Back to Journals » Journal of Inflammation Research » Volume 15
Therapeutic Effects of Silibinin Against Polycystic Ovary Syndrome Induced by Letrozole in Rats via Its Potential Anti-Inflammatory and Anti-Oxidant Activities
Authors Marouf BH , Ismaeel DO, Hassan AH , Ali OJ
Received 25 June 2022
Accepted for publication 29 August 2022
Published 9 September 2022 Volume 2022:15 Pages 5185—5199
DOI https://doi.org/10.2147/JIR.S379725
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Ning Quan
Bushra Hassan Marouf,1 Dana Omer Ismaeel,2 Ali Hussein Hassan,3,4 Othman Jalal Ali5,6
1Department of Pharmacology and Toxicology- College of Pharmacy, University of Sulaimani, Sulaimani, Kurdistan Region, Iraq; 2Department of Surgery and Theriogenology- College of Veterinary Medicine, University of Sulaimani, Sulaimani, Kurdistan Region, Iraq; 3Department of Basic Sciences- College of Dentistry, University of Sulaimani, Sulaimani, Kurdistan Region, Iraq; 4Department of Medical Laboratory Sciences- Komar University of Science and Technology, Sulaimani, Kurdistan Region, Iraq; 5Department of Surgery and Theriogenology- College of Veterinary Medicine- University of Sulaimani, Sulaimani, Kurdistan Region, Iraq; 6Department of Anaesthesia, College of Health Science, Cihan University of Sulaimaniya, Sulaimani, Kurdistan Region, Iraq
Correspondence: Bushra Hassan Marouf, Department of Pharmacology and Toxicology, College of Pharmacy, University of Sulaimani, Sulaimani, 46001, Kurdistan Region, Iraq, Tel +964 7701562796, Email [email protected]
Background: Current therapies for polycystic ovary syndrome (PCOS) are accompanied by unwanted effects. Silibinin; a flavonolignan has pleiotropic activities and favorable safety profile.
Purpose: To investigate the efficacy of silibinin on estrous cyclicity, inflammation, oxidative stress and ovarian morphology in letrozole-induced PCOS in rats.
Methods: Forty-eight female Wistar albino rats were divided into 2 sets. Rats of the first set (n = 12), assigned as a negative control (NC) received only the vehicle, rats of the second set (n = 36), assigned as PCOS rats, were given letrozole 1mg/Kg orally for 21 days. On day 21, six rats from the first set and six rats from the second set were euthanized for confirmation of PCOS-induction. The remaining animals from the first set assigned as group 1, those in the second set (n = 30) were equally divided into 5 groups and treated daily for 19 days as follows: group 2 (positive control) received only the vehicle, group 3 treated with metformin 300mg/Kg orally, groups 4 and 5 treated respectively with 100 and 200 mg/Kg silibinin intraperitoneally (IP), and group 6 treated with a combination of metformin 300mg/Kg orally and silibinin 100mg/Kg IP. On day 40, blood samples were examined for luteinizing hormone (LH), testosterone (TS) and estradiol (EST) levels, the anti-inflammatory and antioxidant parameters, ovarian and uterine morphology.
Results: Silibinin alone or in combination with metformin was found to be effective in restoring the regularity of estrous cycle by ameliorating the abnormal alterations of LH, TS, EST, tumor necrosis factor (TNF)-α, and oxidative status and by resuming the appearance of corpora lutea and decreasing or even total absence of cystic follicles in the ovaries.
Conclusion: Silibinin was effective in restoring estrous regularities and alleviating hormonal and histomorphological abnormalities of the ovarian and uterine tissues, this could be due to its anti-androgenic, anti-inflammatory and antioxidant properties.
Keywords: inflammation, estrous cycle, PCOS, silybin, testosterone
Introduction
Polycystic ovary syndrome (PCOS) is a common endocrinopathy of the women in reproductive age. It is a proinflammatory state, emerging data suggests that chronic low-grade inflammation underpins the development of metabolic aberration and ovarian dysfunction in the disorder.1,2 Most importantly, there is a strong association between hyperandrogenism and inflammation in PCOS that has been the focus of many investigations.3,4
General clinical and physiological features of PCOS include enlargement of ovaries, formation of multiple cysts, hyperandrogenism, disturbance of menstrual cycle, hirsutism, acne, anovulation or oligoamenorrhea, miscarriage, and infertility.5,6 According to the Rotterdam criteria, the identification of at least two of the following three characteristics is required for the diagnosis of PCOS: oligo-anovulation, ultrasonography images of polycystic ovaries and hyperandrogenism.7 The pathophysiology of PCOS is not well defined, however the abnormalities in neuroendocrine aspects such as increased in pulse frequency of gonadotropin-releasing hormone and consequently luteinizing hormone (LH) release and relative follicle stimulating hormone (FSH) deficiency are considered as a universal finding.8 The elevation of LH level enhances the biosynthesis of androgen in the theca cells and the FSH deficiency reduces the conversion of androgen into estrogen by granulosa cells and impedes maturation of the follicle and subsequently impairs ovulation.9 All these disturbances in hormones that are accompany PCOS lead to ovarian dysfunction and infertility. Furthermore, the existence of chronic subclinical inflammation of the ovaries may cause alteration in metabolic, hormonal and structural architecture eventually progresses toward the development of PCOS.10
Another contributing factor that is related to the development of PCOS and its relevant symptoms in the previous studies is oxidative stress.11 Several investigations clarified the impact of free radicals and oxidative stress in the pathogenesis of PCOS by alteration of steroidogenesis in the ovaries, which subsequently leads to hyperandrogenemia, dysregulation of follicular maturation, and infertility.12,13 Moreover, other factors such as obesity, insulin resistance, and cardiovascular risks have been shown to be linked to oxidative stress in PCOS women.14
Current therapies for PCOS are the use of many medications including metformin and clomiphene however, they are commonly accompanied by unwanted adverse effects. Therefore, a new treatment strategy with less side effects, more affordable, and with more pleiotropic activity is required. Previous studies have documented the efficacy of several herbal medications such as resveratrol,15 quercetin,16 thymoquinone17 and Ficus deltoidea18 in restoring function of the ovaries, insulin sensitivity and hormonal profile in experimentally induced PCOS. These studies suggested that the herbal medicines with ovulatory and hypoglycemic effects might be considered as an alternative therapy for PCOS.
