Back to Journals » Journal of Inflammation Research » Volume 16
Therapeutic Effect of Acupotomy at Sanheyang for Cartilage Collagen Damage in Moderate Knee Osteoarthritis: A Rabbit Model
Authors Li Y, Hou Y, Sun J, Wei J, Chai Y, Guo M, Wang R
Received 15 February 2023
Accepted for publication 2 May 2023
Published 24 May 2023 Volume 2023:16 Pages 2241—2254
DOI https://doi.org/10.2147/JIR.S400956
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yuanyuan Li, Yimin Hou, Jiwei Sun, Jiabi Wei ,† Yemao Chai, Mengwei Guo, Rongguo Wang
School of Acupuncture-Moxibustion and Tuina, Beijing University of Chinese Medicine, Beijing, 100029, People’s Republic of China
†Jiabi Wei passed away on March 21, 2023
Correspondence: Rongguo Wang, School of Acupuncture-Moxibustion and Tuina, Beijing University of Chinese Medicine, No. 11, Bei San Huan Dong Lu, Chaoyang District, Beijing, 100029, People’s Republic of China, Tel +86 18518287139, Email [email protected]
Objective: Acupotomy based on the meridian-sinew theory of traditional Chinese medicine has benefits in treating knee osteoarthritis (KOA). The current study aims to prove that acupotomy at the sinew points of Sanheyang protect the knee joint and alleviate the progression of moderate KOA by evaluating KOA symptoms, cartilage structure, and analyzing the changes of cytokines in rabbit cartilage.
Methods: The model used was mono-iodoacetate-induced moderate KOA in the rabbit’s right leg. Rabbits were divided into the model group, the acupotomy group, and the control group, with each group receiving two parts of treatment for 2 weeks and 4 weeks. We evaluated pain in the knee joint and range of motion. The articular cartilage sections were stained with Safranin O/Fast Green and Masson. We used immunohistochemistry and real-time PCR to detect the protein and mRNA expressions of collagen prototype II (COL-II), matrix metalloproteinase 13 (MMP13), and integrin-β 1 (ITG-β 1).
Results: Compared with the model group, the acupotomy group had higher body weight, lower pain score, higher range of motion, lower Mankin score, and significantly lower protein and mRNA expression of MMP13. After 4 weeks of treatment, Col-II expression in the acupotomy group was significantly higher than that in the model group and the expression of ITG-β 1 in the model group was abnormally increased.
Conclusion: Acupotomy at Sanheyang improved the pain symptoms and range of joint motion in rabbits with moderate KOA, and could protect Col-II by regulating MMP13, which may be related to ITG-β 1-mediated mechanical force transmission, thus reducing the damage to cartilage structure and delaying the progression of moderate KOA.
Keywords: acupotomy, cartilage, gastrocnemius, knee osteoarthritis, sinew
Introduction
The Global Burden of Diseases, Injuries, and Risk Factors Study 2017 (GBD 2017) has reported that the prevalence of knee osteoarthritis is increasing.1 Musculoskeletal disorders are a major contributor to the need for rehabilitation and can benefit from rehabilitation.2 Knee osteoarthritis (KOA) is one of the musculoskeletal diseases where rehabilitation is essential. Acupotomy is found to have a beneficial effect in the treatment of KOA, and can improve joint pain and regulate cartilage degeneration.3–6 However, most KOA treatments using acupotomy focus on the patellar tendon, and emphasize the exercise of the quadriceps muscle.7,8 Gastrocnemius also plays an important role in the stability of the knee joint. Gastrocnemius recession has been shown to improve knee extension with total knee arthroplasty knee flexion contracture.9 Therefore, it is necessary to consider the role of gastrocnemius in KOA. Gastrocnemius is closely related to the musculature bladder meridian in the theory of traditional Chinese medicine (TCM). Hence, in this study, we focused on the therapeutic effect of treating KOA at the sinew points on the musculature bladder meridian.
