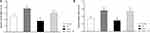Back to Journals » Journal of Experimental Pharmacology » Volume 12
Therapeutic Effects of Spirulina platensis Against Adolescent Stress-Induced Oxidative Stress, Brain-Derived Neurotrophic Factor Alterations and Morphological Remodeling in the Amygdala of Adult Female Rats
Authors Moradi-Kor N , Ghanbari A , Rashidipour H, Bandegi AR, Yousefi B, Barati M , Kokhaei P, Rashidy-Pour A
Received 3 December 2019
Accepted for publication 26 February 2020
Published 19 March 2020 Volume 2020:12 Pages 75—85
DOI https://doi.org/10.2147/JEP.S237378
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bal Lokeshwar
Nasroallah Moradi-Kor,1,2 Ali Ghanbari,2 Hadi Rashidipour,3 Ahmad Reza Bandegi,4 Behpour Yousefi,5 Mehdi Barati,6 Parviz Kokhaei,7 Ali Rashidy-Pour2
1Student Research Committee, Semnan University of Medical Sciences, Semnan, Iran; 2Research Center of Physiology, Semnan University of Medical Sciences, Semnan, Iran; 3School of Veterinary Medicine, Islamic Azad University, Garmsar, Iran; 4Laboratory of Endocrine Research, Research Center of Physiology, School of Medicine, Semnan University of Medical Sciences, Semnan, Iran; 5Department of Anatomical Sciences, School of Medicine, Semnan University of Medical Sciences, Semnan, Iran; 6Department of Immunology, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran; 7Cancer Research Center, Semnan University of Medical Sciences, Semnan, Iran
Correspondence: Ali Rashidy-Pour
Research Center of Physiology, University of Medical Sciences, Semnan 15131-38111, Iran
Tel +98-9121140221
Email [email protected]
Objective: The amygdala structural and functional abnormalities have been implicated in numerous neuropsychiatric and neurodevelopmental disorders. Given the important role of the amygdala in stress responses and the susceptibility of the females to adolescent stress, the present study investigated the beneficial effects of Spirulina platensis microalgae (SP) as a neuroprotective supplement against adolescent stress-induced oxidative stress, brain-derived neurotrophic factor (BDNF) alterations, molecular and morphological remodeling in the basolateral amygdala (BLA) of adult female rats.
Methods: During the adolescent period (PNDs 30– 40) rats were subjected to restraint stress (2 h/day for 10 days). Then, the animals were subjected to 15 days treatment (PNDs 41– 55) with SP (200 mg/kg/day) followed by biochemical (BDNF and stress oxidative markers), molecular (BDNF and its receptor tropomyosin receptor kinase B [TrkB] mRNA expression), and morphological (dendritic length and spines) assessments in the BLA.
Results: The study revealed that adolescent stress decreased BDNF levels and reduced apical dendritic length and branch points of pyramidal neurons in the BLA. In addition, chronic stress significantly increased oxidative stress parameters and decreased BDNF and TrkB mRNA expression in the BLA. Treatment with SP alleviated both biochemical, molecular, and neuroanatomical deficits that induced by adolescent stress.
Conclusion: Our findings provide important evidence that SP as a non-pharmacological intervention during adolescent period can protect against chronic stress-induced neuroanatomical biochemical, and molecular deficits in adulthood, and thus, reduce stress-related disorders.
Keywords: adolescent stress, amygdala, BDNF, dendritic remodeling, Spirulina, oxidative markers
Introduction
Studies have demonstrated that stressful experiences, which occur during critical periods of brain development (pre-pubertal period) persistently change the responses to stressors as well as associate with increasing the vulnerability to stress-related disorders later in adulthood.1,2 Pre-pubertal period is a time of continued brain maturation, particularly in limbic and cortical regions that characterized by changes in brain structure and function.3,4 During this period substantial remodeling occurs in brain areas such as the prefrontal cortex (PFC), hippocampus, and amygdala which are involved in emotional and learning processes.5 It has been suggested that the HPA axis in adolescent rat is fully developed, while other key brain areas (limbic and cortical regions) are still undergoing significant maturation processes.6,7
Rodent models have indicated more information about amygdala function and its role in emotional stress regulation. The amygdala facilitates the encoding of emotional memories by coordination with other brain areas particularly the prefrontal cortex and hippocampus.8 The amygdala through interacting with other brain structures obviously regulates stress response and plays a key role in emotional and stress-related behavior.9 Exposure to acute or chronic stress can induce morphological and functional changes in the amygdala and these changes play an important role in stress-induced neuropsychiatric disorders.10,11 Animal studies have revealed that stress influences synaptic plasticity, morphological remodeling, neurotoxicity, and neurogenesis in the brain and all these stress-derived physiological effects can potentially influence cognitive functions.12,13