Silibinin is a flavonolignan extracted from milk thistle, with free radical scavenging and antioxidant ability.19 It is extensively used for the management of metabolic and inflammatory disorders.20 It possesses anti-inflammatory, antioxidant and anticancer properties with therapeutic effect against liver disorders, various cancers, and gynecological diseases.21–23 Antioxidant, anti-inflammatory, insulin sensitizing, hypoglycemic, hypolipidemic effect and improvement of glucose metabolism dysfunction by silibinin in an in vivo study with non-alcoholic fatty liver disease (NAFLD) and other metabolic dysfunction has placed silibinin as a potential therapy in PCOS-related metabolic and reproductive disorders.20 This pleiotropic molecule exerts multifaceted mechanisms including suppression of pro-inflammatory cytokines including tumor necrosis factor (TNF-α), interleukins through inhibiting nuclear factor kappa-B (NF-κB) activity in human cells24 and it has been suggested by in silico studies to have an agonistic activity on estrogen receptor β (ERβ) through selective binding.23,25 Despite of these aforementioned studies on silibinin, the attempts for treating PCOS with silibinin are lacking. Therefore, silibinin could be regarded as an anti-oxidative agent with anti-inflammatory and estrogenic activity that can modulate the hormonal and metabolic dysfunction associated with PCOS. The aim of the present work was to investigate the efficacy of silibinin in alleviation of reproductive, biochemical and structural alteration associated with letrozole-induced PCOS in albino rats.
Materials and Methods
Chemical Reagents
Silibinin (Silybin) 98% was obtained from Glentham Life Sciences Ltd, UK. Letrozole was obtained from DENK PHARMA GmbH-Germany, Metformin which was commercially available as Glucophage from Merck Sante, Germany. Luteinizing hormone (LH), Testosterone (TS), Estradiol (EST) hormones, Rat tumor necrosis factor (TNF)-α, Rat Total Antioxidant Capacity (TAC BT-LAB) kits enzyme-linked immunosorbent assay (ELISA), were purchased from BT-LAB Bioassay Technology Laboratory Jiaxing Korain Biotech Co., Ltd (Zhejiang-China).
The Study Design, Animals and Ethical Consideration
Forty-eight female Wister Albino rats of 10–12 weeks (weighing 150 ± 20g) were included in the study, they were kept in the animal house of the College of Pharmacy-University of Sulaimani. The rats were being housed in plastic cages and acclimatized to the standardized environment with a 12-h-light–dark cycle, at a temperature of 23± 2°C, a relative humidity of 50± 5% for one week. They were freely accessible to the standard pellet diet and water ad libitum throughout the experimental study. The protocol of the study was approved by the Ethical Committee of the College of Pharmacy-University of Sulaimani with a Registration number PH23-21 in 31.08.2021. The procedures of the experiment were performed in accordance with the standard guidelines for the care and use of experimental animals by the committee for the purpose of control and supervision of experiments on animals and the national institutional animal care.
Treatment Groups and Induction of PCOS
The animals have been monitored for 3 consecutive estrus cycles by vaginal cytology analysis to investigate the relative proportion of leukocytes, epithelial and cornified cells. Rats that showed regular estrus cycle in a cyclical pattern every 4–5 days were included in the study and divided into two sets: the first set (n = 12) was assigned as negative control rats (NC) and left without PCOS induction whereas the second set (n = 36) was assigned as PCOS rats. Induction of PCOS was achieved by administration of water-dissolved letrozole to all rats (1mg/Kg body weight (BW)) by the gavage tube once daily for 21 days.26,27 Seven days before the end of PCOS induction (ie, from day 15 to day 21), vaginal smears were collected from all rats each morning (07:30 a.m. to 08:30 a.m.), stained by Giemsa stain and examined microscopically to confirm the PCOS induction.27,28 The body weight of all animals was recorded at the beginning and twice weekly throughout the experiment.
At the end of PCOS induction period on day 21, six rats from the NC set and another six from the PCOS set were randomly selected and euthanized for confirmation of PCOS-induction. The PCOS was confirmed by blockade of estrous cycle in the diestrus phase, increasing the levels of luteinizing hormone, (LH) and testosterone (TS), decreasing estradiol (EST) level and absence of corpora lutea and appearance of multiple cystic follicles in the ovaries.
The remaining NC rats (n = 6) were assigned as group 1; received only the vehicle (distilled water) and the remaining PCOS rats (n = 30) were randomly divided into 5 equal groups (6 rats each) and treated daily for 19 days as follows: group 2, assigned as positive control (PC), was left without treatment and received only the vehicle (distilled water), group 3 (treatment control) treated with metformin 300mg/Kg orally, groups 4 and 5 treated respectively with 100 and 200 mg/Kg silibinin IP, and group 6 treated with a combination of metformin 300mg/Kg orally and silibinin 100mg/Kg IP. The doses of silibinin have been selected based on the previous studies.29,30 During the treatment period, vaginal smear cytology was obtained daily from all rats and examined microscopically in order to monitor the estrous cycle cyclicity and to identify the estrous phases.