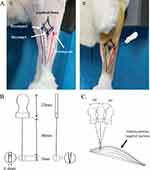
In TCM theory, the “sinew-bone balance” is an important guarantee to maintain the normal function of joints. It is mentioned in Su Wen from the Huang Di Nei Jing that “all sinews belong to the joint” and “the knee is a gathering place of sinews.” Therefore, “imbalance of sinews and bones” is the key pathogenesis of KOA. Damage to either bones or sinews is bound to affect the other. KOA patients have a high proportion of lesions in the musculature bladder meridian, which need to be treated. Therefore, we selected three sinew points on the musculature bladder meridian that are located on the gastrocnemius and near the knee joint, namely Heyangci, Heyangnei, and Heyangwai, collectively referred to as Sanheyang. This experiment studied the therapeutic effect of acupotomy at Sanheyang in rabbits with moderate KOA. The rabbit model of knee osteoarthritis was set up using the mono-iodoacetate (MIA) method.10 Since this study mainly focuses on the structural damage of cartilage and articular pain, the histological and morphological changes of knee joint caused by MIA model are similar to those of human osteoarthritis and it is suitable for the study of both aspects.11
KOA often results in cartilage wear and degeneration. When articular cartilage is damaged, collagen prototype II (COL-II), the most important collagen in cartilage, is reduced.12,13 Matrix metalloproteinase 13 (MMP13) in the cartilage matrix is a key enzyme in the decomposition of Col-II.14,15 The changes in Col-II and MMP13 are observed to explore the degree of cartilage degeneration. In addition, integrin has been proved to be a mechanical sensitive factor, and chondrocyte surface contains more integrin heterodimers that contain β1 subunit.16,17 The changes of ITG-β1 often reveal the abnormal stress of articular cartilage. In this study, we observed whether the acupotomy treatment done at Sanheyang could relieve joint pain and improve the range of joint motion in rabbits with KOA. The present study also sought to investigate the cartilage morphology and the changes in Col-II, MMP13, and ITG-β1 in the joint, to determine whether the treatment had a certain protective effect on the knee cartilage.
Materials and Methods
Experimental Design
The experiment was approved by the Animal Ethics Committee of the Beijing University of Chinese Medicine (Ethics Reference No. BUCM – 4 – 2020112003 - 4070). The animals were raised in the Experimental Animal Center of Liangxiang Campus, Institute of Traditional Chinese Medicine, Beijing University of Chinese Medicine. In this research, 36 male New Zealand white rabbits (six months old) were randomly divided into three groups: the model group, the acupotomy group, and the control group. The rabbits in the model group and acupotomy group were administered MIA (Sigma, St. Louis, USA) at the knee joint. All the rabbits were fed flexibly for a week and had free access to food and water. Rabbits in the model group and acupotomy group were injected with 0.2 mL of MIA solution in the right knee. Rabbits in the control group were injected with 0.2 mL normal saline. Two weeks after modeling, six rabbits from each group were randomly selected for two weeks of treatment and another six rabbits were selected for four weeks of treatment. Only the acupotomy group received acupotomy treatment, while the model and control groups underwent the same fixation and grasping procedure. All the rabbits were sacrificed after treatment, at two weeks (each group: n = 6) and 4 weeks (each group: n = 6), respectively. In each of the following specific experiments, the number of rabbits in each group was 6 in each time period.
Rabbit Model of KOA
Rabbits were anesthetized with intramuscular injection of Zoletil®50 (1 mL/kg) in the left gluteus maximus. The MIA solution used for the model group and acupotomy group was made by dissolving MIA with 0.2 mL normal saline at 2.5 mg/kg. After being anesthetized completely, the rabbits were placed in the supine position and the hair at the right knee joint was shaved. The knee joint was flexed to around 60°. The knee joint area was disinfected with iodophor, and a surgical hole towel was placed. The needle was inserted from the depression below the lateral patellar ligament at an angle of about 45° between the needle and the femoral extension line, and the MIA solution was injected into the knee joint cavity. The area was pressed with a cotton ball for 2 minutes to stop bleeding. The rabbit knee joint was gently passively flexed and extended 10 times, so that the solution infiltrated into the knee cavity. For 2 weeks after modeling, rabbits were made to run for 30 minutes every day; the feeding and other conditions remained unchanged.
