. Milestones in melanocytes/melanogenesis. J Invest Dermatol. 2011;131(E1):E1. doi:10.1038/skinbio.2011.1
123. Kawakami A, Fisher DE. The master role of microphthalmia-associated transcription factor in melanocyte and melanoma biology. Laboratory Investigation. 2017;97(6):649–656. doi:10.1038/labinvest.2017.9
124. Zhou J, Ren T, Li Y, et al. Oleoylethanolamide inhibits α-melanocyte stimulating hormone-stimulated melanogenesis via ERK, Akt and CREB signaling pathways in B16 melanoma cells. Oncotarget. 2017;8(34):56868. doi:10.18632/oncotarget.18097
125. Magina S, Esteves-Pinto C, Moura E, et al. Inhibition of basal and ultraviolet B-induced melanogenesis by cannabinoid CB 1 receptors: a keratinocyte-dependent effect. Arch Dermatol Res. 2011;303(3):201–210. doi:10.1007/s00403-011-1126-z
126. Kim J, In S, Park Y, Park M, Kim E, Lee S. Deposition of JWH-018, JWH-073 and their metabolites in hair and effect of hair pigmentation. Anal Bioanal Chem. 2013;405(30):9769–9778. doi:10.1007/s00216-013-7423-y
127. Hwang YS, Kim Y-J, Kim MO, et al. Cannabidiol upregulates melanogenesis through CB1 dependent pathway by activating p38 MAPK and p42/44 MAPK. Chem Biol Interact. 2017;273:107–114. doi:10.1016/j.cbi.2017.06.005
128. Turner CE, Elsohly MA, Boeren EG. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J Nat Prod. 1980;43(2):169–234. doi:10.1021/np50008a001
129. Stahl V, Vasudevan K. Comparison of efficacy of cannabinoids versus commercial oral care products in reducing bacterial content from dental plaque: a preliminary observation. Cureus. 2020;12(1):e6809.
130. Ali E, Almagboul A, Khogali S, Gergeir U. Antimicrobial Activity of Cannabis sativa L. Chinese Medicine. 2012;3(1): 61–64. doi:10.4236/cm.2012.31010
131. Rashid F, Butt FA, Nasreen S. In vitro antimicrobial and antioxidant activities of two medicinal plants against some clinically important bacteria. FUUAST J Biol. 2016;6(1):103–107.
132. Lone TA, Lone RA. Extraction of cannabinoids from Cannabis sativa L. plant and its potential antimicrobial activity. Universal J Med Dentistry. 2012;1(4):51–55.
133. Appendino G, Gibbons S, Giana A, et al. Antibacterial cannabinoids from Cannabis sativa: a structure− activity study. J Nat Prod. 2008;71(8):1427–1430. doi:10.1021/np8002673
134. Wassmann CS, Højrup P, Klitgaard JK. Cannabidiol is an effective helper compound in combination with bacitracin to kill Gram-positive bacteria. Sci Rep. 2020;10(1):4112. doi:10.1038/s41598-020-60952-0
135. Goldminz AM, Au SC, Kim N, Gottlieb AB, Lizzul PF. NF-kappaB: an essential transcription factor in psoriasis. J Dermatol Sci. 2013;69(2):89–94. doi:10.1016/j.jdermsci.2012.11.002
136. Esposito G, De Filippis D, Maiuri MC, De Stefano D, Carnuccio R, Iuvone T. Cannabidiol inhibits inducible nitric oxide synthase protein expression and nitric oxide production in beta-amyloid stimulated PC12 neurons through p38 MAP kinase and NF-kappaB involvement. Neurosci Lett. 2006;399(1–2):91–95. doi:10.1016/j.neulet.2006.01.047
137. Khaksar S, Bigdeli MR. Intra-cerebral cannabidiol infusion-induced neuroprotection is partly associated with the TNF-alpha/TNFR1/NF-small ka, CyrillicB pathway in transient focal cerebral ischaemia. Brain Injury. 2017;31(13–14):1932–1943. doi:10.1080/02699052.2017.1358397
138. Pucci M, Rapino C, Di Francesco A, Dainese E, D’Addario C, Maccarrone M. Epigenetic control of skin differentiation genes by phytocannabinoids. Br J Pharmacol. 2013;170(3):581–591. doi:10.1111/bph.12309
139. Wilkinson JD, Williamson EM. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J Dermatol Sci. 2007;45(2):87–92. doi:10.1016/j.jdermsci.2006.10.009
140. Holland ML, Allen JD, Arnold JC. Interaction of plant cannabinoids with the multidrug transporter ABCC1 (MRP1). Eur J Pharmacol. 2008;591(1–3):128–131. doi:10.1016/j.ejphar.2008.06.079
141. von Schaper E. Bial incident raises FAAH suspicions. Nat Biotechnol. 2016;34(3):223.