On day 40, the rats were euthanized and blood was obtained from the heart, kept in blood collection tube (gel tube), centrifuged at 5000 rpm for 10 min and the serum was collected for biochemical and hormonal analysis and the ovaries and uteri were taken out for histopathological assessments.
Hormonal, Inflammatory, Antioxidant Biomarkers and Histopathological Analysis
Serum TS, LH and EST were evaluated using commercially available ELISA kits. The serum level of Tumor necrosis factor (TNF-α), Total Antioxidant Capacity (TAC) were determined using enzyme-linked immunosorbent assay (ELISA).
The ovaries and uteri of all the rats were fixed in 10% neutral buffered formalin for 48 hours and undergone routine histological processing, paraffin embedding, sectioning at 5 µm thickness, staining with hematoxylin and eosin (H&E), and examining by the different magnification powers of light microscopy to assess their morphological characteristics. In addition, serial H&D-stained ovarian and uterine sections were used for analyzing the numbers of ovarian follicles and corpora lutea, endometrial thickness and numbers of endometrial glands. To avoid repetition of ovarian follicles counting, only follicles that contain the oocyte’s nucleolus were counted. Five fields in each uterine section image were selected for analyzing the endometrial thickness and numbers of uterine glands using the ImageJ imaging software. For confirmation of the histopathological analysis, the ovarian and uterine morphology was assessed by 2 independent histopathologists blinded to the treatment.
Statistical Analysis
The data were expressed as mean ± Standard Error of Mean (SEM). GraphPad Prism 9.4.0 (San Diego, CA, USA) was used to describe the results statistically. Comparison of mean was done by using one-way analysis of variance (ANOVA), two-way ANOVA followed by Tukey’s test post hoc test. The data of estrous cycle pattern were analyzed by comparing the most representative rat in each group.
Results
PCOS-Induction and Estrous Cycle Pattern
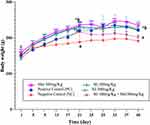
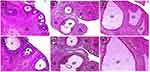
The induction of PCOS was confirmed based on the results of 7 days-vaginal cytology analysis from day 15 to 21, the body weight and hormonal changes on day 21. Significant increase in the body weight (Figure 1), hormonal changes (Table 1) and blockade of the estrous cycle in the diestrus phase (Figure 2B) were regarded as a positive PCOS induction.31 Before Ltz-treatment all the included animals had a normal estrus cycle which was evident by showing four regular phases in sequential pattern: proestrus (PE), estrus (ES), metestrus (ME) and one or two phases of diestrus (DE) every 4–5 days (Figure 2A). The irregularity of the cycle initiated approximately few days before day 15 of Ltz-treatment and complete block of the estrus cycle (100%) was obtained on day 15 ± 1 in all Ltz-treated groups displayed as constant diestrus phase with dominant leukocyte in microscopical evaluation.
 |
Table 1 Sex Hormone Changes in Ltz-Induced PCOS on Day 21 in the Rats |
 |
Figure 2 (A–F) Estrous cycle changes in one most representative rat in each group during PCOS induction and treatment period. As reported by Myondo et al29 (A) NC; negative control; (B) PC; positive control, received only distilled water after PCOS-induction; (C) Met 300mg/Kg received metformin 300mg/Kg after PCOS-induction; (D–F) received silibinin 100, 200mg/Kg and 100mg/Kg silibinin with 300mg/Kg metformin respectively after PCOS-induction. Abbreviations: PE, Proestrus; ES, estrus; ME, metestrus; DE, diestrus. |
Serum levels of TS and LH on day 21 were increased by 214%, 161% respectively and EST was decreased by 75% in the PC (Ltz-treated) group as compared with the NC group (Table 1).
Effect of Silibinin on Various Parameters in the Animals with Ltz- Induced PCOS
Estrous Cycle
In the NC-group, all the rats showed a regular estrous cycle throughout the study period which displayed as four estrous phases in a row of PE (round and nucleated epithelial cells were mainly seen), ES (cornifield squamous epithelial cells were predominantly seen), ME (a mixture of cornified squamous epithelial and leukocytes were mainly seen) and two phases of DE (which is characterized by presence of nucleated epithelial cells and predominant leukocytes) with the observation interval of 40 days. On the 27th day of the experiment, in the Metformin-treated group the nucleated epithelial cells were observed in 5 out of 6 rats under microscopical evaluation; this observation indicated the proestrus phase which lasts for 4 days, then the estrus cycle resumed and continued three times in all rats thereafter (Figure 2C). In silibinin 100mg/Kg treated group PE phase appeared on day 28th and changed into DE phase which is characterized by presence of nucleated epithelial cells and predominant leukocytes, this occurred twice then followed by a regular estrus cycle that continued three times in all the rats (Figure 2D). The conversion to the PE phase was observed on day 28 in Silibinin 200mg/Kg (Figure 2E). The better outcome has been seen in silibinin 100mg/Kg + Met 300mg/Kg as epithelial cells have been observed in half of the animals on 27th day of the experiment then the regular estrous cycle was resumed and continued three times later (Figure 2F).
Body Weight
Treatment with Ltz for 21 days led to significant increase (p < 0.05) in the body weight in all groups when compared to NC group (Figure 1).
After the treatments with metformin 300mg/Kg, two different doses of silibinin, and the combination of Sil 100mg + Met 300mg/Kg for 19 days (ie, at day 40), the combined treatment of Sil 100mg/Kg+ Met 300mg/Kg reduced the body weights significantly compared to the PC (p < 0.05). Non-significant differences were observed between the silibinin groups with PC and the other treatment group (Figure 3).
