Back to Journals » Cancer Management and Research » Volume 12
Therapeutic Effect of Trastuzumab in Neoadjuvant-Treated HER2-Positive Breast Cancer with Low Infiltrating Level of Tumor-Infiltrating Lymphocytes
Authors Liu S , Mou E , Zeng S , Wang L , Dong H , Ji J , Yang H , Li J , Wang H , Li H , Xu J
Received 2 February 2020
Accepted for publication 15 April 2020
Published 5 May 2020 Volume 2020:12 Pages 3145—3153
DOI https://doi.org/10.2147/CMAR.S248071
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Shiwei Liu,1 Exian Mou,1 Shiyan Zeng,1 Lu Wang,2 Hao Dong,3 Juan Ji,3 Hong Yang,3 Junjie Li,1 Hao Wang,1 Hui Li,1 Jia Xu1
1Department of Breast Surgery, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, School of Medicine, University of Electronic Science and Technology of China, Chengdu, Sichuan Province, People’s Republic of China; 2Department of Operating Room, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, School of Medicine, University of Electronic Science and Technology of China, Chengdu, Sichuan Province, People’s Republic of China; 3Department of Pathology, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, School of Medicine, University of Electronic Science and Technology of China, Chengdu, Sichuan Province, People’s Republic of China
Correspondence: Hui Li; Jia Xu
Department of Breast Surgery, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, School of Medicine, University of Electronic Science and Technology of China, No. 55, Section 4, South Renmin Road, Chengdu, Sichuan Province, People’s Republic of China
Tel/Fax +86-02885420456
Email [email protected]; [email protected]
Purpose: The aim of the present study was to investigate the effect of trastuzumab on the pathological complete response (pCR) rate and event-free survival (EFS) in neoadjuvant-treated HER2-positive breast cancer with a low infiltrating level of tumor-infiltrating lymphocytes (TILs).
Patients and Methods: The infiltrating level of TILs was evaluated in hematoxylin and eosin-stained slides from diagnostic needle biopsies, and a low infiltrating level of TILs was defined as TILs < 10%. Data of 179 HER2-positive patients with a low infiltrating level of TILs were retrospectively reviewed and compared according to whether trastuzumab was administered or not. The associations of clinicopathological characteristics with pCR or EFS were assessed in univariate and multivariate analyses. EFS was estimated by the Kaplan–Meier method and compared by the log rank test.
Results: Of 179 patients, the overall pCR rate was 20.1%, and 74 patients (41.3%) received trastuzumab. Patients treated with trastuzumab showed a pCR rate of 20.3% compared with 20.0% for those without trastuzumab (P = 0.965). Trastuzumab administration was not associated with pCR in univariate (P = 0.965) and multivariate (P = 0.994) analyses. Negative status of hormone receptor (HR) (P < 0.001) and histological grade 3 (P = 0.007) were independent predictors for pCR in multivariate analyses. Trastuzumab usage had no significant impact on EFS in univariate (P = 0.916) and multivariate (P = 0.431) analyses, and pCR was the only independent predictor for favorable EFS (P = 0.012) in multivariate analyses.
Conclusion: In neoadjuvant-treated HER2-positive breast cancer with a low infiltrating level of TILs, additional trastuzumab had no significant influence on the pCR rate and EFS. HR-negative status and histological grade 3 were independently associated with higher pCR rates, and pCR was the only independent predictor for improved survival. Our findings may help identify patients who are resistant to trastuzumab, thereby guiding the de-escalating choice of anti-HER2 therapy.
