Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 15
Therapeutic Management of Patients with FLT3 + Acute Myeloid Leukemia: Case Reports and Focus on Gilteritinib Monotherapy
Authors Bocchia M, Carella AM, Mulè A, Rizzo L, Turrini M, Abbenante MC, Cairoli R, Calafiore V, Defina M, Gardellini A, Luzi G, Patti C, Pinazzi MB, Riva M, Rossi G, Sammartano V, Rigacci L
Received 28 October 2021
Accepted for publication 4 April 2022
Published 22 April 2022 Volume 2022:15 Pages 393—407
DOI https://doi.org/10.2147/PGPM.S346688
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Monica Bocchia,1 Angelo Michele Carella,2 Antonino Mulè,3 Lorenzo Rizzo,4 Mauro Turrini,5 Maria Chiara Abbenante,2 Roberto Cairoli,4 Valeria Calafiore,3 Marzia Defina,1 Angelo Gardellini,5 Giovanni Luzi,6 Caterina Patti,3 Maria Beatrice Pinazzi,6 Marta Riva,4 Giovanni Rossi,2 Vincenzo Sammartano,1 Luigi Rigacci6
1Hematology Unit, Azienda Ospedaliera Universitaria Senese, University of Siena, Siena, Italy; 2Division of Hematology with Hematologic Intensive Care Unit and Cellular Therapies, Department of Medical Science, Fondazione IRCCS Casa Sollievo Della Sofferenza, Foggia, Italy; 3UOC Hematology and Oncology, Ospedali Riuniti Villa Sofia-Cervello, Palermo, Italy; 4Department of Haematology, Niguarda Cancer Center, ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy; 5Division of Hematology, Valduce Hospital, Como, Italy; 6UOC Hematology and Stem Cell Transplant Unit, Ospedale S, Camillo, Rome, Italy
Correspondence: Angelo Michele Carella, Division of Hematology with Hematologic Intensive Care Unit and Cellular Therapies, Department of Medical Science, Fondazione IRCCS Casa Sollievo della Sofferenza, Viale Cappuccini, San Giovanni Rotondo, Foggia, 71013, Italy, Tel +390882410054, Fax +390882410322, Email [email protected]
Abstract: Acute myeloid leukemia is a malignant disorder of the bone marrow, characterized by differentiation, clonal expansion, and uncontrolled proliferation of malignant myeloid progenitor cells and by several molecular and genetic abnormalities. A mutation of FMS-like tyrosine kinase 3 gene can be observed in about one-third of cases of acute myeloid leukemia. Two FLT3 inhibitors are actually approved for FLT3 mutated acute myeloid leukemia: midostaurin, a multikinase first generation inhibitor with lower affinity for FLT3 binding, and gilteritinib fumarate, a potent second-generation inhibitor of both FLT3-ITD and TKD. Gilteritinib is a new effective and well-tolerated drug for patients with relapsing or refractory FLT3-positive acute myeloid leukemia. Thanks to its efficacy, low toxicity, its good manageability (oral formulation), this drug is suitable for all the patients, including elderly frail patient with concomitant therapies or pre-existing or underlying diseases, and can be used also in the outpatient setting, reducing risks and costs related to the hospitalization. We report and discuss seven cases of different patients with FLT3 positive acute myeloid leukemia successfully managed with gilteritinib in the real clinical practice.
Keywords: acute myeloid leukemia, FLT3, midostaurin, gilteritinib
Introduction
Acute myeloid leukemia (AML) is a malignant disorder of the bone marrow, characterized by differentiation, clonal expansion, and uncontrolled proliferation of malignant myeloid progenitor cells and by several molecular and genetic abnormalities that cause variable clinical outcomes.1,2 AML is the most common form of acute leukemia: it represents about 80% of acute leukemia in adults, with a median age at diagnosis of 67 years3,4 and its incidence increases with age.1
AML is associated to a poor prognosis: in fact, the 5-year overall AML survival rate is 15%, Survival can vary substantially, ranging from 35–40% in adults aged 60 and below to as low as 5–15% in older patients.3,5
According to the American Cancer Society’s estimates for leukemia, in 2020 were diagnosed about 60,530 new cases of leukemia with 23,100 deaths from the disease, In particular, the new cases of AML were about 19,940 (most in adults) with about 11,180 deaths.6 In USA, the age-adjusted incidence of AML is 4.3 per 100,000 annually.1 The incidence of AML in Europe is 3.5 cases per 100,000 population per year.7 In Italy, the AIRTUM-AIOM report states that in 2020 about 8.000 new diagnosis of leukemia were expected; in particular, the estimated incidence of AML in Italy is similar to the European one (3.5 cases per 100,000 population per year).8
AML is a very complex disease, making its management difficult, because several molecular and cytogenetic abnormalities can involve critical genes of normal cell development, cellular survival, proliferation and maturation. In fact, multiple malignant subclones, characterized by specific genetic mutations which may have an impact on the patients’ prognosis and can represent a therapeutic target, exist in most AML patients.9 For this reason, the early definition of the genetic and molecular pattern of the single patient can help to define prognosis and to individualize the treatment approach.10
A mutation of FMS-like tyrosine kinase 3 gene (FLT3, which encodes a receptor type tyrosine kinase that plays a key role in cell survival and proliferation) can be observed in about one-third of cases of AML.11,12 Among the two forms of FLT3 mutations, the FLT3-ITD (internal tandem duplication) is indicative of high or intermediate risk AML and is associated with highly proliferative disease, shorter duration of remissions, and increased rates of disease relapse, while the role of FLT3-TKD (tyrosine kinase domain) mutation is uncertain.2,3,13,14 Such as other genetic alterations, FLT3 mutation has led to the development of specific targeted therapies.2 The first generation FLT3 inhibitors (such midostaurin and sunitinib) are multikinase inhibitors with lower affinity for FLT3 binding and off-target effects that may increase their toxicity profile. Second generation FLT3 inhibitors are more potent against FLT3 than the first generation ones.2 Gilteritinib fumarate, the first second generation FLT3 inhibitor available on the market, is a potent inhibitor of both FLT3-ITD and TKD, interfering with signaling and proliferation and inducing apoptosis in leukemic cells expressing FLT3; besides, gilteritinib is a strong inhibitor of other molecular target, such as AXL, anaplastic lymphoma kinase (ALK) and leukocyte receptor tyrosine kinase (LTK).2,3
According to the ESMO Clinical Practice Guidelines 2020 for diagnosis, treatment and follow-up of AML in adult patients, the 7 + 3 + midostaurin regimen is recommended in FLT3-ITD or FLT3-TKD positive patients. For primary refractory and relapsed AML patients not eligible for standard chemotherapy, the therapeutic options must control the disease progression and reduce the treatment-related mortality; in FLT3-mutated patients, the Guidelines recommend gilteritinib in monotherapy.15
In this publication, seven patients with FLT3 positive refractory/relapsed (R/R) AML treated with gilteritinib in Italian reference centers will be discussed, in order to describe some real-life experiences with this new oral target drug in terms of efficacy, safety, manageability and quality of life. In particular, each center reported one (2 cases for Como center) of the AML cases treated with gilteritinib monotherapy during the period December 2019 – December 2021: 5 cases in Siena center, 4 cases in S. Giovanni Rotondo center, 10 cases in Palermo center, 8 cases in Milan center, 5 cases in Como center, 4 cases in Rome center, Molecular genetic testing and supportive care, including prophylactic anti-infectious treatment, were performed according to 2017 ELN recommendations and institute policy. No approval was required for this research by an institutional review board or ethics committee, because gilteritinib was used on label in all cases. All patients gave own consent to the publication of their clinical data in anonymous form for scientific and educational purposes.