Acupotomy Therapy
The rabbits in the acupotomy group received acupotomy therapy every three days. During the treatment, the rabbits were fixed in the prone position in the rabbit-fixture, the hair in the popliteal fossa at the rear of the right knee joint and right posterior calf was shaved, and the entire area was disinfected with iodophor. The three sinew points in the musculature bladder meridian (Heyangci: at the back of the calf, below the midpoint of the lower margin of the popliteal fossa, at the level of the lower margin of the flat fibular head; Heyangnei: at the posterior side of the calf, inside and above the Heyangci, the lower margin of the popliteal fossa; Heyangwai: at the back of the calf, the lower margin of the popliteal fossa, the inside of the fibula capitula) (Figure 1A①) were located by palpation of the calf. The operator wore sterile gloves and used a disposable sterile acupotomy needle-knife (0.4 × 40 mm, Jiangsu Huayou Medical Devices Co Ltd) for the treatment. The blade was run parallel to the gastrocnemius muscle, and the acupotomy needle-knife was inserted vertically (Figure 1A② and B). After the needle-knife entered about 5 mm of the muscle layer, the sinew points were dredged longitudinally thrice (Figure 1C). After the procedure, the area was pressed with a sterile cotton ball for 1 min to stop bleeding. All the rabbits convalesced in the cage under the same conditions as before and they were not made to run as in the modeling phase.
Behavioral Assessment
We used electronic weighing scales to measure the weight of the rabbits before and after modeling and after treatment. After the treatment, pain scores were measured according to the assessment criteria of the modified Lequesne index18 (criteria for evaluation: 0 points for no pain; 1 point for only after walking some distance; 2 points for early after initial walking and increasingly with continued walking; 3 points for after initial walking, not increasingly). The pain score was calculated by averaging the scores evaluated by two researchers. All rabbits were anesthetized in the same manner as modeling prior to sampling. After complete anesthesia, the rabbits were placed in the left decubitus position. One researcher fixed the hip joint and passively moved the right knee joint, while the other researcher measured and recorded the maximum range of knee joint motion angle using a digital protractor (2176-200, Insize). The joint motion angle was measured three consecutive times and recorded, and the average value was taken for statistical calculation. The researchers who measured the experimental data were blind to the present study.
Histopathological Analysis
Rabbits were sacrificed by injecting Zoletil®50 (3 mL/kg) into the ear vein. The lateral condyle of the femur of the right knee was fixed with 4% paraformaldehyde for 72 h, and then put into EDTA decalcifying solution (PH 7.2, E1171, Solarbio) until the decalcification was complete. After decalcification, the femoral condyle was amputated, and paraffin embedded for sagittal observation. The section thickness was set to 6 μm.
The sections were stained with Safranin O/Fast Green (G1371, Solarbio) and the Mankin score19 was calculated after observation to evaluate the cartilage injury of the right knee joint. Masson (G1340, Solarbio) staining was used to observe collagen in cartilage, and Image Pro Plus 6.0 (IPP 6.0) was used to calculate the area of blue staining region in cartilage. Two researchers evaluated the scores and the calculation of the blue staining area, and the average values were taken for statistical analysis.
Immunohistochemical Analysis
All immunohistochemical sections were heated at 65°C for 1 h. After dewaxing and rehydration, sodium citrate antigen repair solution was used for antigen repair in a 98°C water bath for 25 min. They were rinsed with Phosphate Buffer Saline (PBS) thrice, for 5 min each time. The primary antibody (Col-II: Proteintech Cat# 28459-1-AP, RRID: AB_2881147; MMP 13: Proteintech Cat# 18165-1-AP, RRID: AB_2144858; ITG-β1: Bioss Cat# bs-0486R, RRID: AB_10856339) was incubated at 4 °C overnight, then rewarmed at room temperature for 1 h and rinsed with PBS. The second antibody (ZSGB-Bio Cat# PV-6001, RRID: AB_2864333) was incubated at 37 °C for 25 min. After rinsing with PBS, DAB (DA1010, Solarbio) was stained for 7 min. Finally, the nuclei were stained with hematoxylin for 5 min and differentiated for 10s. After gradient alcohol and xylene, the tablets were sealed with neutral gum. For each section, the cartilage area at tibiofemoral joint was selected for 40X field observation under the microscope. IPP 6.0 was used to calculate the sum of integrated optical density (IOD) of Col-II and MMP13 and the average optical density (AOD) of ITG. The mean values of the results were calculated by two experimenters.
Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
The medial femoral condylar cartilage of the rabbit’s right leg was taken, and total mRNA was extracted with Trizol (15596026, Ambion, America). Total mRNA was reversely transcribed into cDNA using the RevertAid First Strand cDNA Synthesis Kit (K1622, Thermo Scientific, America). Finally, RT-qPCR was performed thrice using the CFX ConnectTM fluorescence quantitative PCR instrument with the PowerUpTM SYBRTM Green Master Mix (A25742, Thermo Scientific, America). The sequences of gene primers (Sangon Biotech Co., Ltd. Shanghai) used for measurement are shown in Table 1. Glyceraldehyde 3-phosphate dehydrogenase mRNA was used as an endogenous control. Relative transcript levels of mRNA were reported using the 2−ΔΔCT method.
 |
Table 1 The Gene Primers |
Statistical Analysis
Statistical analysis was done using IBM SPSS 20.0 (IBM, Chicago, IL, USA) software. Measurement data are expressed as mean ± standard deviation (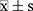 ). If the data conformed to the normal distribution and the homogeneity of variance, the two groups of data were compared by t-test, and the multiple groups of data were analyzed by one-way ANOVA with least significant difference (LSD) test. Otherwise, the two groups of data were compared by Mann–Whitney test, and the multiple groups of data were analyzed by independent-samples Kruskal–Wallis test. P < 0.05 was considered to be statistically significant.
). If the data conformed to the normal distribution and the homogeneity of variance, the two groups of data were compared by t-test, and the multiple groups of data were analyzed by one-way ANOVA with least significant difference (LSD) test. Otherwise, the two groups of data were compared by Mann–Whitney test, and the multiple groups of data were analyzed by independent-samples Kruskal–Wallis test. P < 0.05 was considered to be statistically significant.
Results
Acupotomy at Sanheyang Improved Joint Pain and Range of Motion in Rabbits with KOA
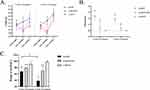
There was no statistical difference in the initial body weight (Figure 2A) of the rabbits between each group at each time point. The rabbits in the acupotomy group and model group lost weight after modeling, but there was no statistical difference in the degree of weight loss. However, the body weight of the rabbits in the control group increased and the degree of weight gain was significantly higher than that of the acupotomy group (2/4 weeks of treatment: P < 0.0001) and model group (2/4 weeks of treatment: P < 0.0001). From completion of modeling to the end of the treatment, the weight of rabbits in all groups increased. But the weight gain of rabbits in the acupotomy group was significantly higher than that of the control (2 weeks of treatment: P < 0.0001; 4 weeks of treatment: P = 0.002) and model (2 weeks of treatment: P < 0.0001; 4 weeks of treatment: P = 0.038) groups, and there was no significant difference between the control and model groups. This was seen in both treatment durations. In addition, we compared the body weight before modeling and after treatment with the two treatment durations, and found that the weight gain of the model group was significantly lesser than that of the control group (2 weeks of treatment: P < 0.0001; 4 weeks of treatment: P = 0.0003) and the acupotomy group (2/4 weeks of treatment: P < 0.0001). However, the weight gain of the control group was significantly higher than that of the acupotomy group after 2 weeks of treatment (P = 0.035), while there was no significant difference between the two groups after 4 weeks of treatment. With respect to the pain score (Figure 2B), the scores in the model group were higher than the acupotomy group (2 weeks of treatment: P = 0.037; 4 weeks of treatment: P = 0.038) and the control group (2 weeks of treatment: P = 0.002; 4 weeks of treatment: P = 0.001) during the same period, but there was no statistical difference between the acupotomy group and the control group.
After 2 weeks and 4 weeks of treatment, the range of motion (Figure 2C) in the control group was significantly higher than that of the acupotomy group (2 weeks of treatment: P = 0.025; 4 weeks of treatment: P < 0.0001) and the model group (2 weeks of treatment: P = 0.0002; 4 weeks of treatment: P < 0.0001), while the degree of joint movement in the acupotomy group was significantly higher than that of the model group (2 weeks of treatment: P = 0.034; 4 weeks of treatment: P < 0.0001). There was no difference in range of motion between the control group after 2 weeks of treatment and after 4 weeks of treatment. The same situation was observed in the acupotomy group. The range of motion in the model group after 4 weeks of treatment was significantly lower than that in the model group for 2 weeks (P = 0.0005).