Adolescent stress increases anxiety behavior,14 remodels corticolimbic structure,15 and alters neural gene expression in adulthood.16,17 It has been reported that depression-related behaviors are associated with reducing the expression of BDNF and its receptor TrkB.18 BDNF is the primary neurotrophin family that has emerged as a major synaptogenesis regulator directly involved in synaptic plasticity underlying cause the learning and memory in the brain.19 In a previous study, we showed that exposure of female rats to restraint stress during adolescence decreased hippocampal BDNF and reduced apical dendritic length and branch points of CA3 pyramidal neurons in adulthood.20 Several lines of studies suggest that adolescent stress alters the morphology of pyramidal neurons in the prelimbic region of the mPFC.1,21,22 It has been reported that chronic immobilization stress induced dendritic atrophy and debranching in CA3 pyramidal neurons of the hippocampus but these events were opposite in the amygdala.15 Several lines of evidence indicated that females typically are more susceptible to stress effects than the male; it seems that the risk of depressive disorders in females is higher than the males.23–25
In the present research, we are looking to find effective and non-pharmacological therapies for alleviating the detrimental effects of adolescent stress. Plants and natural products have been used as therapeutic agents worldwide. On the search for non-pharmacological compounds, Spirulina platensis (SP) is considered as a novel and natural agent with potent antioxidant properties. SP is a marine microalgae which belongs to the class of cyanobacteria widely used as traditional intervention, known to contain various antioxidants, especially phycocyanin and basic necessary nutrients has been used as a natural food supplement.26,27 SP exerts various effects including anti–inflammatory, antioxidant, antimicrobial, antitumoral, and immunomodulatory properties.28–31 Studies suggest that Spirulina has protective effects in the treatment of neurodegenerative disorders.32,33 It has been reported that Spirulina reduces oxidative stress in the hippocampus and protects against damaging neurobehavioral effects of systemic kainic acid.34 SP enhanced the beneficial effect of exercise on oxidative stress and lipid profiles in rats.35 The antioxidant property of Spirulina is ascribed to phycocyanin component, which is a potent antioxidant. It has been shown that administration of SP alleviates neuropathic pain induced by chronic constriction injury (CCI) through increasing FRAP levels in male rats.36 Previous findings from our group indicate that SP has protective effects against adolescent stress-induced deficits in learning and memory, hippocampal BDNF and morphological remolding in adult female rats.20 In another study, we showed that SP prevents scopolamine-induced memory impairment in young female rats.37
To date, the effects of SP on biochemical, molecular, and morphological changes in basolateral (BLA) amygdala in the adult brain have not been investigated. Therefore, the present study investigated the effects of SP against adolescent stress-induced oxidative stress, BDNF alterations, molecular and morphological remodeling in the BLA of female rats in adulthood.
Materials and Methods
Animals
Wistar female adolescent rats (30 days old, 60–70 g) obtained from the breeding colony of the Semnan University of Medical Sciences (SUMS), Semnan, Iran. Animals were maintained at 12-h light/dark cycle and in a controlled room temperature (22 ± 2°C) and, housed 5 to 6 per a cage (27×21×14 cm) given free access to food and water ad libitum. All experiments were approved by the Ethics Committee of Semnan University of Medical Sciences (IR.SEMUMS.REC.1394.208). The experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental Groups
Rats randomly were distributed into 4 following groups (10 animals in each group). Control group: rats received only oral physiological saline using gavage tube; SP group: rats received 200 mg/kg SP per day for 15 days using gavage tube (PNDs 41–55); Stress group: rats exposed to 10 days restraint stress (PNDs 30–40) and Stress + SP group: rats exposed to stress and then received SP for 15 days (PNDs 41–55). Biochemical assessments began at the PND 61 after the termination of all intervention procedure. In each group, half of the animals were used for histology assessment and half of them were used for measurement of oxidative stress markers (left BLA) and BDNF levels, and BDNF and TrkB mRNA expression (right BLA) (Figure 1, Timeline of experiments).
Spirulina platensis
Spirulina platensis (SP) was purchased as pure powder from Setendorf Company, Jask, Bandar Abbas, Iran (Product No: SPM-12462016). Spirulina administrated at a dose of 200 mg/kg per day by oral gavage for 15 days (PNDs 41–55). The volume of gavage solution was 1 mL per rat. The control and stress rats received distilled water alone and solutions were refreshed daily. The dose of SP was chosen based on dose-response effects of SP in our previous study.20
Stress Induction
Rats were exposed to restraint stress (2 h/day for 10 days, PNDs 30–40) between 10:00 Am and 12:00 Am.15,20 During the restraint, each rat was restrained in a clear polyethylene cylinder. The cylinder size was adjusted according to the size of the rats. A hole in the front of the cylinder was set to allow the animals to breathe freely.
BDNF Measurements
The rats were decapitated, and the BLA was dissected and then immediately frozen at −70°C until used for preparation of homogenates. The tissues were homogenate in cold lysis buffer and the supernatants, which were obtained after centrifugation at 12,000g for 20 min at −4°C, were used for BDNF assay. The BDNF protein levels were assessed using Rat BDNF ELISA kits (Hangzhou Eastbiopharm Co., LTP) according to the manufacturer’s recommendations.