Case Reports
Case Report 1
In April 2019, a 72-year-old man with a newly diagnosed AML was transferred to our Hematology Unit. Cytogenetic analysis did not show abnormalities, while the FLT3-ITD mutation (allelic ratio (AR) < 0.5) was detected on molecular evaluation, thus classifying the patient at the intermediate risk according to the 2017 ELN recommendations. Despite his age, patient presented in good clinical conditions (ECOG 1) and was considered eligible for standard chemotherapy. As patient was FLT3-ITD positive, we decided to start an induction chemotherapy with 3 + 7 regimen in combination with the FLT3 inhibitor midostaurin. The therapy was moderately tolerated due to the occurrence of febrile neutropenia and QTc interval prolongation, the latter requiring a dose reduction of midostaurin. Post-induction bone marrow (BM) evaluation revealed a 20% blast persistence with the presence of FLT3-ITD mutation (AR < 0.5); for this reason, a second cycle of 3 + 7 + midostaurin regimen was started on June 2019. However, midostaurin was discontinued after only 3 days for QTc interval prolongation and the onset of paroxysmal cough that resolved completely after drug discontinuation. BM assessment showed a morphologic complete remission (CR) with an incomplete haematological recovery due to severe and persistent platelet (PLT) count < 10 x 109/L and the persistence of the FLT3-ITD mutation after molecular evaluation (AR < 0.5). Due to the poor tolerance and the moderate outcome after the intensive chemotherapy approach, we decided to continue the treatment with the hypomethylating agent azacitidine. Along this treatment, the patient required weekly PLT transfusions due to the persistence of a severe thrombocytopenia, yet maintaining good values of hemoglobin and absolute neutrophil count. BM assessments performed after cycles IV, VIII and X of azacitidine showed the presence of blasts (about 10%), with a further increase (20%) together with a rise in the allelic burden of the FLT3-ITD mutation in the last evaluation (AR 0.9). As a consequence, azacitidine therapy was discontinued on May 2020 and the oral FLT3 inhibitor gilteritinib was started. The new drug was well tolerated and the patient did not experience any adverse events; in particular, no signs or symptoms of differentiation syndrome neither QTc interval prolongation were observed. After the second cycle of gilteritinib, PLT count increased and the patient became independent from PLT transfusions. A BM assessment performed after cycle VIII showed a morphologic complete remission without FLT3-ITD mutation on molecular evaluation for the first time since diagnosis. At the last follow-up (April 2021), 2 years after the diagnosis and 11 months after the start of gilteritinib, the patient refers a good compliance to this target therapy, is in good clinical condition, transfusion-independent and without any clinical evidence of leukemia.
Case Report 2
In 2019, a 73-year-old woman came to our attention from the emergency room, for persistent asthenia from several weeks. Blood tests showed a severe pancytopenia (white blood count - WBC 1400/mmc, hemoglobin - Hb 8.4 g/dl, PLT 29.000/mmc): for this reason, a venous peripheral blood smear was performed, showing 40% of blasts, possible of myeloid lineage. Despite the good general conditions, the patient was hospitalized in our department in order to continue the diagnostic work-up. Interestingly, according to the medical history, the patient was previously treated with chemotherapy in 2010 for a high-grade sarcoma; other relevant comorbidities were glaucoma and an anxious-depressive syndrome. The bone marrow aspirate confirmed the diagnosis of AML (flow cytometry positive for 55.5% of CD45+, CD13+, CD33+, CD34+, CD36+, CD117+ HLADR+ MPO dim blasts), with chromosome 11 trisomy at conventional cytogenetic and the presence of a FLT3-ITD mutation. Finally, the patient was not considered eligible for allogeneic transplantation or intensive chemotherapy based on age, comorbidities and clinical conditions. For this reason, the patient was enrolled in a trial; at the time of randomization, the patient was candidated for a monotherapy with azacitidine (75 mg/m2 sc for 7 days every 28 days); the first cycle was administered on July 2019.
After 6 cycles of treatment, which was well tolerated, our patient had normal blood tests; the bone marrow showed a flow cytometry positive for 0.7% of myeloblasts CD45dim, CD13+, CD33+, CD34+ and CD117+; a cytogenetics with chromosome 11 trisomy in just one metaphase out of 20; and the absence of any FLT3 mutation. These results were compatible with a complete remission: for this reason, the patient continued the treatment. Unfortunately, before the ninth cycle the patient had a pancytopenia; therefore, an urgent bone marrow evaluation showed a morphological relapse of AML. The flow cytometry showed 55% myeloid blasts CD45dim/+, CD13+, CD33+, CD34+, CD117+, and the FLT3-ITD mutation was still detectable.
After the bone marrow confirmation of relapse, a cytoreductive therapy with hydroxyurea (2500 mg tablets/day) associated to gilteritinib (120 mg – 3 tablets of 40 mg/day) was started for the progressive increase of the WBC count. The blood count before the first cycle of gilteritinib was: WBC 14,160/mmc, Neutrophils 400/mmc, Myelocytes 9540/mmc, Hb 9.6 g/dl, PLTs 7000/mmc. After only 4 days, the WBC count reduced, so the concomitant cytoreductive therapy was stopped. The blood count at day 4 was: WBC 6590/mmc, Neutrophils 290/mmc, Myelocytes 4290/mmc, Hb 9.5 g/dl, PLTs 17,000/mmc; it is noteworthy that the patient did not need a transfusion support from the start of the therapy and the liver and kidney function remained within the normal limits.
At the first disease evaluation after two cycles of therapy, the bone marrow blasts reduced to 3%; the blood count (WBC 2350/mmc, Neutrophils 1040/mmc, Hb 8.6 g/dl, PLTs 31,000/mmc) was indicative for a complete remission with incomplete haematological recovery. Indeed, after 4 cycles, the patient achieved a partial normalization of the blood count independently from transfusion support.
Unfortunately, the blasts count of bone marrow increased (6%) at the beginning of the sixth cycle, meaning a possible initial loss of response; However, based on the clinical conditions, the good tolerability of the treatment and the absence of valid therapeutic alternatives, it was decided to go ahead with the ongoing therapy switching the maximum dosage of gilteritinib: 200 mg (or 5 tablets of 40 mg)/day. This new schedule was subjectively well tolerated and no side effects were observed.