Changes Were Observed in the Articular Cartilage
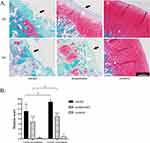
By observing the Safranin O/Fast Green-stained sections (Figure 3A), we found that the thickness of cartilage in all control groups was normal, the surface was regular, the chondrocytes were evenly arranged, the matrix was uniformly red stained, and the tide line was clearly visible. After 2 weeks of treatment, the model group showed cartilage layer wear, irregular surface, chondrocytes were few and clustered, the fading degree of matrix was more serious, and tide lines were barely discernible. Compared with the model group, the acupotomy group retained more cartilage, the surface was relatively flat, the matrix was less faded, and the tide line basically disappeared. After 4 weeks of treatment, the model group cartilage wear was more severe, the chondrocytes were disordered, the matrix fading was severe, and the tide line disappeared. While the cartilage layer was preserved more and the red staining of the matrix remained more in the acupotomy group, the arrangement of cells was chaotic, and the tide line disappeared. After the completion of the treatment stage, the Mankin score of the model group was significantly higher than that of the acupotomy group (2 weeks of treatment: P = 0.001; 4 weeks of treatment: P < 0.0001) and the control group (2/4 weeks of treatment: P < 0.0001), and the score of the acupotomy group was significantly higher than that of the control group (2 weeks of treatment: P = 0.001; 4 weeks of treatment: P < 0.0001) (Figure 3B).
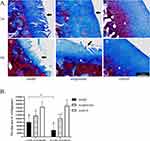
Masson staining (Figure 4A) showed that the cartilage of the model group and acupotomy group was severely worn and the blue staining was lighter than that of the control group in the corresponding period. The cartilage wear of the model group was more serious than that of acupotomy group. By analyzing and comparing the blue staining area of the cartilage in each group with Masson staining (Figure 4B), we found that at the corresponding period the blue stain area in the control group was significantly larger than that in the acupotomy group (2 weeks of treatment: P = 0.011; 4 weeks of treatment: P = 0.0004) and model group (2 weeks of treatment: P = 0.0002; 4 weeks of treatment: P < 0.0001), while the blue stain area in the acupotomy group was only significantly larger than that in the model group only after 4 weeks of treatment (P = 0.0004). Compared with the two time points, only the blue staining area of the model group decreased significantly (P = 0.025).
More Retention of Col-II and Higher mRNA Expression of Col-II in MIA Rabbit Cartilage After 4 Weeks of Treatment
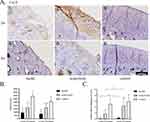
Irrespective of 2 weeks or 4 weeks of treatment, the immunohistochemistry showed that the sum of IOD of Col-II (Figure 5A and B) was significantly higher in the control group than in the acupotomy group (2 weeks of treatment: P = 0.0004; 4 weeks of treatment: P = 0.009) and the model group (2 weeks of treatment: P < 0.0001; 4 weeks of treatment: P < 0.0001). It was significantly higher in the acupotomy group than in the model group after 4 weeks of treatment (P = 0.012), but there was no significant difference after 2 weeks of treatment.
While the mRNA expression of COL-II (Figure 5C) in the model group was significantly lower than that in control group (2 weeks of treatment: P < 0.0001; 4 weeks of treatment: P = 0.002) during the same period. But there was no statistically significant difference when comparing the mRNA expression of COL-II in the acupotomy group with the model group and control group at both points in time. The mRNA expression of COL-II in the acupotomy group was significantly higher than that in the model group only after 4 weeks of treatment (P = 0.045). Comparing the mRNA level after 2 weeks with 4 weeks of treatment, the Col-II mRNA in the model group (P = 0.041) and the acupotomy group (P = 0.031) were significantly increased.
The Treatment Reduced the Abnormally High Expression of MMP 13 and Corresponding mRNA in KOA Rabbits
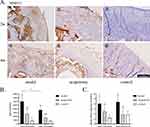
In the same treatment phase, the sum of IOD of MMP 13 (Figure 6A and B) in the model group was significantly higher than that in the acupotomy group (2 weeks of treatment: P = 0.001; 4 weeks of treatment: P = 0.0004) and the control group (2/4 weeks of treatment: P < 0.0001), while it was significantly higher in the acupotomy group than in the control group (2 weeks of treatment: P = 0.04; 4 weeks of treatment: P = 0.04).