Molecular Study (RT-PCR)
The mRNA expression of BDNF and its receptor TrkB was determined by using RT-PCR method. The animals were decapitated after anesthesia and their brains were removed and the BLA rapidly dissected out on ice and flash frozen at –80°C. RNA was reverse transcribed (RT) into cDNA by using a commercial cDNA synthesis kit (Bioneer Inc., Seoul, South Korea) and stored at –20°C until further use. The primers were synthesized by Shenggong Biotech Limited Company (Shanghai, China). The sequences of the primers are presented in Table 1. Accuracy of the primers was confirmed by direct sequencing of target sequences. PCR was performed using a thermal cycler (ABI 7900HT, 96 wells, SYBR Green) consisting of denaturation at 95°C for 2 min, 45 additional cycles at 94°C for 30 s, and then 45 s of primer annealing at 58°C (for BDNF), 56.5°C (for TrkB), and 53°C (for GAPDH), followed by a final extension at 72°C for 8 min. All amplification was performed on Applied Biosystems RT-PCR (ABI 7900HT Fast Real-Time PCR System, US). Gene expression was normalized to the levels of GAPDH as a reference gene in all reactions and gene expression was calculated by using the 2−ΔΔCt method. All primers were designed using AlleleID® version 7.5. Primer Select program (PREMIER Biosoft, USA) and synthesized by Bioneer Inc. (Seoul, South Korea). Accuracy of the primers was confirmed by direct sequencing of target sequences. All tests were conducted in duplicate for each sample.
 |
Table 1 Primer Sequences Used in Quantitative Reverse Transcriptase Polymerase Chain Reaction |
Histology and Dendritic Analysis
After completion of stress protocol and SP intervention, the animals were decapitated after anesthesia and their brains were removed quickly. The blocks of tissue containing the amygdala were dissected and processed for rapid Golgi staining technique as described previously.20 Analysis of apical dendritic length and branch points of pyramidal neurons in the BLA was performed using an image J (1.48 version) software. For each animal, an average apical dendritic length and branch points within a 120-µm thick section of each dendritic tree of 6–8 selected pyramidal neurons was calculated. From each experimental group, five animals randomly were selected for morphology analysis.
Measurements of Oxidative Stress Markers
FRAP and MDA Activity Assay
Animals were decapitated under anesthesia and their brains were quickly removed, cleaned with ice-cold saline and stored at –80°C. For ferric reducing antioxidant power (FRAP) and malondialdehyde (MDA) assays, a fraction of tissue was homogenized (1:10 w/v) in cold 1.15% KCl. Homogenates were prepared in a ratio of 100 mg tissue to 1 mL phosphate buffer (50 mmol/l; pH 7.5) containing 1 mM EDTA. The supernatants obtained after centrifugation at 20,000×g for 15 min at 4°C were used for biochemical analysis. The level of total protein in supernatants was determined by the Bradford method using bovine serum albumin as a standard.38
Enzymes Activity Assay
SOD and GPx activities were measured in the supernatant by using ZellBio kits (Zellbio GmbH, Ulm, Germany). After adding reagents, samples, and standards into the wells, absorbance was measured at 0 and 2 mins with an ELISA microplate reader (AD Touch Reader, apDia, Belgium) at 412 nm. The concentration of SOD and GPx was expressed as IU/mg protein. The sensitivity of the assay for SOD and GPx was 1U/mL and 5U/mL, respectively.
Statistical Analysis
Results are expressed as mean ± S.E.M. The data were analyzed two-way ANOVA using SPSS software which followed by Tukey post-hoc test for multiple comparisons. The accepted level of significance for all tests was p < 0.05.
Results
Amygdala BDNF Levels
As shown in Figure 2, a two-way ANOVA analysis revealed significant effects of stress (F1, 16 = 14.260, P = 0.002), significant effect of treatment (F1, 16 = 12.584, p = 0.003) and no significant interaction between two factors (F1, 16 = 0.260, p = 0.617). Between-group comparisons indicated that the BDNF levels in the SP treated groups were significantly higher than the stress group (P = 0.001). BDNF levels in the stress group were also significantly lower than those of the control group (P = 0.036).
BDNF and TrkB mRNA Expression in BLA
BDNF mRNA Expression
A two-way ANOVA on amygdala BDNF mRNA expression (Figure 3A) revealed no significant effect of stress (F1, 11 = 0.651, p = 0.437), a significant effect of treatment (F1, 11 = 21.597, p = 0.001), and no significant interaction between two factors (F1, 11 = 0.116, p = 0.740). Paired comparisons showed that amygdala BDNF mRNA expression in the stress group was significantly lower than control group (p = 0.01). Also, BDNF mRNA expression in the stressed treated group with SP were significantly higher than the stress group (p = 0.001).
TrkB mRNA Expression
Two-way ANOVA on amygdala TrkB mRNA expression (Figure 3B) revealed significant effect of stress (F1, 11 = 6.001, p = 0.032), significant effect of treatment (F1, 11 = 16.156, p = 0.002) and no significant interaction between two factors (F1, 11 = 0.003, p = 0.955). Paired comparisons showed that TrkB mRNA expression in the stress group was significantly lower than the SP group (p=0.01). Also, TrkB mRNA expression in the stressed treated group with SP was significantly higher than the stress group (p=0.001).