The therapy was taken continuously throughout the treatment period, except for a short period (during a hospitalization for sepsis and pneumonia occurred at mid fourth cycle), when the drug was stopped for less than a week due to the intravenous antibiotic therapy and a grade 4 neutropenia. The patient accessed Day Hospital already feverish with blurred respiratory symptoms and pathological rhonchi and crackles in both lungs. A chest computerized tomography (CT) scan showed “in the anterior segment of the right upper lobe and in the lingular area, some nuanced mantle alterations […] Frosted glass associated with interstitial thickening, of inflammatory significance.”
COVID 19 testing was negative. The patient was treated with piperacillin/tazobactam for a total of 14 days, obtaining a rapid defervescence and the reduction of the inflammation parameters. During the hospitalization, the patient also had an accidental trauma to the nape without any acute bleeding documented by brain CT. Moreover, during the treatment with gilteritinib the patient was hospitalized for another uncomplicated covid-negative interstitial pneumonia and treated with iv meropenem and vancomycin without stopping gilteritinib. Both hospitalizations lasted less than 3 weeks without sequelae.
After 7 cycles of 28 days of therapy (October 2020) the patient was hospitalized for a fungal suspected pneumonia, and treated with isavuconazole (chosen due to minor interaction with gilteritinib) without any relevant toxicity. Unfortunately, she died after a week due to respiratory complications without clear signs of peripheral progression of the underlying haematological disease.
During all the treatment no adverse events related to the FLT3 inhibitor were reported. Hepatic and renal function were always within normal limits and the QT interval was never prolonged. It is also important to point out the concomitant use of drugs with well-known interactions with gilteritinib, such as fluoroquinolones and thiazoles, without withdrawal of the drug or dose adjustment, or any clinical or laboratory adverse effects.
Case Report 3
A 70-year-old female patient without a significant medical history accessed to our Department with fever and generalized weakness. Physical examination at baseline was unremarkable. A complete blood count showed a white blood cell count of 50.5 × 109/L, hemoglobin 8.7 g/dL, and platelet count 124 × 109/L. Peripheral blood smear showed several monocytes with blasts. Bone marrow examination showed an hypercellular marrow with 80% blasts. At immunophenotypic analysis by flow cytometry, blasts were positive for CD34, CD117, DR, CD13, CD7, CD33. Karyotype analysis of bone marrow cells showed a 47, XX, +21 [20] karyotype; trisomy 21 was considered as acquired since the patient showed no phenotypic characteristics of Down syndrome. Molecular study showed the presence of a FLT3-ITD mutation; the FLT3-ITD allelic ratio was > 0,5 (high). No other mutations were detected, but WT1 was overexpressed (15.060 WT1/ABL 104). Consequently, the diagnosis was high risk AML, according to 2017 ELN risk stratification by genetics.
Leukocytosis was immediately treated with hydroxycarbamide, allopurinol and iv hyperhydration (prophylaxis for the tumor lysis syndrome). A standard induction 3 + 7 chemotherapy regimen with daunorubicin and cytarabine plus midostaurin was started 7 days after diagnosis. The clinical course was complicated by an episode of neutropenic fever, which required a treatment with broad-spectrum antibiotics. The patient developed a grade 4 oral mucositis during the nadir of neutrophils: for this reason, a total parental nutrition was administered for a total of 14 days. At day 28 from chemotherapy, when neutrophil count started to rise again, a cellulitis on the left wrist was observed: the new cycle of antibiotic therapy allowed the progressive resolution of symptoms.
The bone marrow evaluation after the induction phase showed a treatment failure with primary refractory disease. Molecular status was re-evaluated and FLT3 ITD mutation was detected in leukemic cells; for this reason, the patient started a second line therapy with gilteritinib (120 mg once a day). At this time, the patient was neutropenic and blood transfusion needs were high (approximately 1 transfusion every 8 days); weakness and fatigue were the main symptoms reported.
Two months after gilteritinib therapy, bone marrow evaluation showed a stable disease with a concomitant improvement of the clinical status: a granulocyte recovery and a transfusion independence were observed and the patient was in good clinical conditions without the need of hospitalizations. During the third month of therapy, the patient experienced a differentiation syndrome, with febrile neutropenia, hypoxia and body weight gain; chest CT showed the presence of pulmonary infiltrates. Reactive C protein and Ferritin levels were high (2000 ng/mL). This adverse event was managed in outpatient setting because clinical conditions were stable and the patient did not need hospitalization: the administration of gilteritinib was temporary interrupted, and corticosteroids associated to broad-spectrum antibiotics were prescribed. Fifteen days later, symptoms resolved (except weakness) but grade 1 anemia and grade 4 thrombocytopenia appeared: gilteritinib was re-introduced. One month later, the patient was transfusion-free and the clinical conditions markedly improved; reactive C protein level decreased but ferritin and LDH levels remained unexpectedly high and WBC progressive increased with monocytosis. The patient obtained a partial remission after 5 months of treatment, but with persisting serum ferritin and LDH inexplicably high levels (> 7000 μg/l and >2000 U/I, respectively) without other signs or symptoms.
Case Report 4
In July 2019, a 46-years-old woman was admitted to our Haematological Unit for an hyperleucocytic AML (WBC 35,000/µL). The immunophenotype analysis on bone marrow aspirate showed > 80% blasts cells positive for CD117, CD13, CD33, CD34, CD38 and aberrant CD7. At the diagnosis, a normal 46 XX karyotype and FLT3-ITD and NPM1B mutations were present. The disease was completely resistant to the induction chemotherapy with the 3 + 7 (daunorubicin 60 mg/m2 days 1,2,3, cytosine arabinoside 100 mg/m2 days 1–7) plus midostaurin (50 mg every 12 hours at days 8–21) regimen. For this reason, at the end of August 2019 a reinduction therapy with the FLA-IDA regimen (fludarabine 50 mg/m2 at days 1–5 + cytosine arabinoside 2000 mg/m2 at days 1–5 + idarubicin 10 mg/m2 at days 3,4,5) was started, with a partial remission due to persistence of 7% blasts on bone marrow.
On September 2019, the patient received a FLA3 chemotherapy (fludarabine 50 mg/m2 at days 1–3 plus cytosine arabinoside 2000 mg/m2 at days 1–3) as a bridge for bone marrow transplantation during the iatrogenic aplastic phase. After the TBF (thiotepa, busulfan, fludarabine) conditioning and a graft versus host disease (GVHD) prophylaxis with post-transplant cyclophosphamide, an haploidentical bone marrow transplantation was performed on October 28th, 2019 (donor: brother).