RT-qPCR experiments showed the mRNA expression of MMP 13 (Figure 6C) in the model group was significantly higher than that in the control group (2 weeks of treatment: P = 0.001; 4 weeks of treatment: P = 0.003), whereas there was no significant difference in MMP 13 mRNA expression between the acupotomy group and control group. Compared with the model group, the mRNA expression of MMP 13 was significantly lower in the acupotomy group (2 weeks of treatment: P = 0.036; 4 weeks of treatment: P = 0.003).
ITG-β1 and Corresponding mRNA of KOA Rabbits Increased at 4 Weeks After Successful Modeling
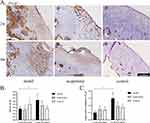
There was no significant difference in the AOD of the ITG-β1 in each group after 2 weeks of treatment, whereas after 4 weeks of treatment, the AOD of the integrin β1 in the model group was significantly higher than that in the acupotomy group (P = 0.032) and the control group (P = 0.003), but there was no significant difference in the expression between the acupotomy group and the control group. The AOD of the model group at 4 weeks was significantly higher than that at 2 weeks (P = 0.005), see Figure 7.
There was no significant difference in the mRNA expression of ITG-β1 among the three groups after 2 weeks of treatment. But the mRNA expression of ITG-β1 in the model group was significantly higher than that in the acupotomy group (P = 0.002) and the control group (P = 0.001) after 4 weeks of treatment. The mRNA expression of the model group was also higher at 4 weeks than at 2 weeks of treatment (P = 0.0004).
Discussion
Osteoarthritis is a multi-factor degenerative disease, and recovery from cartilage damage is difficult. Therefore, delaying the progression of the disease and carrying out therapeutic intervention for patients as soon as possible are the keys to protect cartilage.20 Studies have shown that high knee extensor strength did not protect against the development of symptomatic KOA.21 It has been shown that gastrocnemius activation affects knee adduction moment in patients with KOA and gastrocnemius properties are also affected by foot posture in patients with KOA.22,23 Therefore, it is important to pay attention to the role of the flexor calf muscle in maintaining the stability of the knee joint. Research has shown that patients with more severe KOA are more likely to have changes in the Achilles tendon, which may be related to reduced gastrocnemius strength.24 The conventional acupotomy therapy for KOA often focuses on the release of the ligament and tendon around the patella. However, the role of the flexor calf muscle in maintaining the stability of the knee joint should also be emphasized. As per the theory of “sinew-bone balance” in TCM, we treated bone disease from the sinews. This study focused on the sinew points located on the musculature bladder meridian and on the gastrocnemius. We found that acupotomy on the Sanheyang could delay the progression of arthritis and relieve pain in KOA rabbits.
There is evidence that the body weight of rabbits changes in response to pain.25,26 In this study, we used the change in body weight along with the pain score to evaluate KOA pain symptoms in rabbits. The knee joint pain was obvious after the injection of MIA-induced KOA in rabbits. The degree of joint pain could be alleviated by releasing Sanheyang with acupotomy. After 2 weeks of treatment, the rabbit body weight of the acupotomy group did not return to the level of the control group but could reach the level of the control group after 4 weeks of treatment, while the weight of the model group did not recover to the level of the control group. Research has shown that gastrocnemius recession can relieve knee flexion contracture.9 The rabbits in the acupotomy group also showed improvement in range of motion. This study suggests that this treatment improved the pain symptoms and joint motion limitation caused by arthritis in KOA rabbits, and the range of joint motion could have been more restricted without treatment.
Observation of the cartilage structure showed that the femoral condylar cartilage of rabbits in the model group was severely worn and reached the level of moderate KOA, while the degree of cartilage damage in the acupotomy group was significantly lesser, and more of the matrix red staining was retained. The cartilage structure damage of both groups further aggravated over time. Col-II and collagenase MMP13 are the indicators that show the structure and destruction of articular cartilage better, so we chose to detect these 2 typical indicators in this study to reveal the therapeutic effect of acupotomy on KOA. The protein and mRNA expressions of Col-II in the cartilage were significantly higher in the acupotomy group than in the model group only after 4 weeks of treatment, which was consistent with the blue staining area of the cartilage shown by Masson staining. However, the protein and mRNA expression of MMP 13 were abnormally increased in both time periods in the model group, while the expression levels of MMP 13 were significantly lower in the acupotomy group. After treatment, the improvement in MMP 13 appeared earlier while the improvement in collagen was relatively late. This suggests that acupotomy at Sanheyang could inhibit the expression of MMP 13 and further ensure the retention of more COL-II in cartilage.