Morphology of BLA Neurons
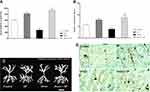
Apical Dendritic Length
A two-way ANOVA on apical dendritic length (Figure 4A) revealed a significant effect of stress (F1, 16 = 23.455, p = 0.0001), a significant effect of treatment (F1, 16 = 372.015, p = 0.0001) and a significant interaction between two factors (F1, 16 = 92.875, p = 0.0001). Paired comparisons showed that dendritic length in the stress group was significantly lower than control group (p = 0.0001). Also dendritic length in the SP treated groups was significantly higher than the stress group (p = 0.0001).
Number of Dendritic Spines
A two-way ANOVA on number of dendritic spines (Figure 4B) revealed a significant effect of stress (F1, 16 = 0.542, p = 0.472), a significant effect of treatment (F1, 16 = 372.015, p = 0.0001) and a significant interaction between two factors (F1, 16 = 6.644, p = 0.020). Paired comparisons showed that number of dendritic spines in the stress group was significantly lower than SP group (p = 0.0001). Also dendritic spines in the SP treated groups were significantly higher than the stress group (p = 0.0001). A photograph of computer-assisted and Golgi stained BLA neurons are presented in Figure 4C and D, respectively. These neurons were selected because they are representative of dendritic lengths near their respective group means.
Activity of Antioxidant Enzymes
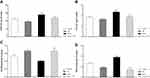
SOD Activity
The data for the SOD level are illustrated in Figure 5A. A two-way ANOVA on SOD level revealed significant effects of stress (F1, 16 = 86.443, p = 0.0001), of treatment (F1, 16 = 64.149, p = 0.0001), but no significant interaction between two factors (F1, 16 = 0.520, p = 0.481). Between-group comparisons indicated that SOD levels of the stress group were significantly higher than those the control, SP, and stress + SP groups (p = 0.0001).
GPX Activity
The data for the GPX level are illustrated in Figure 5B. Two-way ANOVA on GPX level revealed significant effects of stress (F1, 16 = 23.890, p = 0.0001) of treatment (F1, 16 = 22.289, p = 0.0001) and a significant interaction between two factors (F1, 16 = 4.573, p = 0.048). Post-hoc comparison revealed that the GPX levels of the stress group were significantly higher than the control, SP, and stress + SP groups (P = 0.001, p = 0.0001).
Oxidative Stress Markers
FRAP Levels
The data for the FRAP level are illustrated in Figure 5C. Two-way ANOVA on FRAP level revealed significant effects of stress (F1, 16 = 5.975, p = 0.026), of treatment (F1, 16 = 50.529, p = 0.0001) and a significant interaction between two factors (F1, 16 = 5.060, p = 0.039). Post-hoc comparison showed that the FRAP levels of the stress group were significantly lower than the control, SP, and stress + SP groups (P = 0.01, p = 0.0001).
MDA Levels
The data for the MDA level are illustrated in Figure 5D. Two-way ANOVA on MDA level revealed significant effects of stress (F1, 16 = 17.610, p = 0.001), of treatment (F1, 16 = 363.342, p = 0.0001) and a significant interaction between two factors (F1, 16 = 87.308, p = 0.0001). Post-hoc comparison showed that the MDA levels of the stress group were significantly higher than the control, SP, and stress + SP groups (p = 0.0001).
Discussion
The present study for the first time investigated the effects of Spirulina platensis against adolescent stress induced biochemical, molecular, and neuroanatomical deficits in adulthood. Findings showed that adolescent stress decreased BDNF levels and reduced apical dendritic length and branch points of BLA neurons in adult female rats. In addition, adolescent stress induced oxidative stress and reduces the BDNF and TrkB mRNA expression in the amygdala. Treatment with Spirulina platensis alleviated these deficits and even exerted the positive effects on BDNF and TrkB mRNA expression and apical dendritic lengths in the amygdala of non-stressed animals.
In this study we found that chronic stress reduces BDNF levels and its mRNA expression in the amygdala, suggesting that downregulation of BDNF and its receptor in amygdala could be a risk factor for anxiety of depressive-like behaviors. The amygdala is one of the limbic structures involved in emotion and changes its structure and function following chronic stress may contribute to mood disorders like depression and anxiety. Recent studies indicated that BDNF may create an important link between stress and mental disorders.19,39 It has been established that stress is a risk factor of mental diseases and has a potent effect on signaling via BDNF.40 Stress is a major risk factor in depression, and modulates BDNF expression. Studies have shown that acute or chronic stress disrupted BDNF signaling in the brain, mainly via decreasing its expression or release.41–43 BDNF is one of the major neurotrophic factors in the brain that promotes neuronal viability during development and in adulthood. The effects of BDNF are mediated by the activation of intracellular signaling pathways upon high affinity binding to the TrkB receptor, a main member of tyrosine kinase receptors.44 Our findings indicated that adolescent stress decreased BDNF and TrkB mRNA expression in the BLA reflecting the potential involvement of BDNF and its receptors in stress-induced depressive disorders. Treatment with Spirulina platensis normalized decreased mRNA expression of BDNF and its content in the BLA. In a recent study, we indicated that the combined treatment of Spirulina platensis with either environmental enrichment or physical exercise significantly increased hippocampal BDNF levels in female rats during adulthood.20 Based on these findings, we assumed that BDNF and its receptor may be a target for adverse effects of stress on the amygdala, resulting that the impairment of amygdala induced by adolescent stress may be involved in the development of depression-like behaviors in the adulthood.