The re-evaluation of disease at 30, 60, 90 days after the transplant showed the achievement and the maintenance of a haematological complete remission, a FLT3-ITD and NPM1B negativity, and a complete chimerism with 100% donor cells. In February 2020, during the cyclosporine reduction phase, a chronic mild gastric GVHD was successfully treated with beclomethasone dipropionate. Unfortunately, 6 months after transplantation the patient developed a disease relapse: for this reason, a third induction line therapy with MEC (mitoxantrone 6 mg/m2 at days 1–6 plus etoposide 80 mg/m2 at days 1–6 and cytosine arabinoside 1000 mg/m2 at days 1–6) regimen was administered starting from May 2020, showing a complete resistance. Moreover, a very severe septic shock caused by resistant to carbapenems (KPC positive) E. coli and K. Pneumoniae strains complicated the chemotherapy.
From June 18th 2020, the patient started gilteritinib 40 mg three times daily, which was stopped on July 30th 2020 due to a CVC-related infection with second septic shock caused by extended spectrum beta lactamase (ESBL) positive K. Pneumoniae. After the resolution of the severe infection, the treatment with gilteritinib was re-started at the dose of 40 mg two times daily due to the persistent grade III neutropenia. During the gilteritinib therapy with a reduced dose, the patient experienced a progressive clinical improvement and was transfusion-free; the neutrophils count progressive recovered according to the progressive reduction blasts count, as well as quantitative PCR for FLT3-ITD and NPM1B mutation on bone marrow with consensual near complete chimerism recovery (Figure 1). After 3.5 months of gilteritinib therapy (October 2020), the patient was in good clinical condition, neutropenia was solved (neutrophils 3700/µL) and noteworthy she achieved the second haematological complete remission with minimal molecular disease positivity for FLT3-ITD e NPM1B mutation and a near complete chimerism (Figure 2). At that time, the patient was considered eligible by another centre for the enrolment in a Phase 1/2 clinical trial on allogeneic CIK (Cytokine induced Killer) cells from haploidentical donor with a reduced risk of induction of GVHD and increased antileukemia activity following the in vitro expansion process. This experimental therapy was administered from December 2nd 2020 to January 13th 2021: the patient is actually GVHD free and waiting for a re-evaluation of the leukemic disease.
 |
Figure 1 Molecular biology clearance. |
 |
Figure 2 Haematological recovery. |
Case Report 5
A 52-year-old woman referred to the emergency room for general fatigue. She had a history of hysterectomy for menorrhagia and pityriasis rosea. The patient’s vital signs were stable and no alterations were recorded after physical examination. Blood tests showed a mild leukocytosis (WBC 10,300/μL) with a relative neutropenia (neutrophils 15%), severe macrocytic anemia (Hb 6.8 g/dL, MCV 101 fl), and a slightly reduced platelet count (131,000/μL). On peripheral blood smear, myeloid blasts represented 13% of leukocytes. Bone marrow aspiration was performed and she was diagnosed with acute myeloid leukemia, with 30% myeloperoxidase (MPO) staining positive myeloblasts and 16% MPO-negative monoblasts. In flow cytometric analysis, the myeloid blast cells resulted positive for CD13, CD33, CD38, CD11,7 and cytoplasmic MPO, while monocytoid elements were positive for CD4, CD33, CD36, CD38, CD64 and partially for CD14 and CD56. The karyotype was normal, and molecular analysis were positive for insertion c.863_864insTCTG in exon 11 of nucleophosmin (NPM1) gene and for nucleotide substitution c.419G>a in exon 4 of isocitrate dehydrogenase 2 gene (IDH2-R140), and negative for other gene mutations (FLT3, RUNX1, TP53, IDH1, CEBPA). The patient received a standard induction therapy consisting of daunorubicin and cytarabine. The treatment was complicated by a severe neutropenic enterocolitis requiring a massive antibiotic therapy, with further evidence of gastrointestinal colonization by carbapenemase-producing Citrobacter freundii and vancomycin-resistant Enterococcus faecium. A hematological and molecular complete remission was achieved 4 weeks after the start of the induction therapy: a consolidation treatment with intermediate-dose cytarabine was decided. However, the patient showed a bone marrow relapse after the second course of consolidation therapy. Molecular retest confirmed the presence of IDH2-R140 mutation, with negativity for NPM1 and FLT3 gene mutations. A salvage therapy with the FLAG-IDA regimen (fludarabine, cytarabine, idarubicin and G-CSF) was started, but the patient failed to respond with a persistence of more than 70% of bone marrow blasts and rapid expansion of peripheral blasts. Molecular tests showed the presence of FLT3-ITD gene mutations with high allelic ratio (0.8). The patient was therefore a candidate for gilteritinib monotherapy, but she developed hypotension, fever and bilateral consolidating pneumonia (Figure 3) with respiratory failure prior to start the drug. A blood test showed a leukocytosis (WBC 60,300/μL), requiring a cytoreductive treatment with hydroxycarbamide, associated to transfusion-dependent anemia and thrombocytopenia. C-reactive protein and procalcitonin values increased up to 405 mg/L and 1.94 ug/L, respectively. Blood and urine cultures were negative, as well as the SARS-CoV-2 test both on the nasopharyngeal swab and bronchoalveolar lavage (BAL). The patient received an empiric broad-spectrum antibiotic therapy with ceftazidime/avibactam and linezolid, without any clinical improvement. A CT scan showed the progression of the underlying pneumonia and an additional BAL test was negative (culture and multiplex PCR assay). High-dose fosfomycin and liposomal amphotericin B were added to the ongoing antibiotic therapy, without any clinical improvement but with a further increase of the inflammation markers. Due to suspect bronchiolitis obliterans with organizing pneumonia, a steroid treatment was started, which resulted in complete regression of the fever and resolution of respiratory failure. Therefore, gilteritinib was administered at a starting dose of 120 mg once daily, with a rapid clearance of peripheral blasts within 72 hours. The antifungal prophylaxis was ultimately changed to isavuconazole to drug-drug interactions. After 7 days of gilteritinib treatment, the patient developed a severe differentiation syndrome, with a rapidly progressing non-itchy maculo-papular skin rash (Figure 4A and B) associated with fever, weight gain and increase of transaminase values. A chest x-ray was negative for pleural effusion, but lung ultrasound showed clear B lines. A steroid therapy with dexamethasone 10 mg bid was initiated without discontinuing gilteritinib therapy, resulting in a gradual resolution of the skin rash (Figure 4C–F). After 18 days, the patient developed a febrile neutropenia, successfully treated with an empiric antibiotic therapy associated with the rapid taper of steroids without gilteritinib withdrawal. After discharge, the patient continued gilteritinib in the out-patient setting, with persistence of pancytopenia and transfusion-dependence. The bone marrow evaluation, performed after 2 months of therapy, showed a partial remission with 13% of blasts, and the patient underwent an allogeneic transplant with myeloablative conditioning from an unrelated HLA-matched donor. The transplant course was regular and a maintenance therapy with gilteritinib was started 3 months after the transplant. 6 months after the allogeneic procedure, no graft-vs-host disease or infectious events were observed, and bone marrow evaluation confirmed the complete remission with full-donor chimerism.