Serum TS, LH, EST Hormone Level
After PCOS induction on day 21, treatment with two different doses of silibinin and combination of the low dose (Sil 100mg/Kg) with Met 300mg/Kg for 19 days (i.e at 40 days of experiment) caused a noticeable decrease in serum level of TS in a non-significant manner (p > 0.05) as compared with the PC group although the decrement level of the combination was less. Meanwhile treatment with Met 300mg/Kg caused a significant (p < 0.05) decrease in serum level of TS. However, all the treatment groups lowered the serum level of LH in a significant manner (p < 0.05) when compared to the PC group. Non-significant changes in serum EST level have been observed in all tested groups (Figure 4).
Serum Pro-Inflammatory and Antioxidant Capacity Biomarkers
Serum total antioxidant capacity (TAC) biomarker was not significantly reduced (p > 0.05) in the PCOS induced rats in the PC group. Treatments with silibinin and its combination with metformin and metformin 300mg/Kg alone restored antioxidant capacity in non-significant manner.
Meanwhile TNF-α was significantly increased on day 21 in the PC group and the other Ltz-induced PCOS groups, and treatment with silibinin, metformin 300mg/Kg and their combination led to significant decrement in the serum level of this parameter in all treated groups (p < 0.05) as shown in Figure 5.
Histopathological Findings
Ovaries
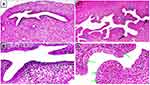
On day 21, ovarian sections of the PCOS rats showed absence of corpora lutea and presence of multiple cystic follicles surrounded by flat, compressed granulosa cell layers and a cortical stroma exhibiting chronic low grade inflammatory reaction of fibrosis and mononuclear inflammatory cells infiltration mainly lymphocytes in comparison with ovaries of the control negative rats which showed normal cortical and medullary morphology represented by antral follicles at different phases of growth and presence of multiple corpora lutea (Figure 6).
Nineteen days following treatment application (on day 40), ovarian sections of group 2 (positive control) were still showing cystic follicles and absence of corpora lutea whereas group 3 (Met 300mg/Kg BW) and group 6 (combination of Sil 100 mg/Kg and Met 300 mg/Kg BW) showed resuming the appearance of multiple corpora lutea, absence of cystic follicles, presence of antral follicles at different growth phases and disappearance of the cortical stromal inflammation. On the other hand, ovarian sections of groups 4 and 5 rats (Sil 100 mg/Kg and Sil 200 mg/Kg BW respectively) showed resuming appearance of corpora lutea; however, few cystic follicles were still apparent but with no evidence of cortical stromal inflammation (Figure 7).
Numbers of Ovarian Follicles and Corpora Lutea
On day 40, a significant decrease (p ≤ 0.05) was observed in numbers of ovarian follicles and corpora lutea in ovaries of positive control rats (group 2) in comparison with those of the negative control (group 1) and treatment rats (groups 3–6). Although non-significant, a decrease in numbers of ovarian follicles was also encountered in rats of group 4 in comparison with those of negative control and treatment groups 3, 5 and 6 (Table 2).
 |
Table 2 Numbers of Ovarian Follicles and Corpora Lutea in Control and Treatment Rats on Day 40 |
Uterus
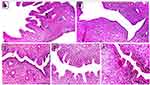
On day 21, uterine sections of the PCOS rats exhibited marked endometrial hyperplasia with profound, irregular endometrial folds toward the uterine lumen, proliferation, convolution and cystic dilatation of endometrial glands and rapid proliferation of endometrial epithelial cells as indicated by epithelial thickening and presence of many normal-looking mitotic figures in comparison to uteri of the control negative rats which showed normal uterine morphology represented by a normal thickened endometrium and normal looking, straight rather than convoluted endometrial glands (Figure 8).
On day 40, uterine sections of group 2 (positive control) showed persistence of endometrial hyperplasia as indicated by proliferation, convolution and cystic dilatation of endometrial glands, whereas group 3 (treatment control) and group 6 (combination of Sil 100 mg/Kg and Met 300 mg/Kg BW) showed normal uterine morphology. On the other hand, uterine sections of groups 4 and 5 rats (Sil 100 mg/Kg and Sil 200 mg/Kg BW respectively) showed mild endometrial hyperplasia represented by short endometrial folds toward the uterine lumen (Figure 9). These data were confirmed by analyzing the endometrial morphology using the image J software as shown in Table 3. On day 40, the endometrium thickness and number of endometrial glands were significantly increased (p ≤ 0.05) in the PC group (group 2) compared to the control negative (group 1) and treatment rats (groups 3, 4, 5 and 6). In addition, non-significant increase in endometrium thickness and number of endometrial glands was seen in treatment groups 4 and 5 in comparison with the negative control and treatment groups 3 and 6.
 |
Table 3 Endometrial Thickness and Numbers of Endometrial Glands in the Control and Treatment Rats on Day 40 |
Discussion
Various experimental animal models for PCOS were documented in the literature, none of these animal models are fully convincing and mimic completely the human PCOS. However, the short length of the estrous cycle of rats makes them ideal models for fertility studies.