Keywords: HER2, trastuzumab, tumor-infiltrating lymphocytes, pathological complete response, event-free survival
Introduction
Neoadjuvant treatment (NAT) was initially applied in primary inoperable breast cancer for tumoral shrinkage and surgical feasibility.1 Increasingly in non-metastatic disease, NAT has been utilized for facilitation of breast conservation,2 and assessment of treatment response by pathological evaluation of surgical specimens.3–5 Substantial evidence reveals associations of long-term outcomes with pathological response to NAT,6–9 and pathological complete response (pCR) has been demonstrated as a surrogate indicator for favorable prognosis.10–12 Furthermore, the survival benefit of postoperative escalating therapy in patients without pCR after NAT has been validated in randomized trials.13,14 However, the pCR rate and the impact of pCR on survival are highly distinct among molecular subtypes of breast cancer.10,11,15
Human epidermal growth factor receptor 2 (HER2)-positive breast cancer represents a distinctive molecular subtype with aggressive biological behavior and poor clinical outcomes.16–18 Trastuzumab, a recombinant monoclonal antibody that targets HER2, plays a fundamental role in HER2-directed treatment.19–21 In the neoadjuvant scenario, addition of trastuzumab has significantly increased the pCR rate and improved prognosis compared with chemotherapy alone.22–25 Moreover, achieving pCR in HER2-positive disease significantly correlates with better survival in terms of event-free survival (EFS) and overall survival.11,26 Nevertheless, more than half of trastuzumab-treated patients show residual disease after NAT.10,11 This heterogeneous sensitivity has prompted investigations into the mechanisms of response and resistance to trastuzumab. However, currently, no clinically meaningful biomarkers can identify the patients who will or will not respond to trastuzumab. This issue will be increasingly critical as the efficacy of trastuzumab is fundamental and irreplaceable despite novel anti-HER2 agents.19–21
Preclinical evidence suggests a key role of immune mechanisms in modulation of the effect of trastuzumab.27–29 The antitumor immune response depends on the infiltration of anti- or proinflammatory immune cells in the tumor microenvironment.27–29 Tumor-infiltrating lymphocytes (TILs), observed as mononuclear immune cells in and around tumor tissue by hematoxylin and eosin (H&E)-stained slides, represent a surrogate marker for antitumor immunity.30,31 In HER2-positive breast cancer treated with NAT, higher infiltrating levels of TILs have been shown to be associated with higher pCR rates and better survival.32–36 Contrarily, in spite of treated with various trastuzumab-containing therapies, patients with low infiltrating levels of TILs generally show lower pCR rates and worse prognosis.32–36 However, little evidence focuses on the benefit of trastuzumab usage in patients with low infiltrating levels of TILs.
The aim of our study was to study the effect of trastuzumab on the pCR rate and EFS in neoadjuvant-treated HER2-positive breast cancer with a low infiltrating level of TILs. TILs were evaluated based on consensual guidelines,30,37 and data for patients with a low infiltrating level of TILs were categorized and compared according to whether trastuzumab was used or not.
Patients and Methods
Patients
A retrospective review of the database at the breast disease center of our hospital was performed with approval from the Ethics Committee of Sichuan Cancer Hospital & Institute. This study was conducted in compliance with the Declaration of Helsinki. Because of the retrospective nature of our study, patient consent was not required by the Ethics Committee, and the data related to our study were anonymized.
Patients with invasive non-metastatic breast cancer who underwent NAT and subsequent radical surgery from 2008 to 2018 were identified. The inclusion criteria of the present study were female sex, age 18–70 years, histologically confirmed HER2 positivity, completion of all predetermined cycles of standard neoadjuvant chemotherapy, available information of baseline clinicopathological characteristics, definite status of postoperative pathological response, complete data of postoperative treatment and follow-up, available H&E-stained slides of core needle biopsies from primary tumors for TILs assessment, and a low infiltrating level of TILs defined by our preestablished cut-off point of TILs density.
For neoadjuvant chemotherapy, all predesigned cycles had to be completed, and combined or sequential chemotherapy regimens were allowed. Trastuzumab was not administered to all patients for reasons of health insurance and medical cost. In trastuzumab-based NAT, a taxane plus carboplatin (TCb), and an anthracycline plus cyclophosphamide followed by a taxane (AC-T) were regarded as standard chemotherapy. Neoadjuvant trastuzumab had to be used concurrently with taxane, and adjuvant trastuzumab had to be continued to complete 1 year of treatment. For patients who did not receive neoadjuvant trastuzumab, an anthracycline plus cyclophosphamide and a taxane (TAC) and AC-T were considered standard chemotherapy. Patients treated without neoadjuvant trastuzumab but with adjuvant trastuzumab were not included in our study.
Pathological Evaluation
Data for routine pathological parameters were reviewed by experienced breast pathologists. HER2 positivity was defined as either a 3+ score by immunohistochemistry or gene amplification by fluorescent in situ hybridization. Tumor histology was assigned according to the 2012 World Health Organization Classification of Tumors of the Breast. Histological grade was determined by the Scarff–Bloom–Richardson classification modified by Elston and Ellis.38 The Ki67 score was defined as the percentage of nuclear stained cells among at least 1000 tumor cells counted, and the median score was used as the cut-off value for statistical analyses. Estrogen receptor (ER) and progesterone receptor (PR) status were considered positive if ≥1% of the cancer cells had nuclear staining.39 Hormone receptor (HR) status was judged positive if the ER and/or PR status was positive, whereas negative status for both ER and PR was considered HR-negative status. Pathological complete response was defined as no evidence of residual invasive disease in the breast and axilla (ypT0/is ypN0).