A growing body of research indicated that stress has profound effects on the morphology of corticolimbic structures including PFC, hippocampus, and amygdala.45–47 Studies revealed that chronic stress alters hippocampal structure including modification of dendritic length and remodeling of dendritic spines in the CA3 region.46,48 Ample pieces of evidence have demonstrated that chronic stress has powerful effects on the branching patterns of apical dendrites in mPFC and amygdala pyramidal cells, causing dendritic retraction in the PL and IL regions of the mPFC21,49 but hypertrophy in the amygdala.15,45 These morphological modifications involve increased corticosterone levels as a recent study showed that chronic treatment with highly physiological levels of cortisone for 10 days induced dendritic hypertrophy in the BLA.50 These findings are opposite with our morphologic results showing that chronic restraint stress reduced apical dendrites length and dendritic spines in the BLA. This apparent discrepancy between these studies may be due to different factors such as duration of stress, sex, and age of animals (for example, female and juvenile rats in our study and male and adult rats in the study of Vyas et al 200215), time of histological assessment, and experimental intervention following the stress. In our study, animals received stress between PNDs 30-40 and then morphological assessment was done between PNDs 55-60. While in the study of Vyas 2002,15 morphological changes of BLA neurons were studied after completion of stress protocols. Clearly, more work needs to be done to elucidate these discrepancies.
The present study showed that treatment with Spirulina platensis alleviated adolescent stress induced dendritic retraction in the BLA pyramidal neurons. To date, there is little information about the effects of Spirulina platensis against stress-induced dendritic remodeling in the amygdala. The positive effects of Spirulina platensis on dendritic remodeling might be mediated through increasing BDNF levels. As we previously showed that Spirulina platensis combined with physical activity and enriched environment could reverse the cognitive-behavioral deficits induced by adolescent stress and increases the hippocampal BDNF in female rats during adulthood.20 It was previously reported that Spirulina platensis through enhancing total antioxidant capacity improves scopolamine induced-memory deficit.36 Recent studies have shown Spirulina platensis reduces oxidative stress in the hippocampus and protects CA3 neurons against damaging of systemic kainic acid.34 Spirulina platensis has different roles, such as anti–inflammatory, anti-oxidative, neuroprotective, and hepatoprotective properties.20,31,32,35 Because of its neuroprotective and antioxidant properties, the findings of the present study suggest that the application of Spirulina platensis as a natural food supplement is useful to prevention the cognitive-behavioral impairment induced by stress.
Our findings indicated that adolescent stress induces oxidative stress in the BLA. Adolescent stress significantly decreased total antioxidant power, increased MDA production and enhanced the activity of antioxidant enzymes in the BLA. Stress through impairment of antioxidant defenses leads to oxidative damage in the central nervous system. Oxidative stress caused by the imbalance between the generation and detoxification of reactive oxygen and nitrogen species.51 A growing body of evidence has demonstrated that the corticolimbic structures such as the PFC, hippocampus, and amygdala are highly vulnerable to oxidative stress.52,53 It has been reported that chronic restraint stress increased plasma levels of corticosterone, induced oxidative stress in the hippocampus and impaired spatial learning and memory.54 Spirulina platensis significantly modulates the levels of oxidative markers in the amygdala. We found that chronic stress increased lipid oxidation in the BLA, and this effect was blocked by Spirulina platensis treatment. We also found a significant reduction in total antioxidant power in the stressed animals, which was prevented by Spirulina platensis intervention. In line with earlier studies, we demonstrated a significant increase in the activities of antioxidant defense enzymes (SOD and GPx) in the BLA following chronic stress. The results of the present study can be interpreted in terms of two mechanisms: an elevation of glucocorticoid levels following chronic stress and a compensatory response to chronic stress. Therefore, the increased SOD and GPx activities observed in the present study are possibly adaptations to chronic stress. We have shown that Spirulina platensis decreases the activities of these enzymes in the BLA. This could be due to the fact that Spirulina platensis leads to reductions of reactive oxygen species, which in turn decreases the need for antioxidant enzymes. Another possibility is that Spirulina platensis directly modulates synthesis of the SOD and GPx, which needs further work to test this assumption. Spirulina platensis contains many carotenoids with powerful antioxidant effects and may protect amygdala neurons from oxidative damage.
Spirulina platensis has protective effects against oxidative stress and this effect is related to C-phycocyanin, an active ingredient of Spirulina, which has significant antioxidant properties.55,56 There have been many reports on the antioxidant properties of Spirulina platensis in different animal models.31,36,57 It has been reported that Spirulina has protective effect against apoptotic cell death induced by free radicals DPPH and ABTS.57 A study has shown Spirulina platensis through its antioxidant properties suppressed vascular ischemic injury in rats.58 Given the wide variety of effects, it is suggested potential application of incorporating Spirulina into food products during pre-pubertal is useful to prevention stress-induced deficits in adulthood.