Letrozole (Ltz) belongs to non-steroidal reversible aromatase inhibitors, it builds a PCOS model in rats resembling the human ones in term of histological and biochemical similarities it also exhibits the main characteristics of human PCOS such as hyperandrogenism, and multiple cysts.32 The mechanism of Ltz-induced PCOS is elucidated by blockade of transformation of testosterone and androstenedione to estradiol and estrone respectively and consequently this leads to androgen accumulation, hormonal alteration, synthesis of intraovarian androgen that ends with appearance of a polycystic ovary, hyperglycemic condition, and metabolic disturbances. Follicular atresia and development of abnormal follicular status are observed due to the access of androgen levels inside the ovary.33
In the present study, the use of Ltz was continued for 21 days27,34 this led to significant increase in the body weight of the animals, estrous cycle irregularity and hormonal abnormalities ie, hyperandrogenisim thus the development of PCOS in the studied animals of the present study might be due to increased TS and LH. It is well known that obesity and visceral adiposity worsen the clinical features of menstrual irregularity and infertility35 and they are correlated with increased serum androgens and LH in female with PCOS.36
In this study treatment of the PCOS with flavonolignan silibinin displayed the potential alleviation of the hormonal abnormalities and restored the normal estrous cyclicity in the rats. Silibinin treated groups alone and in combination with metformin, did not display any significant increase in body weight compared with the animals in the different groups, which suggested that silibinin might have the suppressive effect on lipid accumulation in adipocytes.37
Furthermore, low and high silibinin doses showed a remarkable anti-inflammatory effect as well as it showed a synergistic effect when it was combined with metformin. Previous studies have reported the suppression effect of silibinin in TNF-α and interferon (IFN-γ)-induced intracellular adhesion molecule (ICAM-1) expression by inhibiting NF-κB activity in human cells.24
Although the etiology of PCOS is complex and remains a mystery, but the evidences support the presence of chronic low-grade inflammation in women with this syndrome38 and it has been postulated that inflammation may also be involved in the development of metabolic alteration and ovarian dysfunction.39 A recent systematic review stated that PCOS individuals have increased levels of inflammatory mediators and the persistent release of inflammatory markers are linked to metabolic complications over time. Furthermore, other studies indicate a relationship between high level of androgens and leukocyte count, so that chronic low grade inflammation may be mediated through androgens concentration. Additionally, various studies investigated the impact of obesity and insulin resistance in increased inflammation, suggesting an enhancing influence of these abnormal metabolic states on PCOS pathogenesis.40
A recent case-control study and previous in vivo studies demonstrated high levels of inflammatory markers in women with PCOS.41,42 The finding of the current study was consistent with the previous results regarding TNF-α elevation during PCOS induction. Silibinin in the present study has ameliorated the serum level of this inflammatory biomarker in a significant manner, the mechanisms associated with the anti-inflammatory effect of silibinin have been clarified in many studies such as the suppression of NF-κB-regulated gene products have been reported by Bannwart et al.43 Furthermore, silymarin; composed of many flavonolignans, the most abundant constituents of it is silibinin, it can inhibit both inflammation and oxidative stress through inhibition of the NF-κB activation and reduction of the production of pro-inflammatory cytokines interleukin- 1β (IL-1β) and TNF-α.44
A previous study also demonstrated that silibinin upregulated estrogen receptor β (ERβ) expression, induced apoptosis, inhibited proliferation, and reduced expression of the pro-inflammatory cytokines IL-17 and TNF-α through ERβ binding in T lymphocytes obtained from female and male healthy donors.23
In the present study silibinin also increased or maintained the total antioxidant capacity of the PCOS-rats in a non-significant manner. It is obvious that oxidative stress is considered as a potential stimulant of PCOS, and serum levels of antioxidants are reduced in patients with PCOS. Therefore, the use of polyphenols with antioxidant activity such as quercetin, berberine, resveratrol in the management of PCOS have been attempted in various studies with positive outcomes.23,45,46 These polyphenol compounds interact with multiple cellular targets to induce antioxidant and anti-inflammatory systems then improve the weight gain, hormone profile and ovarian follicular cell architecture in PCOS such as activation of silent information regulator 1 (SIRT1) and AMP activated protein kinase (AMPK) activation in resveratrol.46
Antioxidant and anti-inflammatory features of silibinin were demonstrated by dose-dependent inhibition of hydrogen peroxide (H2O2) release and production of TNF-α, interleukin-10 (IL-10), transforming growth factor beta (TGF-β) and prostaglandin E2 (PGE2) by peripheral blood monocytes from healthy individuals stimulated with lipopolysaccharide.47
Investigation of gonadal hormone levels such as TS, LH, and EST is encouraged for the diagnosis of PCOS. It is obvious that the elevated serum level of TS and LH and low EST, progesterone, and FSH are the most comparable and consistent hormonal hallmarks to diagnose PCOS in women.48 In this study, Ltz-induced PCOS rats displayed elevated levels of TS and LH, and low level of EST, compared with control. These results are consistent with the findings of the previous researches that produced PCOS-model by Ltz- administration.27,49
Silibinin in the present study potentially ameliorated the hormonal and metabolic alteration associated with PCOS and it was evidenced by a noticeable reduction in serum TS level, significant decrease in LH, a non-significant change in body weights and restoration of regularities of the estrous cycle in the albino rats after it was blocked in the diestrus phase following Ltz-PCOS-induction. These effects might be associated with reduction in the levels of LH and TS.