In this study, we also found that the Col-II mRNA expression in KOA rabbits increased significantly after 4 weeks of treatment than after 2 weeks, but the IOD value did not change. However, the IOD results showed that MMP 13 expression in the model group decreased after 4 weeks compared with 2 weeks, while the mRNA expression showed no significant difference. We hypothesize that increased MMP13 expression damages COL-II during KOA, while articular cartilage tends to increase Col-II expression to compensate for cartilage injury. It can be observed that MMP 13 expression at mRNA level was always higher than normal during the progression of KOA, which caused the cartilage collagen to continuously decompose and cartilage to be further damaged. The total amount of MMP13 protein in cartilage decreased when the cartilage layer became thinner and worn. Similarly, the total amount of COL-II protein did not increase due to cartilage loss. In conclusion, the IOD value of the corresponding protein expression was also related to the retention of chondrocyte and cartilage matrix.
At the end of 4 weeks of treatment, we observed an abnormal increase in ITG-β1 in the model group. In vitro studies found that arthritic chondrocytes increased ITG-β1.27 Studies have also shown that ITG-β1 mediates the adhesion of chondrocytes to cartilage and blocking ITG-β1 significantly increases cell detachment from cartilage.28,29 This suggests that the expression of ITG-β1 in cartilage should be maintained at a certain level. ITG-β1 is an indispensable part of sensing mechanical forces and conducting subordinate responses, but its excessive increase may lead to further cartilage damage. In this research, after 4 weeks of treatment, the model group showed an abnormal increase in ITG-β1, while the acupotomy group maintained the same level as the control group. This suggests that our treatment could maintain ITG-β1 levels relatively equal to those of normal cartilage. A study has demonstrated that ITG-β1 chondrocyte occurs in metabolically active chondrocytes based on cell cycle analysis, which showed a significantly high percentage of active chondrocytes (S phase) in the maximum-damaged zone.30 This suggested that the articular cartilage of KOA rabbits in the model group was in a state of massive cartilage damage accompanied by active metabolism of chondrocytes after 4 weeks of treatment, so the abnormal increase of ITG-β1 could be detected. The degree of articular cartilage injury in acupotomy group was lighter, and the metabolic state of cells was relatively stable, so there was no abnormal expression of ITG-β1. We could not determine whether the abnormal increase in ITG in the model group was a response to the body’s attempt to repair cartilage and whether this response accelerated the progression of arthritis. Specific experiments are needed to further explore this issue.
Moreover, studies have shown that integrins combine with extracellular matrix ligands on the outside of chondrocytes to form focal adhesion complexes, selectively converting the response force signals into the cells, and in turn transmitting the force signals experienced in the cells to the extracellular matrix.31 Focal adhesion complex can activate focal adhesion kinase (FAK) and then activate a series of downstream signaling molecules mediated by it, among which FAK has been confirmed to activate the mitogen-activated protein kinase (MAPK) pathway.32 The activation of p38MAPK signaling pathway in MAPK signal transduction pathway plays a key role in inducing MMP13 expression.33 Therefore, the high expression of ITG-β1 in KOA rabbits in the model group may induce the excessive expression of MMP13 through FAK-p38MAPK pathway, resulting in the decomposition of Col-II, and eventually leading to the continuous aggravation of cartilage damage. Acupotomy at “Sanheyang” can prevent the over-expression of ITG-β1 by adjusting the mechanical balance of sinews and bones and may inhibit activation of FAK-p38MAPK pathway, thereby reducing MMP13 expression and Col-II loss.