Our study has a few limitations. One limitation is the lack of sex hormone measurements during estrus cycle. It has been proved hormonal changes that occur with the estrus cycle may be correlated with alterations in both neuronal and behavioral indices in females.59 However, due to the use of animals of the same age kept in similar conditions, the estrous cycle can be largely synchronized. Another limitation is the lack of an intact group (receiving no stress and gavage) as control. Although we used “no stress + gavage group” as a control for “stress + gavage group”, but the former group might display some of the stress effects and thus, the SP effects might be reduced.
Conclusion
In summary, our study is the first to demonstrate the beneficial effects of Spirulina platensis against adolescent stress-induced oxidative stress, BDNF alterations, molecular and morphological remodeling in the amygdala of female rats. Our findings revealed that chronic stress decreased BDNF levels and reduced apical dendritic length and branch points of pyramidal neurons in BLA. In addition to biochemical and morphological changes, we observed that chronic stress induced oxidative stress and altered BDNF and TrkB mRNA expression in the amygdala. Based on our current and previous findings, it can be concluded that adolescent stress through reducing important neuronal growth factors and remodeling the limbic structures causes neuropsychiatric disorders in adulthood. So given the criticality of the adolescence period and sensitivity of the females to stress, our findings provide important evidence that neuroprotective herbal supplement such as Spirulina platensis during pre-pubertal period can be used as a non-pharmacological therapy to alleviate the harmful effects of stress in adulthood.
Acknowledgments
The authors gratefully acknowledge the deputy of research and technology of Semnan University of Medical Sciences and the staff of the Research Center of Physiology for their support throughout the study. In addition, Mr. Nasroallah Moradi-Kor carried out this work in partial project fulfillment of the requirements to obtain the Ph.D. degree in Neuroscience.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from Semnan University of Medical Sciences and Iran National Science Foundation (#95836886).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37(1):39–47. doi:10.1016/j.psyneuen.2011.04.015
2. Brydges NM, Jin R, Seckl J, Holmes MC, Drake AJ, Hall J. Juvenile stress enhances anxiety and alters corticosteroid receptor expression in adulthood. Brain Behav. 2014;4(1):4–13. doi:10.1002/brb3.2014.4.issue-1
3. Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2013;249:162–171. doi:10.1016/j.neuroscience.2012.10.048
4. Barha CK, Brummelte S, Lieblich SE, Galea LA. Chronic restraint stress in adolescence differentially influences hypothalamic-pituitary-adrenal axis function and adult hippocampal neurogenesis in male and female rats. Hippocampus. 2011;21(11):1216–1227. doi:10.1002/hipo.20829
5. Kanwal J, Jung Y, Zhang M. Brain plasticity during adolescence: effects of stress, sleep, sex and sounds on decision making. Anat Physiol. 2016;6:e135. doi:10.4172/2161-0940.1000e135
6. Brydges NM, Wood ER, Holmes MC, Hall J. Prepubertal stress and hippocampal function: sex‐specific effects. Hippocampus. 2014;24(6):684–692. doi:10.1002/hipo.v24.6
7. Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi:10.1016/S0149-7634(00)00014-2
8. Zhang X, Yin G, Cui R, Zhao G, Yang W. Stress-induced functional alterations in Amygdala: implications for neuropsychiatric diseases. Front Neurosci. 2018;12:367. doi:10.3389/fnins.2018.00367
9. Sharp BM. Basolateral amygdala and stress-induced hyperexcitability affect motivated behaviors and addiction. Transl Psychiatry. 2017;7:e1194. doi:10.1038/tp.2017.161
10. Jie F, Yin G, Yang W, et al. Stress in regulation of GABA amygdala system and relevance to neuropsychiatric diseases. Front Neurosci. 2018;12(562). doi:10.3389/fnins.2018.00562
11. Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi:10.1016/j.neuron.2005.09.025
12. Horovitz O, Tsoory M, Hall J, Jacobson-Pick S, Richter-Levin G. Post-weaning to pre-pubertal (‘juvenile’) stress: a model of induced predisposition to stress-related disorders. Neuroendocrinology. 2012;95(1):56–64. doi:10.1159/000331393
13. Perez-cruz C, Müller-keuker JI, Heilbronner U, Fuchs E, Flügge G. Morphology of pyramidal neurons in the rat prefrontal cortex: lateralized dendritic remodeling by chronic stress. Neural Plast. 2007;2007:1–14. doi:10.1155/2007/46276
14. Avital A, Richter-Levin G. Exposure to juvenile stress exacerbates the behavioural consequences of exposure to stress in the adult rat. Int J Neuropsychopharmacol. 2005;8(2):163–173. doi:10.1017/S1461145704004808
15. Vyas A, Mitra R, Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22(15):6810–6818. doi:10.1523/JNEUROSCI.22-15-06810.2002
16. Jacobson-pick S, Elkobi A, Vander S, Rosenblum K, Richter-levin G. Juvenile stress-induced alteration of maturation of the GABAA receptor α subunit in the rat. Int J Neuropsychopharmacol. 2008;11(7):891–903. doi:10.1017/S1461145708008559
17. Tsoory M, Guterman A, Richter‐levin G. “Juvenile stress” alters maturation‐related changes in expression of the neural cell adhesion molecule L1 in the limbic system: relevance for stress-related psychopathologies. J Neurosci Res. 2010;88(2):369–380. doi:10.1002/jnr.22203
18. Badowska-szalewska E, Spodnik E, Klejbor I, Morys J. Effects of chronic forced swim stress on hippocampal brain-derived neurotrophic factor (BDNF) and its receptor (TrkB) immunoreactive cells in juvenile and aged rats. Acta Neurobiol Exp. 2010;70:370–381.