142. Van Esbroeck AC, Janssen AP, Cognetta AB, et al. Activity-based protein profiling reveals off-target proteins of the FAAH inhibitor BIA 10-2474. Science. 2017;356(6342):1084–1087. doi:10.1126/science.aaf7497
143. Bai J, Zheng Y, Wang G, Liu P. Protective effect of D-limonene against oxidative stress-induced cell damage in human lens epithelial cells via the p38 pathway. Oxid Med Cell Longev. 2016;2016.
144. Gulluni N, Re T, Loiacono I, et al. Cannabis essential oil: A preliminary study for the evaluation of the brain effects. Evidence-Based Complementary Alternative Medicine. 2018;2018.
145. Klauke A-L, Racz I, Pradier B, et al. The cannabinoid CB2 receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. European Neuropsychopharmacol. 2014;24(4):608–620. doi:10.1016/j.euroneuro.2013.10.008
146. Bahi A, Al Mansouri S, Al Memari E, Al Ameri M, Nurulain SM, Ojha S. β-Caryophyllene, a CB2 receptor agonist produces multiple behavioral changes relevant to anxiety and depression in mice. Physiol Behav. 2014;135:119–124. doi:10.1016/j.physbeh.2014.06.003
147. Gertsch J, Leonti M, Raduner S, et al. Beta-caryophyllene is a dietary cannabinoid. Proc Nat Acad Sci. 2008;105(26):9099–9104. doi:10.1073/pnas.0803601105
148. Katsuyama S, Mizoguchi H, Kuwahata H, et al. Involvement of peripheral cannabinoid and opioid receptors in β‐caryophyllene‐induced antinociception. European j Pain. 2013;17(5):664–675. doi:10.1002/j.1532-2149.2012.00242.x
149. Raduner S, Majewska A, Chen J-Z, et al. Alkylamides from Echinacea are a new class of cannabinomimetics Cannabinoid type 2 receptor-dependent and-independent immunomodulatory effects. J Biological Chemistry. 2006;281(20):14192–14206. doi:10.1074/jbc.M601074200
150. Chicca A, Raduner S, Pellati F, et al. Synergistic immunomopharmacological effects of N-alkylamides in Echinacea purpurea herbal extracts. Int Immunopharmacol. 2009;9(7–8):850–858. doi:10.1016/j.intimp.2009.03.006
151. Hohmann J, Rédei D, Forgo P, et al. Alkamides and a neolignan from Echinacea purpurea roots and the interaction of alkamides with G-protein-coupled cannabinoid receptors. Phytochemistry. 2011;72(14–15):1848–1853. doi:10.1016/j.phytochem.2011.06.008
152. Hou -C-C, Chen C-H, Yang N-S, et al. Comparative metabolomics approach coupled with cell-and gene-based assays for species classification and anti-inflammatory bioactivity validation of Echinacea plants. J Nutr Biochem. 2010;21(11):1045–1059. doi:10.1016/j.jnutbio.2009.08.010
153. Hudson JB. Applications of the Phytomedicine Echinacea purpurea (Purple Coneflower) in Infectious Diseases. BioMed Research International. 2012. doi:10.1155/2012/769896
154. Hu C, Kitts DD. Studies on the antioxidant activity of Echinacea root extract. J Agric Food Chem. 2000;48(5):1466–1472.
155. Hassanzadeh P. The CB 1 receptor-mediated endocannabinoid signaling and NGF: the novel targets of curcumin. Neurochem Res. 2012;37(5):1112–1120. doi:10.1007/s11064-012-0716-2
156. Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The essential medicinal chemistry of curcumin: miniperspective. J Med Chem. 2017;60(5):1620–1637. doi:10.1021/acs.jmedchem.6b00975
157. Siemoneit U, Koeberle A, Rossi A, et al. Inhibition of microsomal prostaglandin E2 synthase‐1 as a molecular basis for the anti‐inflammatory actions of boswellic acids from frankincense. Br J Pharmacol. 2011;162(1):147–162. doi:10.1111/j.1476-5381.2010.01020.x
158. Rempel V, Fuchs A, Hinz S, et al. Magnolia extract, magnolol, and metabolites: activation of cannabinoid CB2 receptors and blockade of the related GPR55. ACS Med Chem Lett. 2013;4(1):41–45. doi:10.1021/ml300235q
159. Shen J-L, Man K-M, Huang P-H, et al. Honokiol and magnolol as multifunctional antioxidative molecules for dermatologic disorders. Molecules. 2010;15(9):6452–6465. doi:10.3390/molecules15096452
160. Chandrasekhar K, Kapoor J, Anishetty S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian J Psychol Med. 2012;34(3):255. doi:10.4103/0253-7176.106022
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