Full-face H&E-stained slides of core needle biopsies of primary tumors were utilized for TILs evaluation based on current consensuses standardized by the International Immuno-Oncology Biomarker Working Group.30,37 All mononuclear cells, including lymphocytes and plasma cells, in the stromal compartment within the tumor border were counted as TILs, and the infiltrating degree of TILs was quantified as the percentage of stromal tissue occupied by TILs. The infiltrating level of TILs was assessed by experienced breast pathologists in a blind manner. A cut-off point of 10% for the TILs percentage was preestablished as a criterion for the inclusion of patients. A low infiltrating level of TILs was defined as TILs < 10% in the present study (Figure 1).
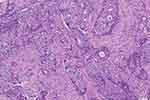 |
Figure 1 Representative image of a low infiltrating level of tumor-infiltrating lymphocytes. |
Statistical Analysis
The chi-square test or Fisher’s exact test was conducted for comparisons of categorical variables. Univariate and multivariate logistic regression analyses were performed to assess the associations of pCR with patient and tumor characteristics, and to calculate odds ratios (ORs) and 95% confidence intervals (CIs). For survival analyses, EFS was defined as the interval from the date of diagnosis to the date of events such as locoregional recurrence, distant metastasis, or death from any cause. Univariate and multivariate Cox proportional hazards regression analyses were conducted to evaluate the associations of EFS with patient and tumor characteristics, and to estimate hazard ratios and 95% CIs. The Kaplan–Meier method was employed to draw survival curves, and EFS was compared by the log rank test. P values < 0.05 were considered statistically significant; all tests were two-sided. Statistical analyses were carried out using GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA) and SPSS version 14.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient and Tumor Characteristics
The entire cohort consisted of 179 consecutive HER2-positive breast cancer patients, including 105 patients (58.7%) treated without trastuzumab and 74 patients (41.3%) with trastuzumab. Patient and tumor characteristics are summarized in Table 1. The median age was 53 (range 28–70) years. Most patients were over 50 years (62.0%), and 64.8% of patients were postmenopausal. The majority of tumors had a clinical tumor size of T1–2 (83.8%), and 52.0% of patients had positive nodal status. Histologically, 96.6% of tumors were invasive ductal disease, and most tumors were histological grade 1–2 (67.6%) or had Ki67 score ≤ 35% (53.1%). HR-positive tumors constituted 68.7% of the entire cohort.
 |
Table 1 Patient and Tumor Characteristics |
As for treatment, 96 patients (53.6%) received sequential neoadjuvant chemotherapy (AC-T), and 83 patients (46.4%) were treated with combined therapy, either TCb or TAC. All chemotherapy regimens were given every 3 weeks for a predefined 6 or 8 cycles. In trastuzumab-containing therapy, a loading dose of 8 mg/kg intravenous trastuzumab followed by 6 mg/kg every 3 weeks was administered for a total of 1 year. The majority of patients underwent mastectomy for the breast (84.4%) or axillary lymph node dissection for the axilla (72.1%). Adjuvant treatment was performed in 66.5% of patients for radiotherapy, and in 65.4% of patients for endocrine therapy, respectively.
Therapeutic Effect of Trastuzumab
All patient and tumor characteristics were compared according to whether trastuzumab was used or not. No significant differences were found among patients treated without or with trastuzumab (Table 1). Overall, 36 patients (20.1%) achieved a pCR, and 48 patients (26.8%) had survival events over a median follow-up of 51 months (range 13–126). The associations of trastuzumab administration with pCR or EFS were investigated along with patient and tumor characteristics in univariate and multivariate analyses. Trastuzumab had no significant impact on the pCR rate in univariate (OR, 1.02; 95% CI, 0.48–2.14; P = 0.965) and multivariate (OR, 1.00; 95% CI, 0.45–2.26; P = 0.994) logistic regression analyses (Table 2). Patients treated without or with trastuzumab showed comparable pCR rates (20.0% vs 20.3%; P = 0.965; Figure 2A). In Cox proportional hazards regression analyses, trastuzumab was not significantly associated with EFS in univariate analysis (hazard ratio, 0.97; 95% CI, 0.55–1.73; P = 0.916), and consistently showed no significant influence on EFS in multivariate analysis (hazard ratio, 0.79; 95% CI, 0.43–1.43; P = 0.431) (Table 3). Patients with trastuzumab usage had similar EFS compared with those without trastuzumab (log rank test, P = 0.916; Figure 3A).