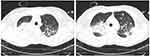 |
Figure 3 Consolidating pneumonia before starting gilteritinib. |
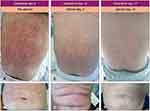 |
Figure 4 Evolution of the skin rash during the differentiation syndrome (A-F). |
Case Report 6
A 76-year-old man referred to emergency room with fatigue and laterocervical swelling. He had a history of duodenocephalopancreatectomy for insulinoma and occult HBV infection. The patient’s vital signs were stable; the physical examination showed a diffuse extraoral swelling in the lower one-third of face extending to left submandibular and laterocervical region. Blood tests showed hyperleukocytosis (WBC 153,000/μL) with normocytic anemia (Hb 9.2 g/dL, MCV 90 fl), and thrombocytopenia (PLT 48,000/μL). On peripheral blood smear; 90% of the leukocytes were monoblasts: for this reason, the diagnosis was acute monoblastic leukemia. Bone marrow aspiration showed 89% of monoblasts positive for CD13, CD33, CD34, CD64, CD117, and partially for CD7 and CD56. The karyotype was normal, but the molecular analysis was positive for ITD of FLT3 gene with high allelic ratio (0.64) and negative for other gene mutations (NPM1, RUNX1, TP53, IDH1, CEBPA). According the Comprehensive Geriatric Assessment, the patient was considered unfit and therefore he was candidate for the hypomethylating agent decitabine. Despite an initial cytoreductive treatment with hydroxycarbamide, he developed a rapid and massive progression of submandibular and laterocervical sarcoma resulting in rapidly progressive inspiratory dyspnea and dysphagia (Figure 5). Thus, an induction chemotherapy with the “2+5” schedule was initiated for debulking purpose. The treatment was complicated by the evolution of cutaneous sarcoma in a submandibular abscess (Figure 6) caused by methicillin-sensitive Staphylococcus aureus; oral midostaurin was not initiated due to severe dysphagia. The patient failed to respond to the induction therapy, with the persistence of more than 70% of bone marrow blasts, the confirm of the FLT3-ITD gene mutation (allelic ratio 0.39), a rapid expansion of peripheral blasts and the growth of new sarcomatous lesions. The patient had an intercurrent septicaemia caused by an ESBL-positive Escherichia coli, which led to a further worsening of the clinical status (ECOG performance status 3). After the resolution of the sepsis, a target therapy with gilteritinib at a starting dose of 120 mg once daily was initiated, resulting in rapid clearance of peripheral blasts and progressive reduction of sarcomatous lesions. Therapy was well tolerated and the patient continued gilteritinib treatment in the out-patient setting. A progressive hemometric recovery with transfusion-independence and normalization of peripheral parameters were observed, as well as a concomitant complete morphological remission after bone marrow evaluation. After 6 months from the start of gilteritinib therapy, the patient was still alive with full recovery of performance status and complete regression of the sarcomatous lesions (Figure 7).
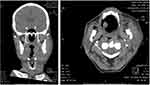 |
Figure 5 CT scans of submandibular and laterocervical sarcoma. |
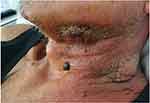 |
Figure 6 Evolution of the cutaneous sarcoma in a submandibular abscess. |
 |
Figure 7 Evolution of the myeloid sarcoma during the treatment with gilteritinib. |
Case Report 7
A previously fit 47-year-old man accessed to our Hospital in May 2020 with a 5 days history of fever, asthenia, fatigue and no respiratory symptoms. Blood counts showed a leukocytosis (WBC 174 x 109/L), 90% myeloid blasts in a peripheral blood smear and hemoglobin 9.2 g/dl; platelet count (105 × 109/L) and coagulation markers were indicative of a disseminated intravascular coagulation (DIC). The diagnosis was confirmed by immunophenotype performed on bone marrow aspirate, where the following antigens were detected on myeloid blasts: CD117+, CD34 +, CD33+, CD13+, MPO+, CD7+, CD38+, CD99+. A male karyotype with a deletion involving the chromosome 12 del (12p13) was found by cytogenetics. Molecular tests based on polymerase chain reaction showed a FLT3-ITD mutation with high allelic ratio (AR: 1.2) and nucleophosmin 1 (NPM1) gene- mutated on the entire leukemic burden (100%). No other additional mutations were detected. The hyperleukocytosis was immediately treated with hydroxyurea and rasburicase (for tumor lysis syndrome prophylaxis) and a two doses of 200 mg cytarabine over the first 48 hours. A combined nasal and pharyngeal swab for SARS-CoV-2 RNA was negative. An induction therapy was performed according to the 3+7 regimen associated to midostaurin at the dosage of 50 mg bid from day +8, complicated by febrile neutropenia and pneumonia. Although the patient received an induction treatment with a midostaurin-based regimen, the remission was not achieved. As there was no availability of a matched related donor, the search for an unrelated donor was started in May 2020.