On the other hand, it has been found that silibinin with and without metformin has no significant effect on restoration of estradiol hormone to its normal level. A previous in vitro study has emphasized on the estrogenic-like effect of silibinin through modulation of expression of ERβ in lymphocyte cells,23 the study demonstrated the anti-inflammatory role of silibinin through ERβ binding, therefor the effect of this phytoestrogen molecule need to be further studied in metabolic and hormonal alteration disorders, because ER is identified as an effective target for the treatment of PCOS. It is obvious that most phytoestrogens exert their physiological effects by regulating ERα and ERβ, indeed, many studies have provided evidences that polyphenols have the potential to control PCOS and other related metabolic disorders through modulation of these receptors.50
On day 21, the PCOS rats showed absence of corpora lutea and appearance of multiple cystic follicles in the ovaries and marked endometrial hyperplasia in the uteri. This finding is consistent with the previous study which confirm the PCOS induction.26 The absence of corpora lutea and the presence of cystic follicles represent an indicator for anovulation status51 due to the increase in biosynthesis of androgens that interfere with the normal process of follicular maturation.27 Subsequently, the absence of corpora lutea results in a lack of progesterone which in turn results in endometrial overgrowth and hyperplasia.52,53
On day 40, rats of groups 4 and 5 (Sil 100 mg/Kg BW and Sil 200 mg/Kg BW respectively) showed resuming the appearance of corpora lutea, persistence of few cystic follicles and mild endometrial hyperplasia. This finding indicates that silibinin treatment improves the resuming of an approximately normal ovarian and uterine structure and function compared to the positive control rats (group 2) which were still showing absence of corpora lutea, persistence of multiple cystic follicles and marked endometrial hyperplasia. However, the best improvement was seen in rats of the standard treatment (group 3, Met 300mg/Kg BW) and group 6 (combination of Sil 100mg/Kg and Met 300mg/Kg BW) which showed resuming the appearance of multiple corpora lutea, absence of cystic follicles, presence of antral follicles at different growth phases and disappearance of endometrial hyperplasia. The enhancement of ovarian histomorphological characteristics might be attributed to an anti-androgenic mechanism54 of the silibinin as evident by the decrease in testosterone hormone level demonstrated in this study and the alleviation of endometrial hyperplasia might be attributed to the improved structural and functional microenvironment of the ovarian and uterine tissues.55,56 The incomplete restoration of ovarian and uterine tissues in rats treated by silibinin alone is probably attributed to insufficient time-duration (19 days) of the treatment. Further investigations are needed to investigate the long-term treatment of silibinin with different doses and route of administration.
The present study was not without limitations. The duration of silibinin therapy was based on the improvement and the restoration of the normal estrous cycle which seems to be not enough for complete alleviation of tissue changes in the ovary and uterus. Therefore, a long-term treatment period is recommended.
Conclusion
This study showed that silibinin therapy alone and in combination with metformin was effective in decreasing the serum levels of total TS, LH, restoring estrous regularities and alleviating histomorphological abnormalities of the ovarian and uterine tissues, this could be due to its anti-androgenic, anti-inflammatory and antioxidant properties. Further investigations in animals and humans are recommended to observe the value of this pleiotropic agent in PCOS. Thus, silibinin can be suggested for more hormonal, immunohistochemical and molecular investigation to explore its potential in the treatment of PCOS.
Acknowledgments
The authors would like to thank the College of Pharmacy, College of Veterinary Medicine and Department of Biology in College of Education at the University of Sulaimani for their support to conduct this research.
Author Contributions
All authors shared substantial contributions to the present work, whether that is in the conception, study design, execution, data acquisition, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work
References
1. Deligeoroglou E, Vrachnis N, Athanasopoulos N, et al. Mediators of chronic inflammation in polycystic ovarian syndrome. Gynecol Endocrinol. 2012;28(12):974–978. doi:10.3109/09513590.2012.683082
2. González F, Rote NS, Minium J, Kirwan JP. Increased activation of nuclear factor κB triggers inflammation and insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(4):1508–1512. doi:10.1210/jc.2005-2327
3. Repaci A, Gambineri A, Pasquali R. The role of low-grade inflammation in the polycystic ovary syndrome. Mol Cell Endocrinol. 2011;335(1):30–41. doi:10.1016/j.mce.2010.08.002
4. González F, Rote NS, Minium J, Kirwan JP. Evidence of proatherogenic inflammation in polycystic ovary syndrome. Metabolism. 2009;58(7):954–962. doi:10.1016/j.metabol.2009.02.022
5. Sirmans S, Pate K. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2013;1. doi:10.2147/CLEP.S37559
6. Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primer. 2016;2(1):16057. doi:10.1038/nrdp.2016.57
7. Azziz R. Diagnosis of polycystic ovarian syndrome: the Rotterdam criteria are premature. J Clin Endocrinol Metab. 2006;91(3):781–785. doi:10.1210/jc.2005-2153
8. Burt Solorzano CM, Beller JP, Abshire MY, Collins JS, McCartney CR, Marshall JC. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids. 2012;77(4):332–337. doi:10.1016/j.steroids.2011.12.007
9. McCartney CR, Eagleson CA, Marshall JC. Regulation of gonadotropin secretion: implications for polycystic ovary syndrome. Semin Reprod Med. 2002;20(4):317–326. doi:10.1055/s-2002-36706
10. Li Q, Du J, Feng R, et al. A possible new mechanism in the pathophysiology of Polycystic Ovary Syndrome (PCOS): the discovery that leukocyte telomere length is strongly associated with PCOS. J Clin Endocrinol Metab. 2014;99(2):E234–E240. doi:10.1210/jc.2013-3685
11. Sulaiman M, Al-Farsi Y, Al-Khaduri M, Saleh J, Waly M. Polycystic ovarian syndrome is linked to increased oxidative stress in Omani women. Int J Womens Health. 2018;10:763–771. doi:10.2147/IJWH.S166461
12. Archibong AE, Rideout ML, Harris KJ, Ramesh A. Oxidative stress in reproductive toxicology. Curr Opin Toxicol. 2018;7:95–101. doi:10.1016/j.cotox.2017.10.004
13. Mohammadi M. Oxidative stress and polycystic ovary syndrome: a brief review. Int J Prev Med. 2019;10:86. doi:10.4103/ijpvm.IJPVM_576_17
14. Zuo T, Zhu M, Xu W. Roles of oxidative stress in polycystic ovary syndrome and cancers. Oxid Med Cell Longev. 2016;2016:1–14. doi:10.1155/2016/8589318
15. Zhang N, Zhuang L, Gai S, et al. Beneficial phytoestrogenic effects of resveratrol on polycystic ovary syndromein rat model. Gynecol Endocrinol. 2021;37(4):337–341. doi:10.1080/09513590.2020.1812569
16. Pourteymour Fard Tabrizi F, Hajizadeh-Sharafabad F, Vaezi M, Jafari-Vayghan H, Alizadeh M, Maleki V. Quercetin and polycystic ovary syndrome, current evidence and future directions: a systematic review. J Ovarian Res. 2020;13(1):11. doi:10.1186/s13048-020-0616-z
17. Taghvaee Javanshir S, Yaghmaei P, Hajebrahimi Z. Thymoquinone ameliorates some endocrine parameters and histological alteration in a rat model of polycystic ovary syndrome. Int J Reprod Biomed. 2018;16(4):275–284.