 |
Table 2 Associations of pCR with Patient and Tumor Characteristics in Univariate and Multivariate Logistic Regression Analyses |
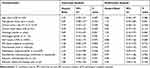 |
Table 3 Associations of EFS with Patient and Tumor Characteristics in Univariate and Multivariate Cox Proportional Hazards Regression Analyses |
Predictive Factors for pCR
The relationship of patient and tumor characteristics with pCR was analyzed in univariate and multivariate logistic regression analyses (Table 2). Univariate analysis suggested that patient’s age along with menopausal status, clinical tumor size, clinical nodal status, tumor histology, Ki67 score, neoadjuvant chemotherapy, and trastuzumab usage showed no significant influence on the pCR rate. Negative HR status (OR, 3.72; 95% CI, 1.74–7.93; P = 0.001) and histological grade 3 (OR, 2.58; 95% CI, 1.22–5.44; P = 0.013) were significantly associated with higher pCR rates. Multivariate analysis confirmed that negative HR status (OR, 5.33; 95% CI, 2.11–13.45; P < 0.001) and histological grade 3 (OR, 3.25; 95% CI, 1.39–7.62; P = 0.007) were independent predictors for pCR.
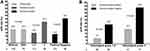
Tumors with negative HR status had a significantly higher pCR rate in comparison with HR-positive disease (35.7% vs 13.0%; P < 0.001; Figure 2A). The pCR rate of histological grade 3 tumors was 31.0%, while tumors with histological grade 1–2 showed a pCR rate of 14.9% (P = 0.012; Figure 2A). We then employed HR status and histological grade as stratified factors for subgroup analysis. Results indicated that the entire population could be categorized into four subgroups showing extremely different pCR rates. Seventeen patients (9.5%) had tumors with negative HR status and histological grade 3, and demonstrated the highest pCR rate of 52.9% (Figure 2B). Eighty-two patients (45.8%) had tumors with positive HR status and histological grade 1–2, and showed the lowest pCR rate of 8.5% (Figure 2B).
Prognostic Factors for EFS
Univariate and multivariate Cox proportional hazards regression analyses were conducted to assess the prognostic value of patient and tumor characteristics on EFS (Table 3). In univariate analysis, patient’s age along with menopausal status, clinical tumor size, clinical nodal status, tumor histology, histological grade, Ki67 score, HR status, trastuzumab administration, neoadjuvant chemotherapy, adjuvant radiotherapy, and adjuvant endocrine therapy were not significantly associated with EFS. However, achieving a pCR had a significant favorable impact on EFS (hazard ratio, 0.35; 95% CI, 0.14–0.89; P = 0.028). In multivariate analysis, pCR was the only independent predictor for improved EFS (hazard ratio, 0.26; 95% CI, 0.09–0.74; P = 0.012). EFS was better among patients with pCR than among those with non-pCR (log rank test, P = 0.022; Figure 3B).
Discussion
The therapeutic efficacy of trastuzumab depends on the adaptive immunity of the immune system, and T lymphocytes function as critical effectors in the adaptive immune response.27,28,40 Early investigations identified T lymphocytes as the main component of TILs in breast cancer.41,42 For clinical utility, recent guidelines have standardized morphological evaluation of TILs based on H&E-stained slides.30,37 In neoadjuvant research using this scoring methodology, a higher infiltrating degree of TILs has been identified and found to be associated with higher pCR rates and better survival in HER2-positive breast cancer.35,43 Nevertheless, the therapeutic effect of trastuzumab in tumors with a low infiltrating level of TILs has not been reported. The present study was conducted to evaluate the therapeutic effect of trastuzumab on the pCR rate and EFS in neoadjuvant-treated HER2-positive disease with a low infiltrating level of TILs.