A bone marrow analysis on day 50 after the induction chemotherapy showed 54% of blast cells and the presence of FLT3-ITD (AR:1.1) and NPM1 mutations (95.5%). On July 2020, the patient started gilteritinib at a standard dose of 120 mg/daily; the blood values were: hemoglobin 11.20 g/dl; platelet count 205 × 109/L and WBC count of 3.18 x 109/L, 25% of myeloid blasts in a peripheral blood smear; aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase levels were normal. After 4 days from gilteritinib treatment, the patient was discharged with a weekly day hospital visit; he did not need platelet and blood transfusion, and no infection and febrile episodes were reported. Considering the well-known cardiovascular and hepatic toxicity of gilteritinib, a comprehensive cardiologic and metabolic evaluation was scheduled every 15 days with a weekly routinary blood test. However, no cardiovascular or hepatic side effects, as well as differentiation syndrome, posterior reversible encephalopathy syndrome or arrhythmias were observed. At the end of the first cycle of gilteritinib, peripheral blood count was normal (hemoglobin 14.60 g/dl; platelet count 216 × 109/L, WBC count 5.84 x 109/L, no myeloid blasts in peripheral blood smear) and the bone marrow examination showed a morphological complete remission. For the first time, a minimal residual disease negativity by flow cytometry was observed in association to a negative FLT3-ITD allelic ratio (AR: 0). Unfortunately, the NPM1 mutation persisted with a value of 1.42%. The patient, in good general condition, continued the therapy with gilteritinib at the standard dose of 120 mg/daily, awaiting allograft from an unrelated donor. After completing the second cycle without any complications, before starting conditioning therapy, a morphological CR and a minimal residual disease negativity were confirmed. However, NPM1 mutation was persistently positive with a value of 2.98% and FLT3-ITD AR became newly positive with a value of 0.2. On September 29th, 2020, the patient underwent a hematopoietic stem cell transplantation (HSCT) from a 10/10 matched (HLA typing of donors and patients was performed for -A, -B, -C, -DRB1 and -DQB1 loci at a high resolution by sequence-based typing and sequence-specific oligonucleotide probe methods), unrelated donor (20 years old woman), after a myeloablative conditioning regimen (thiotepa 10 mg/kg, 3 days of iv busulfan 9.6 mg/kg plus 3 days of fludarabine 150 mg/m2 and 2 days of antithymocyte globulin 5 mg/kg). The graft-versus-host- disease prophylaxis consisted of cyclosporine and methotrexate. Peripheral blood stem cells were infused on day 0; the number of nucleated cells was 10.90 x 108/kg and the number of CD34-positive cells and CD3+ cells infused was 8.57 x 106/kg and 131.88 x 106/kg respectively. The combination of blood group and serological status of CMV was: 0+ donor versus A+ recipient and negative donor versus positive recipient, respectively. The Hematopoietic cell transplantation comorbidity index (HCT-CI) score was low-0; the age adjusted risk level was intermediate-1 and the ECOG performance status was 0. From day −1 the patient has abdominal pain and watery diarrhea; microbiological tests (stool sample) documented the presence of Clostridium Difficile toxins. The patient was treated with oral vancomycin 125 mg four times a day for 10 days, with e complete resolution of the infection. From day + 7; the patient started a prophylaxis for Cytomegalovirus infection with letermovir 240 mg os per day. Neutrophil (>0.5 x 109/L) and platelet (>20,000 x 109/L) engraftment were achieved on days 17 and 14, respectively. One month after the transplant, a bone marrow aspirate confirmed a morphological CR, a minimal residual disease negative by flow cytometry and the negativity of NPM1 and FLT3-ITD AR mutations (Figure 8). No acute graft versus host disease were observed after transplant and the chimerism detected on day 30 on peripheral blood and bone marrow aspirate was of full-donor origin. After the discharge (day + 32), the patient did not show hepatic, renal and cardiac toxicity. Subsequently, blood tests, including cytomegalovirus (CMV), HHV6 and Epstein-Barr virus (EBV)-DNA, were performed weekly. A bone marrow evaluation after 3 months from Allo HSCT showed any evidence of disease recurrence; the chimerism detected on day 90 on peripheral blood and bone marrow was of full donor origin. After 90 days, enlarged and tender lymph nodes on both sides of retro-auricular, submandibular, neck region, axillae and groins were observed; the EBV-DNA load was 28,532 copies/mL. A diagnosis of probable Epstein-Barr virus-related post-transplant lymphoproliferative disorder EBV-PTLD was made, and a rituximab (375 mg/m2 weekly) was immediately started, together a reduction of the cyclosporine dose.
 |
Figure 8 NPM1/FLT3-ITD assays. |
Discussion
Patients with R/R AML has limited treatment options and often require high-dose salvage chemotherapy for controlling the disease. In the last years, several agents became available for this life-threatening disease, including some target therapy focused on specific mutations (such as FLT3 or IDH1 and 2).
Gilteritinib, a potent, rationally designed, second generation inhibitor of both FLT3 and AXL, represents a valid alternative for FLT3 mutated AML, a high-risk population, and has been approved in the European Union as monotherapy for the treatment of adult patients with relapsed or refractory AML with a FLT3 mutation.2,3,12 FDA approved gilteritinib on November 28, 2018 for treatment of relapsed or refractory acute myeloid leukemia with a FLT3 mutation as detected by an FDA-approved test.16
A non-randomized, single-arm, open-label Phase I/II study evaluated seven dose escalations of gilteritinib (from 20 mg/day to 450 mg/day) in 252 patients with R/R AML, most of them with FLT3 mutations. Gilteritinib was generally well-tolerated and the maximum tolerated dose was 300 mg/day. However, the dose of 120 mg/day was chosen for further trials as a balance between potent FLT3 inhibition and safety, also allowing the dose adjustment without compromising efficacy and safety.17
The pivotal Phase III open-label, multicenter, randomized ADMIRAL study (2215-CL-0301) evaluated the efficacy and safety of gilteritinib versus salvage chemotherapy in 371 patients with AML and FLT3 mutation who were refractory (at least to one cycle of induction chemotherapy) or relapsed (achieved a complete remission/complete remission with incomplete hematologic recovery [CRi]/complete remission with incomplete platelet recovery [CRp] after first-line AML therapy. Patients were randomized to gilteritinib (120 mg orally once daily, n = 247) or salvage chemotherapy (low-dose cytarabine; azacitidine; mitoxantrone, etoposide and intermediate-dose cytarabine; fludarabine, cytarabine and granulocyte colony-stimulating factor with idarubicin; n = 124). The primary end points were overall survival (OS) and the rate of complete remission (CR) with full or partial hematologic recovery. Secondary end points were: event-free survival (EFS) and the rate of patients with CR.18
The median OS was significantly longer in patients treated with gilteritinib respect to patients receiving chemotherapy: 9.3 months vs 5.6 months, respectively (hazard ratio [HR] for death, 0.64; 95% confidence interval [CI], 0.49 to 0.83; P<0.001). The superiority of gilteritinib was confirmed by the second primary endpoint too: In fact, CR rate with full or partial hematologic recovery was 34.0% in the gilteritinib group and 15.3% in the chemotherapy group (risk difference, 18.6 percentage points; 95% CI, 9.8 to 27.4). As far as secondary endpoints are concerned, the median EFS was 2.8 months for gilteritinib and 0.7 months for chemotherapy (HR for treatment failure or death, 0.79; 95% CI, 0.58 to 1.09) and the CR rate 21.1% and 10.5%, respectively (risk difference, 10.6 percentage points; 95% CI, 2.8 to 18.4). It is noteworthy that the transplantation rate was significantly higher in patients treated with gilteritinib compared to those treated with chemotherapy: 25.5% vs 15.3% (unstratified p = 0.033).18
Perl et al recently published a 2 years update of the ADMIRAL trial in order to evaluate the long-term outcome of the treatment with gilteritinib. After a median follow-up of 37.1 months, the median OS was 9.3 months for gilteritinib and 5.6 months for the control group (HR=0.665; 95% CI: 0.518, 0.853; two-sided P=0.0013). Besides, the 2-year estimated survival rate was 20.6% for gilteritinib and 14.2% for the control group. It is noteworthy that 26 out of 49 patients treated with gilteritinib still alive after ≥ 2 years has no relapses; 18 of these patients underwent transplantation and 16 restarted gilteritinib as post-HSCT maintenance therapy. These data confirm the higher efficacy of gilteritinib versus the standard therapy in terms of sustained disease remission and survival.19