18. Haslan MA, Samsulrizal N, Hashim N, Zin NSNM, Shirazi FH, Goh YM. Ficus deltoidea ameliorates biochemical, hormonal, and histomorphometric changes in letrozole-induced polycystic ovarian syndrome rats. BMC Complement Med Ther. 2021;21(1):291. doi:10.1186/s12906-021-03452-6
19. Marouf BH, Zalzala MH, Al-Khalifa II, Aziz TA, Hussain SA. Free radical scavenging activity of silibinin in nitrite-induced hemoglobin oxidation and membrane fragility models. Saudi Pharm J. 2011;19(3):177–183. doi:10.1016/j.jsps.2011.03.006
20. Tong WW, Zhang C, Hong T, et al. Silibinin alleviates inflammation and induces apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes and has a therapeutic effect on arthritis in rats. Sci Rep. 2018;8(1):3241. doi:10.1038/s41598-018-21674-6
21. Kavitha CV, Deep G, Gangar SC, Jain AK, Agarwal C, Agarwal R. Silibinin inhibits prostate cancer cells- and RANKL-induced osteoclastogenesis by targeting NFATc1, NF-κB, and AP-1 activation in RAW264.7 cells: SILIBININ INHIBITS PCA- AND RANKL-INDUCED OSTEOCLASTOGENESIS. Mol Carcinog. 2014;53(3):169–180. doi:10.1002/mc.21959
22. Kim TH, Woo JS, Kim YK, Kim KH. Silibinin induces cell death through reactive oxygen species–dependent downregulation of Notch-1/ERK/Akt signaling in human breast cancer cells. J Pharmacol Exp Ther. 2014;349(2):268–278. doi:10.1124/jpet.113.207563
23. Dupuis ML, Conti F, Maselli A, et al. The natural agonist of estrogen receptor β silibinin plays an immunosuppressive role representing a potential therapeutic tool in rheumatoid arthritis. Front Immunol. 2018;9:1903. doi:10.3389/fimmu.2018.01903
24. Chen YH, Chen CL, Liang CM, et al. Silibinin inhibits ICAM-1 expression via regulation of N-linked and O-linked glycosylation in ARPE-19 Cells. BioMed Res Int. 2014;2014:1–13. doi:10.1155/2014/701395
25. El-Shitany NA, Hegazy S, El-desoky K. Evidences for antiosteoporotic and selective estrogen receptor modulator activity of silymarin compared with ethinylestradiol in ovariectomized rats. Phytomedicine. 2010;17(2):116–125. doi:10.1016/j.phymed.2009.05.012
26. Kafali H, Iriadam M, Ozardalı I, Demir N. Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch Med Res. 2004;35(2):103–108. doi:10.1016/j.arcmed.2003.10.005
27. Ndeingang EC, Defo Deeh PB, Watcho P, Kamanyi A. Phyllanthus muellerianus (Euphorbiaceae) restores ovarian functions in letrozole-induced polycystic ovarian syndrome in rats. Evid Based Complement Alternat Med. 2019;2019:1–16. doi:10.1155/2019/2965821
28. Guo Y, Qi Y, Yang X, et al. Association between polycystic ovary syndrome and gut microbiota. PLoS One. 2016;11(4):e0153196. doi:10.1371/journal.pone.0153196
29. Chen CL, Chen JT, Liang CM, Tai MC, Lu DW, Chen YH. Silibinin treatment prevents endotoxin-induced uveitis in rats in vivo and in vitro. PLoS One. 2017;12(4):e0174971. doi:10.1371/journal.pone.0174971
30. Lee B, Choi GM, Sur B. Silibinin prevents depression-like behaviors in a single prolonged stress rat model: the possible role of serotonin. BMC Complement Med Ther. 2020;20(1):70. doi:10.1186/s12906-020-2868-y
31. Mannerås L, Cajander S, Holmäng A, et al. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148(8):3781–3791. doi:10.1210/en.2007-0168
32. Ghafurniyan H, Azarnia M, Nabiuni M, Karimzadeh L. The effect of green tea extract on reproductive improvement in estradiol valerate-induced polycystic ovarian syndrome in rat. Iran J Pharm Res IJPR. 2015;14(4):1215–1233.