In the adjuvant FinHER trial, a significant interaction between TILs as a continuous parameter and trastuzumab therapy was reported, and higher infiltrating levels of TILs were found to be associated with increased trastuzumab benefit of survival.44 Further analyses used a cut-off point of 50% to define a clinical subtype with a high immune infiltrate. Tumors with TILs ≥ 50% obtained a significant survival improvement with additional trastuzumab, while trastuzumab usage had no significant impact on survival for those with TILs < 50%.44 On the contrary, in the adjuvant N9831 trial, patients with TILs ≥ 60% did not derive survival benefit from the addition of trastuzumab, whereas those with TILs < 60% showed significantly better survival with additional trastuzumab.45 The associations between the infiltrating level of TILs and trastuzumab benefit remain controversial in HER2-positive breast cancer in the adjuvant setting.
In the neoadjuvant scenario, we found no evidence on the interaction between trastuzumab benefit and the infiltrating level of TILs. To our knowledge, our study is the first that investigated the therapeutic effect of trastuzumab on the pCR rate and EFS in neoadjuvant-treated HER2-positive tumors with a low infiltrating degree of TILs. The contradictory results of the FinHER and N9831 trials revealed the importance of a predefined cut-off point of TILs density in defining a clinical subtype with low immune infiltrate. Tumors with TILs ≥ 50% or 60% have been found to be infrequent and have excellent survival with fewer survival events in HER2-positive breast cancer.32,35,43,45 The survival benefit of trastuzumab might not be significant in survival analyses for tumors with TILs ≥ 50% or 60%. On the other hand, tumors with TILs < 50% or 60% might have enough density of TILs for a trastuzumab effect, and, therefore, TILs < 50% or 60% might be unreasonable for representation of low immune infiltrate. Our study chose a relatively low cut-off point of 10%, and TILs < 10% was defined as a low level of immune infiltrate. Results indicated that the entire population had a pretty low pCR rate of 20.1%, and the pCR rate and EFS were comparable among patients treated with or without trastuzumab.
In predictive and prognostic analyses, trastuzumab administration was not associated with either the pCR rate or EFS. Among parameters in predicting pCR, the infiltrating degree of TILs has been widely accepted as a powerful indicator.32–36,46 However, predictive factors for pCR in HER2-positive tumors with a low infiltrating level of TILs have been unknown. Our findings suggested a significant interaction of pCR with HR status and histological grade. Moreover, we identified a special clinical subtype with positive HR status and histological grade 1–2, which constituted 45.8% of our cohort with a pretty low pCR rate of 8.5%. Further research should focus on this common subtype in tumors with a low infiltrating level of TILs, which showed strong resistance to treatment. For prognostic factors, although the prognostic significance of pCR has been intensively studied,10,12,26 this prognostic impact has not been validated in HER2-positive disease with a low infiltrating level of TILs. Our results indicated that only pCR independently correlated with favorable EFS in this special clinical subtype.
Our study has some limitations. For reasons of health insurance and medical cost, not all patients received trastuzumab in our cohort, and the present study was performed to study the therapeutic efficacy of trastuzumab. However, no patient in our study received pertuzumab, which had not been approved for NAT in our country before our research. Our findings should be carefully applied in HER2-positive breast cancer treated with dual HER2 blockade. Additionally, the small-sample, retrospective design of our study emphasizes the need for further prospective studies with larger populations of patients. Finally, currently, no validated cut-off point of TILs density has been determined in predictive or prognostic studies. The infiltrating level of TILs < 10% was used to define tumors with low immune infiltrate in our study. Nevertheless, further research on the accurate cut-off point of TILs density in defining this special clinical subtype is warranted.
Conclusion
In neoadjuvant-treated HER2-positive breast cancer with a low infiltrating level of TILs (TILs < 10%), the pCR rate and EFS were comparable regardless of whether trastuzumab was administered or not. In this special subgroup, HR-negative status and histological grade 3 were independent predictors for pCR, and pCR was independently associated with the benefit of EFS.
Disclosure
The authors report no conflicts of interest in this work.