A retrospective analysis performed on patients with R/R AML enrolled in the 2 previously discussed trials evaluated whether a prior TKI therapy with midostaurin or sorafenib could affect the clinical outcome in terms of response and survival, In the phase I/II trial, 23% of FLT3-mutation-enriched patients treated with 120- or 200-mg gilteritinib received a prior TKI (sorafenib). The results showed that the CR rates were similar in patients pretreated or not with a TKI (42% vs 43%, respectively). The ADMIRAL trial confirmed the efficacy of gilteritinib in pretreated patients (about 12% of the global population): in fact, CR rates were similar in patients pretreated or not with TKIs (48% vs 55%m respectively). These figures were higher than those observed in the control group (21% and 22%, respectively). Besides, in patients pretreated with TKIs the median OS was higher in the gilteritinib group respect to the control group (6.5 vs 4.7 months, respectively). The Author conclude that patients with R/R AML who received prior TKIs (midostaurin or sorafenib) were able to achieve remission with gilteritinib.20
A very recently published paper described a retrospective analysis on 113 patients with R/R FLT3 mutated AML previously treated with FLT3 inhibitors enrolled in 11 US centers. All the patients received gilteritinib alone or as combination therapy. The composite CR rate (CRc, CR + CRi + CRp) with gilteritinib was 48.7%; the CRc rate in patients pretreated with 7+3 and midostaurin with or without consolidation was 58% and the median OS was 7.8 months. Again, these real-life data confirm the effectiveness of gilteritinib also after a previous treatment with midostaurin, which is now widely used as standard intensive induction and consolidation or posttransplant FLT3 inhibitor maintenance.21
The safety data of gilteritinib on 522 patients who received at least one dose of gilteritinib, (integrated R/R AML safety population) showed that the incidence of grade ≥ 3 drug-related treatment-emergent adverse events (TEAEs) was 60.2% and 52.3% in gilteritinib and chemotherapy groups, respectively. The most frequent TEAEs with gilteritinib were the increase of blood creatine phosphokinase, alanine aminotransferase, aspartate aminotransferase and alkaline phosphatase, diarrhea, fatigue and nausea.3 Actually, gilteritinib is the second FLT3 inhibitor approved for the treatment of AML.22
These clinical data were confirmed in the clinical practice by our case reports. In fact, the administration of gilteritinib monotherapy in R/R AML patients allowed to improve the clinical status in all the patients, independently from age and co-morbidities; in some cases, platelet transfusions independence was reached. Furthermore, gilteritinib has an acceptable and manageable toxicity, also in patients previously intolerant to midostaurin.
For out of 7 patients aged > 70 years: due to the good manageability and low toxicity, gilteritinib represents an optimal choice also in the delicate setting of the elderly patient, not suitable for allogeneic stem cells transplantation and with several comorbidities. In one case (73 years old woman), the dose of gilteritinib was increased to 200 mg/day without any safety problem. In these patients, gilteritinib proved to be an effective and well-tolerated drug, which can also be managed in combination with other concomitant therapies, pre-existing or related to the underlying disease. Besides, gilteritinib, due to the oral formulation, can be safely used in the outpatient setting, avoiding hospitalizations, which can be deleterious in the COVID-19 era, reducing risks and costs related to the hospital access and significantly improving patient’s quality of life.
In the COVID-19 context, gilteritinib could be used for patients with newly diagnosed AML infected with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)/COVID-19. Wilson et al reported the case a 27‐year‐old male with a 3‐day history of fever (>39 C), swollen, erythematous elbows and no respiratory symptoms and a de novo FLT3-mutated AML with a complete remission after an induction therapy with gilteritinib during hospitalization for COVID-19. According to the Authors, gilteritinib can be considered as a treatment option FLT3-mutated AML patients and severe COVID-19, where a prolonged period of chemotherapy-induced pancytopenia could adversely affect outcomes.23
Gilteritinib is also effective against extramedullary disease.24 In fact, our 76 years old patient, who developed a rapid and massive progression of submandibular and laterocervical sarcoma with rapidly progressive inspiratory dyspnea and dysphagia, obtained the complete regression of the sarcomatous lesions after 4 months from the start of gilteritinib. Kida et al published the case of a 56-year-old man with FLT3-ITD mutated AML undergone to a myeloablative conditioning regimen, followed by peripheral blood stem cell transplantation. After 180 days, he has an extensive chronic graft associated to several subcutaneous tumors, diagnosed as myeloid sarcoma and successfully treated with gilteritinib.25
A differentiation syndrome with gilteritinib was reported in 3% of patients. Of the 11 patients who experienced differentiation syndrome, 9 recovered after treatment or after dose interruption of gilteritinib.3 An episode of differentiation syndrome occurred in 2 outpatients: this adverse event required the temporary suspension of therapy with rapid worsening of the blood count. Resumption of treatment with Gilteritinib resulted in rapid haematological recovery with persistence of elevated serum levels of ferritin and LDH. High serum ferritin is found in a large spectrum of conditions, both genetic and acquired, associated or not with iron overload; it is well known that both acute and chronic inflammation are associated with hyper ferritinemia. Gilteritinib has been shown that it is able to induce two distinct marrow responses in FLT3 mutated AML: responses with or without differentiation.26 Gilteritinib therapy promoted differentiation of leukemic blasts and this condition caused a systemic inflammatory state with high serum ferritin level and increased LDH.
Finally, gilteritinib is a well-tolerated and effective drug also for patients with FLT3-positive AML eligible for an allogeneic bone marrow transplantation as bridge therapy, maintenance therapy or bridge to a second bone marrow transplant, as suggested by the case report by Kamitani (as well as case report n. 4). A 38-year-old FLT3-ITD positive AML patient underwent a bone marrow transplantation with bone marrow remission obtained on day 32. The relapse, occurred four months after transplantation and resistant to salvage chemotherapy, was treated with gilteritinib. Following the stabilization of the disease, the patient received a cord blood transplantation three months after relapse, with a second remission.27 Gilteritinib can be also useful in already transplanted patients as possible bridge therapy towards experimental rescue strategies.
Conclusion
Based on literature and case reports, we can conclude that gilteritinib in monotherapy is a new effective and well-tolerated treatment with manageable adverse effects for patients with relapsing or refractory FLT3-positive AML. Thanks to its good manageability combined with the low toxicity, this drug is suitable for all the patients, including elderly frail patient with concomitant therapies or pre-existing or underlying diseases. Due to the oral formulation, gilteritinib can be used also in the outpatient setting, reducing risks and costs related to the hospitalization. Besides, gilteritinib can be a valid therapeutic option for bridge to transplantation or other experimental rescue strategies.
Clinical trials evaluating the use of gilteritinib in first-line induction setting with intensive chemotherapy and azacitidine (in particular, preliminary data of the phase III LACEWING trial confirm the safety and feasibility of gilteritinib plus azacitidine in patients with newly diagnosed FLT3‐mutated AML not eligible for induction therapy, suggesting an important new treatment approach for these patients),28 in the maintenance setting and in the R/R setting with atezolizumab and venetoclax alone or plus azacitidine are ongoing.2
Acknowledgments
The authors thank the patients for providing their consent to use medical information.