33. Franks S, Hardy K. Androgen action in the ovary. Front Endocrinol. 2018;9:452. doi:10.3389/fendo.2018.00452
34. Kakadia N, Patel P, Deshpande S, Shah G. Effect of Vitex negundo L. seeds in letrozole induced polycystic ovarian syndrome. J Tradit Complement Med. 2019;9(4):336–345. doi:10.1016/j.jtcme.2018.03.001
35. Ozcan Dag Z, Dilbaz B. Impact of obesity on infertility in women. J Turk Ger Gynecol Assoc. 2015;16(2):111–117. doi:10.5152/jtgga.2015.15232
36. Khmil M, Khmil S, Marushchak M. Hormone imbalance in women with infertility caused by polycystic ovary syndrome: is there a connection with body mass index? Open Access Maced J Med Sci. 2020;8(B):731–737. doi:10.3889/oamjms.2020.4569
37. Suh HJ, Cho SY, Kim EY, Choi HS. Blockade of lipid accumulation by silibinin in adipocytes and zebrafish. Chem Biol Interact. 2015;227:53–62. doi:10.1016/j.cbi.2014.12.027
38. Duleba AJ, Dokras A. Is PCOS an inflammatory process? Fertil Steril. 2012;97(1):7–12. doi:10.1016/j.fertnstert.2011.11.023
39. Spritzer PM, Lecke SB, Satler F, Morsch DM. Adipose tissue dysfunction, adipokines, and low-grade chronic inflammation in polycystic ovary syndrome. Reproduction. 2015;149(5):R219–R227. doi:10.1530/REP-14-0435
40. Rudnicka E, Suchta K, Grymowicz M, et al. Chronic low grade inflammation in pathogenesis of PCOS. Int J Mol Sci. 2021;22(7):3789. doi:10.3390/ijms22073789
41. Alissa EM, Algarni SA, Khaffji AJ, Al Mansouri NM. Role of inflammatory markers in polycystic ovaries syndrome: in relation to insulin resistance. J Obstet Gynaecol Res. 2021;47(4):1409–1415. doi:10.1111/jog.14684
42. Mohammadi S, Kayedpoor P, Karimzadeh-Bardei L, Nabiuni M. The effect of curcumin on TNF-α, IL-6 and CRP expression in a model of polycystic ovary syndrome as an inflammation state. J Reprod Infertil. 2017;18(4):352–360.
43. Bannwart CF, Nakaira-Takahagi E, Golim MA, et al. Downregulation of nuclear factor-kappa B (NF-κB) pathway by silibinin in human monocytes challenged with Paracoccidioides brasiliensis. Life Sci. 2010;86(23–24):880–886. doi:10.1016/j.lfs.2010.04.005
44. Bijak M. Silybin, a major bioactive component of milk thistle (Silybum marianum L. Gaernt.)—chemistry, bioavailability, and metabolism. Molecules. 2017;22(11):1942. doi:10.3390/molecules22111942
45. Ashkar F, Hassan Eftekhari M, Tanideh N, et al. Effect of hydroalcoholic extract of Berberis integerrima and resveratrol on ovarian morphology and biochemical parameters in Letrozole-induced polycystic ovary syndrome rat model: an experimental study. Int J Reprod Biomed IJRM. 2020;18:637–650. doi:10.18502/ijrm.v13i8.7505
46. Furat Rencber S, Kurnaz Ozbek S, Eraldemır C, et al. Effect of resveratrol and metformin on ovarian reserve and ultrastructure in PCOS: an experimental study. J Ovarian Res. 2018;11(1):55. doi:10.1186/s13048-018-0427-7
47. Bannwart CF, Peraçoli JC, Nakaira-Takahagi E, Peraçoli MTS. Inhibitory effect of silibinin on tumour necrosis factor-alpha and hydrogen peroxide production by human monocytes. Nat Prod Res. 2010;24(18):1747–1757. doi:10.1080/14786410903314492
48. Bednarska S, Siejka A. The pathogenesis and treatment of polycystic ovary syndrome: what’s new? Adv Clin Exp Med off Organ Wroclaw Med Univ. 2017;26(2):359–367. doi:10.17219/acem/59380
49. Mvondo MA, Mzemdem Tsoplfack FI, Awounfack CF, Njamen D. The leaf aqueous extract of Myrianthus arboreus P. Beauv. (Cecropiaceae) improved letrozole-induced polycystic ovarian syndrome associated conditions and infertility in female Wistar rats. BMC Complement Med Ther. 2020;20(1):275. doi:10.1186/s12906-020-03070-8
50. Rani R, Hajam YA, Kumar R, Bhat RA, Rai S, Rather MA. A landscape analysis of the potential role of polyphenols for the treatment of Polycystic Ovarian Syndrome (PCOS). Phytomed Plus. 2022;2(1):100161. doi:10.1016/j.phyplu.2021.100161
51. Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;28(1):117–149. doi:10.1210/er.2006-0022
52. Chandra V, Kim JJ, Benbrook DM, Dwivedi A, Rai R. Therapeutic options for management of endometrial hyperplasia. J Gynecol Oncol. 2016;27(1):e8. doi:10.3802/jgo.2016.27.e8
53. Sanderson PA, Critchley HOD, Williams ARW, Arends MJ, Saunders PTK. New concepts for an old problem: the diagnosis of endometrial hyperplasia. Hum Reprod Update. 2016. doi:10.1093/humupd/dmw042
54. Rajan RK, Balaji B, Balaji B. Soy isoflavones exert beneficial effects on letrozole-induced rat polycystic ovary syndrome (PCOS) model through anti-androgenic mechanism. Pharm Biol. 2017;55(1):242–251. doi:10.1080/13880209.2016.1258425
55. Wang Q, Guo X, Li L, Gao Z, Ji M. Treatment with metformin and sorafenib alleviates endometrial hyperplasia in polycystic ovary syndrome by promoting apoptosis via synergically regulating autophagy. J Cell Physiol. 2020;235(2):1339–1348. doi:10.1002/jcp.29051
56. Yang Y, Liu J, Xu W. Naringenin and Morin reduces insulin resistance and endometrial hyperplasia in the rat model of polycystic ovarian syndrome through enhancement of inflammation and autophagic apoptosis. Acta Biochim Pol. 2022. doi:10.18388/abp.2020_5722
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.