References
1. De Lena M, Zucali R, Viganotti G, Valagussa P, Bonadonna G. Combined chemotherapy-radiotherapy approach in locally advanced (T3b-T4) breast cancer. Cancer Chemother Pharmacol. 1978;1(1):53–59. doi:10.1007/bf00253147
2. Karakatsanis A, Tasoulis MK, Wärnberg F, Nilsson G, MacNeill F. Meta-analysis of neoadjuvant therapy and its impact in facilitating breast conservation in operable breast cancer. Br J Surg. 2018;105(5):469–481. doi:10.1002/bjs.10807
3. Provenzano E, Bossuyt V, Viale G, et al. Standardization of pathologic evaluation and reporting of postneoadjuvant specimens in clinical trials of breast cancer: recommendations from an international working group. Mod Pathol. 2015;28(9):1185–1201. doi:10.1038/modpathol.2015.74
4. Zhang M, Wei W, Liu J, et al. Comparison of the effectiveness and toxicity of neoadjuvant chemotherapy regimens, capecitabine/epirubicin/cyclophosphamide vs 5-fluorouracil/epirubicin/cyclophosphamide, followed by adjuvant, capecitabine/docetaxel vs docetaxel, in patients with operable breast cancer. Onco Targets Ther. 2016;9:3443–3450. doi:10.2147/OTT.S104431
5. Wei J, Luo Y, Fu D. Early and late outcomes of bevacizumab plus chemotherapy versus chemotherapy alone as a neoadjuvant treatment in HER2-negative nonmetastatic breast cancer: a meta-analysis of randomized controlled trials. Onco Targets Ther. 2018;11:9049–9059. doi:10.2147/OTT.S186816
6. Ogston KN, Miller ID, Payne S, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003;12(5):320–327. doi:10.1016/s0960-9776(03)00106-1
7. Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25(28):4414–4422. doi:10.1200/JCO.2007.10.6823
8. Mittendorf EA, Vila J, Tucker SL, et al. The neo-bioscore update for staging breast cancer treated with neoadjuvant chemotherapy: incorporation of prognostic biologic factors into staging after treatment. JAMA Oncol. 2016;2(7):929–936. doi:10.1001/jamaoncol.2015.6478
9. Choi M, Park YH, Ahn JS, et al. Assessment of pathologic response and long-term outcome in locally advanced breast cancers after neoadjuvant chemotherapy: comparison of pathologic classification systems. Breast Cancer Res Treat. 2016;160(3):475–489. doi:10.1007/s10549-016-4008-4
10. Minckwitz von G, Untch M, Blohmer J-U, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–1804. doi:10.1200/JCO.2011.38.8595
11. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi:10.1016/S0140-6736(13)62422-8
12. Takada M, Ishiguro H, Nagai S, et al. Survival of HER2-positive primary breast cancer patients treated by neoadjuvant chemotherapy plus trastuzumab: a multicenter retrospective observational study (JBCRG-C03 study). Breast Cancer Res Treat. 2014;145(1):143–153. doi:10.1007/s10549-014-2907-9
13. Masuda N, Lee S-J, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–2159. doi:10.1056/NEJMoa1612645
14. von Minckwitz G, Huang C-S, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–628. doi:10.1056/NEJMoa1814017
15. Zhao Y, Dong X, Li R, et al. Evaluation of the pathological response and prognosis following neoadjuvant chemotherapy in molecular subtypes of breast cancer. Onco Targets Ther. 2015;8:1511–1521. doi:10.2147/OTT.S83243
16. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi:10.1126/science.3798106
17. Wu Z, Xu S, Zhou L, et al. Clinical significance of quantitative HER2 gene amplification as related to its predictive value in breast cancer patients in neoadjuvant setting. Onco Targets Ther. 2018;11:801–808. doi:10.2147/OTT.S157634
18. Chen L, Weng YM, Hu MX, Peng M, Song QB. Effects of HER2 status on the prognosis of male breast cancer: a population-based study. Onco Targets Ther. 2019;12:7251–7260. doi:10.2147/OTT.S209949
19. Loibl S, Gianni L. HER2-positive breast cancer. Lancet. 2017;389(10087):2415–2429. doi:10.1016/S0140-6736(16)32417-5
20. Ma W, Zhao F, Zhou C, et al. Targeted neoadjuvant therapy in the HER-2-positive breast cancer patients: a systematic review and meta-analysis. Onco Targets Ther. 2019;12:379–390. doi:10.2147/OTT.S183304
21. Feng F, Zhang T, Yin F, et al. Efficacy and safety of targeted therapy for metastatic HER2-positive breast cancer in the first-line treatment: a Bayesian network meta-analysis. Onco Targets Ther. 2019;12:959–974. doi:10.2147/OTT.S187739
22. Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. JCO. 2005;23(16):3676–3685. doi:10.1200/JCO.2005.07.032