19. Maghsoudi N, Ghasemi R, Ghaempanah Z, Ardekani AM, Nooshinfar E, Tahzibi A. Effect of chronic restraint stress on HPA axis activity and expression of BDNF and Trkb in the hippocampus of pregnant rats: possible contribution in depression during pregnancy and postpartum period. Basic Clin Neurosci. 2014;5(2):131.
20. Moradi-Kor N, Ghanbari A, Rashidipour H, Yousefi B, Bandegi AR, Rashidy-Pour A. Beneficial effects of Spirulina platensis, voluntary exercise and environmental enrichment against adolescent stress induced deficits in cognitive functions, hippocampal BDNF and morphological remolding in adult female rats. Horm Behav. 2019;112:20–31. doi:10.1016/j.yhbeh.2019.03.004
21. Ghalandari-shamami M, Nourizade S, Yousefi B, Vafaei AA, Pakdel R, Rashidy-Pour A. Beneficial effects of physical activity and crocin against adolescent stress induced anxiety or depressive-like symptoms and dendritic morphology remodeling in prefrontal cortex in adult male rats. Neurochem Res. 2019;44(4):917–929. doi:10.1007/s11064-019-02727-2
22. Moench KM, Wellman CL. Differential dendritic remodeling in prelimbic cortex of male and female rats during recovery from chronic stress. Neuroscience. 2017;357:145–159. doi:10.1016/j.neuroscience.2017.05.049
23. Wang J, Korczykowski M, Rao H, et al. Gender difference in neural response to psychological stress. Soc Cogn Affect Neurosci. 2007;2(3):227–239. doi:10.1093/scan/nsm018
24. Sotiropoulos I, Silva J, Kimura T, et al. Female hippocampus vulnerability to environmental stress, a precipitating factor in Tau aggregation pathology. J Alzheimer’s Dis. 2015;43(3):763–774. doi:10.3233/JAD-140693
25. Gross CM, Flubacher A, Tinnes S, et al. Early life stress stimulates hippocampal reelin gene expression in a sex‐specific manner: evidence for corticosterone‐mediated action. Hippocampus. 2012;22(3):409–420. doi:10.1002/hipo.v22.3
26. Mazo V, Gmoshinskiĭ I, Zilova I. Microalgae Spirulina in human nutrition. Vopr Pitan. 2004;73(1):45–53.
27. Borowitzka M. Micro-algae as sources of fine chemicals. Microbiol Sci. 1986;3(12):372–375.
28. Dartsch PC. Antioxidant potential of selected Spirulina platensis preparations. Phytother Res. 2008;22(5):627–633. doi:10.1002/(ISSN)1099-1573
29. Estrada JP, Bescós PB, Del Fresno AV. Antioxidant activity of different fractions of Spirulina platensis protean extract. Il farmaco. 2001;56(5–7):497–500. doi:10.1016/S0014-827X(01)01084-9
30. Deng R, Chow TJ. Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae Spirulina. Cardiovasc Ther. 2010;28(4):e33–e45. doi:10.1111/j.1755-5922.2010.00200.x
31. Nasirian F, Dadkhah M, Moradi-kor N, Obeidavi Z. Effects of Spirulina platensis microalgae on antioxidant and anti-inflammatory factors in diabetic rats. Diabetes Metab Syndr Obesity. 2018;11:375. doi:10.2147/DMSO.S172104
32. Chattopadhyaya I, Gupta S, Mohammed A, Mushtaq N, Chauhan S, Ghosh S. Neuroprotective effect of Spirulina fusiform and amantadine in the 6-OHDA induced Parkinsonism in rats. BMC Complement Altern Med. 2015;15(1):296. doi:10.1186/s12906-015-0815-0
33. Koppula S, Kumar H, More SV, Kim BW, Kim IS, Choi D-K. Recent advances on the neuroprotective potential of antioxidants in experimental models of Parkinson’s disease. Int J Mol Sci. 2012;13(8):10608–10629. doi:10.3390/ijms130810608
34. Pérez-juárez A, Chamorro G, Alva-sánchez C, Paniagua-castro N, Pacheco-rosado J. Neuroprotective effect of Arthrospira (Spirulina) platensis against kainic acid-neuronal death. Pharm Biol. 2016;54(8):1408–1412. doi:10.3109/13880209.2015.1103756
35. Mazzola D, Fornari F, Vigano G, Oro T, Costa JAV, Bertolin TE. Spirulina platensis Enhances the beneficial effect of exercise on oxidative stress and the lipid profile in rats. Br Archiv Biol Technol. 2015;58(6):961–969. doi:10.1590/S1516-89132015060216
36. Safakhah HA, Tamimi F, Bandegi AR, Ghanbari A. Hypoalgesic effect of Spirulina platensis on the sciatic neuropathic pain induced by chronic constriction injury in male rats. Biomed Res Ther. 2018;5(9):2671–2679. doi:10.15419/bmrat.v5i9.477
37. Vafaei AA, Dadkhah M, Moradi N. Spirulina Plathensis microalgae prevents scopolamine-induced memory impairment in young female Wistar rats. koomesh. 2019;21(3):549–556.
38. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi:10.1016/0003-2697(76)90527-3
39. Dwivedi Y. Involvement of brain-derived neurotrophic factor in late-life depression. Am J Geriat Psychiat. 2013;21(5):433–449. doi:10.1016/j.jagp.2012.10.026
40. Gray JD, Milner TA, McEwen BS. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013;239:214–227. doi:10.1016/j.neuroscience.2012.08.034
41. Neto FL, Borges G, Torres-sanchez S, Mico JA, Berrocoso E. Neurotrophins role in depression neurobiology: a review of basic and clinical evidence. Curr Neuropharmacol. 2011;9(4):530–552. doi:10.2174/157015911798376262
42. Bath KG, Schilit A, Lee FS. Stress effects on BDNF expression: effects of age, sex, and form of stress. Neuroscience. 2013;239:149–156. doi:10.1016/j.neuroscience.2013.01.074
43. Serra MP, Poddighe L, Boi M, et al. Expression of BDNF and trkB in the hippocampus of a rat genetic model of vulnerability (Roman low-avoidance) and resistance (Roman high-avoidance) to stress-induced depression. Brain Behav. 2017;7(10):e00861–e00861. doi:10.1002/brb3.2017.7.issue-10
44. Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1545–1564. doi:10.1098/rstb.2006.1894
45. Pillai AG, de Jong D, Kanatsou S, et al. Dendritic morphology of hippocampal and amygdalar neurons in adolescent mice is resilient to genetic differences in stress reactivity. PLoS One. 2012;7(6):e38971. doi:10.1371/journal.pone.0038971
46. McEwen BS. Stress-induced remodeling of hippocampal CA3 pyramidal neurons. Brain Res. 2016;1645:50–54. doi:10.1016/j.brainres.2015.12.043
47. Shansky RM, Morrison JH. Stress-induced dendritic remodeling in the medial prefrontal cortex: effects of circuit, hormones and rest. Brain Res. 2009;1293:108–113. doi:10.1016/j.brainres.2009.03.062
48. Wood GE, Young LT, Reagan LP, Chen B, McEwen BS. Stress-induced structural remodeling in hippocampus: prevention by lithium treatment. Proc Natl Acad Sci. 2004;101(11):3973–3978. doi:10.1073/pnas.0400208101
49. Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cerebral Cortex. 2009;19(10):2479–2484. doi:10.1093/cercor/bhp003
50. Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci. 2008;105(14):5573–5578. doi:10.1073/pnas.0705615105
51. Salim S. Oxidative stress and the central nervous system. J Pharmacol Exp Ther. 2017;360(1):201–205. doi:10.1124/jpet.116.237503
52. Fontella FU, Siqueira IR, Vasconcellos APS, Tabajara AS, Netto CA, Dalmaz C. Repeated restraint stress induces oxidative damage in rat hippocampus. Neurochem Res. 2005;30(1):105–111. doi:10.1007/s11064-004-9691-6
53. Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434. doi:10.1038/nrn2639
54. Ghadrdoost B, Vafaei AA, Rashidy-pour A, et al. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur J Pharmacol. 2011;667(1–3):222–229. doi:10.1016/j.ejphar.2011.05.012
55. Ravi M, De SL, Azharuddin S, Paul S. The beneficial effects of Spirulina focusing on its immunomodulatory and antioxidant properties. Nutr Diet Suppl. 2010;2:73–83.
56. Hoseini S, Khosravi-darani K, Mozafari M. Nutritional and medical applications of Spirulina microalgae. Mini Rev Med Chem. 2013;13(8):1231–1237. doi:10.2174/1389557511313080009
57. Chu W-L, Lim Y-W, Radhakrishnan AK, Lim P-E. Protective effect of aqueous extract from Spirulina platensis against cell death induced by free radicals. BMC Complement Altern Med. 2010;10(1):53. doi:10.1186/1472-6882-10-53
58. Thaakur S, Sravanthi R. Neuroprotective effect of Spirulina in cerebral ischemia–reperfusion injury in rats. J Neural Transm. 2010;117(9):1083–1091. doi:10.1007/s00702-010-0440-5
59. Rasia-filho A, Fabian C, Rigoti K, Achaval M. Influence of sex, estrous cycle and motherhood on dendritic spine density in the rat medial amygdala revealed by the Golgi method. Neuroscience. 2004;126(4):839–847. doi:10.1016/j.neuroscience.2004.04.009
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.