The following collaborators contributed to the paper: Nicola Cascavilla, S. Giovanni Rotondo; Fabrizia Colasante, S. Giovanni Rotondo; Rosa Greco, Milan; Francesca Guidotti, Como; Michela Lamanda, Rome; Elena Maino, Como; Valentina Mancini, Milan; Emanuela Merla, S. Giovanni Rotondo; Maria Marta Minervini, S. Giovanni Rotondo; Luca Pappalettera, Milan; Anna Proia, Rome; Michelina Santopietro, Rome; Lucia Savino, S. Giovanni Rotondo; Anna Sicuranza, Siena; Martina Simoncelli, Siena; Michelle Zancanella, Como.
Funding
This study was supported by Astellas.
Disclosure
Dr Lorenzo Rizzo reports personal fees from Editree Srl, during the conduct of the study. The authors reports no other conflicts of interest in this work.
References
1. Shallis RM, Wang R, Davidoff A, et al. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 2019;36:70–87. doi:10.1016/j.blre.2019.04.005
2. Chew S, Mackey MC, Jabbour E. Gilteritinib in the treatment of relapsed and refractory acute myeloid leukemia with a FLT3 mutation. Ther Adv Hematol. 2020;11:2040620720930614. doi:10.1177/2040620720930614
3. Tzogani K, Røshol H, Olsen HH, et al. The European medicines agency review of gilteritinib (xospata) for the treatment of adult patients with relapsed or refractory acute myeloid leukemia with an FLT3 mutation. Oncologist. 2020;25(7):e1070–e1076. doi:10.1634/theoncologist.2019-0976
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387
5. Haematology TL. Editorial, “Closing in on targeted therapy for acute myeloid leukaemia”. Lancet Haematology. 2019;6:e1. doi:10.1016/S2352-3026(18)30223-0
6. American Cancer Society. Key Statistics for Acute Myeloid Leukemia (AML). Available from: https://www.cancer.org/cancer/acute-myeloid-leukemia/about/key-statistics.html.
7. Miranda-Filho A, Piñeros M, Ferlay J, et al. Epidemiological patterns of leukaemia in 184 countries: a population-based study. Lancet Haematol. 2018;5:e14–24. doi:10.1016/S2352-3026(17)30232-6
8. AIOM AIRTUM. I numeri del cancro in Italia; 2020. Available from: https://www.aiom.it/wp-content/uploads/2020/10/2020_Numeri_Cancro-operatori_web.pdf.
9. Löwenberg B, Rowe JM. Introduction to the review series on advances in acute myeloid leukemia (AML). Blood. 2016;127(1):1. doi:10.1182/blood-2015-10-662684
10. De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441. doi:10.1038/bcj.2016.50
11. Daver N, Schlenk RF, Russell NH, et al. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019;33(2):299–312. doi:10.1038/s41375-018-0357-9
12. Levis M, Perl AE. Gilteritinib: potent targeting of FLT3 mutations in AML. Blood Adv. 2020;4(6):1178–1191. doi:10.1182/bloodadvances.2019000174
13. Nguyen B, Williams AB, Young DJ, et al. FLT3 activating mutations display differential sensitivity to multiple tyrosine kinase inhibitors. Oncotarget. 2017;8(7):10931–10944. doi:10.18632/oncotarget.14539
14. Zhao J, Song Y, Liu D. Gilteritinib: a novel FLT3 inhibitor for acute myeloid leukemia. Biomark Res. 2019;7(1):19. doi:10.1186/s40364-019-0170-2
15. Heuser M, Ofran Y, Boissel N, et al. Acute myeloid leukaemia in adult patients: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(6):697–712. doi:10.1016/j.annonc.2020.02.018
16. Pulte ED, Norsworthy KJ, Wang Y, et al. FDA approval summary: gilteritinib for relapsed or refractory acute myeloid leukemia with a FLT3 mutation. Clin Cancer Res. 2021;27(13):3515–3521. doi:10.1158/1078-0432.CCR-20-4271
17. Perl AE, Altman JK, Cortes J, et al. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1–2 study. Lancet Oncol. 2017;18(8):1061–1075. doi:10.1016/S1470-2045(17)30416-3
18. Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381(18):1728–1740. doi:10.1056/NEJMoa1902688
19. Perl AE, Larson RA, Podoltsev NA, et al. Follow-up of patients with R/R FLT3-mutation-positive AML treated with gilteritinib in the Phase 3 ADMIRAL trial. Blood. 2022;26:2021011583. doi:10.1182/blood.2021011583
20. Perl AE, Altman JK, Hosono N, et al. Clinical outcomes in patients with relapsed/refractory acute myeloid leukemia treated with gilteritinib who received prior midostaurin or sorafenib. Blood. 2020;136(Supplement 1):22–23. doi:10.1182/blood-2020-136395
21. Numan Y, Abdel Rahman Z, Grenet J, et al. Gilteritinib clinical activity in relapsed/refractory FLT3 mutated acute myeloid leukemia previously treated with FLT3 inhibitors. Am J Hematol. 2022;97(3):322–328. doi:10.1002/ajh.26447
22. Marjoncu D, Andrick B. Gilteritinib: a novel FLT3 inhibitor for relapsed/refractory acute myeloid leukemia. Adv Pract Oncol. 2020;11(1):104–108.
23. Wilson AJ, O’nions J, Subhan M. Successful remission induction therapy with gilteritinib in a patient with de novo FLT3 -mutated acute myeloid leukaemia and severe COVID-19. Br J Haematol. 2020;190(4):e189–e191. doi:10.1111/bjh.16962
24. Kumode T, Rai S, Tanaka H, et al. Targeted therapy for medullary and extramedullary relapse of FLT3-ITD acute myeloid leukemia following allogeneic hematopoietic stem cell transplantation. Leuk Res Rep. 2020;14:100219. doi:10.1016/j.lrr.2020.100219
25. Kida M, Kuroda Y, Kido M, et al. Successful treatment with gilteritinib for isolated extramedullary relapse of acute myeloid leukemia with FLT3-ITD mutation after allogeneic stem cell transplantation. Int J Hematol. 2020;112(2):243–248. PMID: 32170661. doi:10.1007/s12185-020-02855-4
26. McMahon CM, Canaani J, Rea B, et al. Gilteritinib induces differentiation in relapsed and refractory FLT3-mutated acute myeloid leukemia. Blood Adv. 2019;3(10):1581–1585. doi:10.1182/bloodadvances.2018029496
27. Kamitani I, Saito T, Yokoyama H, et al. Successful bridge therapy of gilteritinib to cord blood transplantation in relapsed acute myeloid leukemia after bone marrow transplantation. J Infect Chemother. 2021;27(4):639–641. doi:10.1016/j.jiac.2020.11.003
28. Solana-Altabella A, Ballesta-López O, Megías-Vericat JE, Martínez-Cuadrón D, Montesinos P. gilteritinib plus azacitidine combination shows promise in newly diagnosed FLT3-Mutated AML. Oncologist. 2021;26(Suppl1):S10. doi:10.1002/onco.13652
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