23. Buzdar AU, Valero V, Ibrahim NK, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13(1):228–233. doi:10.1158/1078-0432.CCR-06-1345
24. Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–384. doi:10.1016/S0140-6736(09)61964-4
25. Zhou P, Jiang Y-Z, Hu X, et al. Clinicopathological characteristics of patients with HER2-positive breast cancer and the efficacy of trastuzumab in the People’s Republic of China. Onco Targets Ther. 2016;9:2287–2295. doi:10.2147/OTT.S97583
26. Broglio KR, Quintana M, Foster M, et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: a meta-analysis. JAMA Oncol. 2016;2(6):751–760. doi:10.1001/jamaoncol.2015.6113
27. Park S, Jiang Z, Mortenson ED, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18(2):160–170. doi:10.1016/j.ccr.2010.06.014
28. Bianchini G, Gianni L. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol. 2014;15(2):e58–e68. doi:10.1016/S1470-2045(13)70477-7
29. Griguolo G, Pascual T, Dieci MV, Guarneri V, Prat A. Interaction of host immunity with HER2-targeted treatment and tumor heterogeneity in HER2-positive breast cancer. J Immunother Cancer. 2019;7(1):90. doi:10.1186/s40425-019-0548-6
30. Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–271. doi:10.1093/annonc/mdu450
31. Savas P, Salgado R, Denkert C, et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nature Publish Group. 2016;13(4):228–241. doi:10.1038/nrclinonc.2015.215
32. Liu S, Duan X, Xu L, et al. Optimal threshold for stromal tumor-infiltrating lymphocytes: its predictive and prognostic value in HER2-positive breast cancer treated with trastuzumab-based neoadjuvant chemotherapy. Breast Cancer Res Treat. 2015;154(2):239–249. doi:10.1007/s10549-015-3617-7
33. Salgado R, Denkert C, Campbell C, et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol. 2015;1(4):448–454. doi:10.1001/jamaoncol.2015.0830
34. Ingold Heppner B, Untch M, Denkert C, et al. Tumor-infiltrating lymphocytes: a predictive and prognostic biomarker in neoadjuvant-treated HER2-positive breast cancer. Clin Cancer Res. 2016;22(23):5747–5754. doi:10.1158/1078-0432.CCR-15-2338
35. Denkert C, Minckwitz von G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. doi:10.1016/S1470-2045(17)30904-X
36. Hwang HW, Jung H, Hyeon J, et al. A nomogram to predict pathologic complete response (pCR) and the value of tumor-infiltrating lymphocytes (TILs) for prediction of response to neoadjuvant chemotherapy (NAC) in breast cancer patients. Breast Cancer Res Treat. 2019;173(2):255–266. doi:10.1007/s10549-018-4981-x
37. Dieci MV, Radosevic-Robin N, Fineberg S, et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: a report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin Cancer Biol. 2018;52(Pt 2):16–25. doi:10.1016/j.semcancer.2017.10.003
38. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 2002;41(3A):154–161.
39. Hammond MEH, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28(16):2784–2795. doi:10.1200/JCO.2009.25.6529
40. Dushyanthen S, Beavis PA, Savas P, et al. Relevance of tumor-infiltrating lymphocytes in breast cancer. BMC Med. 2015;13(1):202. doi:10.1186/s12916-015-0431-3
41. Chin Y, Janseens J, Vandepitte J, Vandenbrande J, Opdebeek L, Raus J. Phenotypic analysis of tumor-infiltrating lymphocytes from human breast cancer. Anticancer Res. 1992;12(5):1463–1466.
42. Whitford P, George WD, Campbell AM. Flow cytometric analysis of tumour infiltrating lymphocyte activation and tumour cell MHC class I and II expression in breast cancer patients. Cancer Lett. 1992;61(2):157–164. doi:10.1016/0304-3835(92)90174-t
43. Stanton SE, Adams S, Disis ML. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol. 2016;2(10):1354–1360. doi:10.1001/jamaoncol.2016.1061
44. Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–1550. doi:10.1093/annonc/mdu112
45. Perez EA, Ballman KV, Tenner KS, et al. Association of stromal tumor-infiltrating lymphocytes with recurrence-free survival in the N9831 adjuvant trial in patients with early-stage HER2-positive breast cancer. JAMA Oncol. 2016;2(1):56–64. doi:10.1001/jamaoncol.2015.3239
46. Ono M, Tsuda H, Shimizu C, et al. Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat. 2011;132(3):793–805. doi:10.1007/s10549-011-1554-7
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.