KOA is a chronic injury disease affecting the quality of life of patients. Acupotomy has certain advantages in treating KOA due to higher acceptability and less pain.34,35 KOA is known to be associated with flexion contracture, and unilateral flexion contracture adversely affects the contralateral knee joint.36–38 We found in our experiment that the range of motion of the knee reduced in KOA rabbits. Therefore, it is necessary to relieve the contracture state. The musculature bladder meridian is described as passing behind the knee and treating the popliteal fossa contracture in TCM. This function is performed by the Sanheyang located on the musculature bladder meridian and on the gastrocnemius, which is an important flexor. In addition to the direct release of muscles, acupotomy treatment at Sanheyang can also improve the knee joint function through meridian-sinew and plays a role in improving various factors in the cartilage. The changes of ITG-β1 in the results of this study suggest that the cartilage in rabbits with KOA was abnormally stressed, and acupotomy treatment could regulate the abnormal expression of ITG-β1. This study demonstrated that acupotomy at Sanheyang could reduce the expression of MMP 13 in moderate KOA to protect Col-II as much as possible and slow the progression of moderate KOA to a more aggressive form by regulating the mechanical state of the knee joint. At the same time, our study showed that electroacupuncture and acupuncture improved pain and joint function, which was partly mediated by reduction in MMP 13.39 Likewise, this treatment of acupotomy also showed a reduction in MMP 13, as well as an alleviation in moderate KOA pain and joint limitation. This suggests that acupotomy therapy could delay the progression of moderate KOA by solely targeting the sinews of the musculature bladder meridian on the gastrocnemius.
Conclusion
Acupotomy at Sanheyang protected Col-II in cartilage by reducing MMP 13 expression in KOA and protected the cartilage structure to a certain extent, to relieve joint pain and improve restricted joint mobility. A probable explanation is that the process of this acupotomy treatment is related to the conduction of mechanical force and improving the abnormal stress state of the knee joint, and ITG-β1 may play a role in this. Our results provide evidence that acupotomy at the sinew points on the gastrocnemius can delay the progression of moderate KOA, and this can contribute to the prevention and treatment of moderate KOA from multiple aspects.
Data Sharing Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request. We declared that materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality.
Ethics Approval and Consent to Participate
The experiment was approved by the Animal Ethics Committee at Beijing University of Chinese Medicine (Ethics Reference No. BUCM – 4 – 2020112003 - 4070). Animal models of disease are subject to the 3Rs of Responsible Research,40 that is: Replace animal experiments with alternatives where possible, Reduce the number of animals used to a scientifically justified minimum and Refine the procedure to minimize animal harm.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding
This work was supported by The Fundamental Research Funds for the Central Universities (No.2020-JYB-ZDGG-064).
Disclosure
The authors declare that they have no competing interests.
References
1. Disease G B D, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi:10.1016/S0140-6736(18)32279-7
2. Cieza A, Causey K, Kamenov K, et al. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2021;396(10267):2006–2017. doi:10.1016/S0140-6736(20)32340-0
3. Lin M, Li X, Liang W, et al. Needle-knife therapy improves the clinical symptoms of knee osteoarthritis by inhibiting the expression of inflammatory cytokines. Exp Ther Med. 2014;7(4):835–842. doi:10.3892/etm.2014.1516
4. Yang YH, Liu TH, Zhang LD, et al. Role of the PERK-eIF2 α -CHOP signaling pathway in the effect of needle knife therapy on knee joint chondrocyte apoptosis. Evid Based Complement Alternat Med. 2019;2019:7164916. doi:10.1155/2019/7164916
5. Zeng GG, Zhang XF, Quan WC, et al. 针刀松解术对膝骨性关节炎局部软组织张力及疼痛的影响 [Effects of needle knife relaxing therapy on tension of local soft tissue and pain of osteoarthritis of knee]. Zhongguo Zhen Jiu. 2008;28(4):244–247. Chinese.
6. Ding Y, Shi X, Wang L, et al. Acupotomy versus sodium hyaluronate for treatment of knee osteoarthritis in rabbits. J Tradit Chin Med. 2017;37(3):404–411. doi:10.1016/S0254-6272(17)30078-X
7. Xu D, Lee M, Huang C, et al. Effect of acupotomy in knee osteoarthritis patients: study protocol for a randomized controlled trial. Trials. 2021;22(1):295. doi:10.1186/s13063-021-05247-z
8. Xie Y, Zhang C, Jiang W, et al. Quadriceps combined with hip abductor strengthening versus quadriceps strengthening in treating knee osteoarthritis: a study protocol for a randomized controlled trial. BMC Musculoskelet Disord. 2018;19(1):147. doi:10.1186/s12891-018-2041-7
9. Rocco J, Putzer D, Nogler M, et al. The effect of gastrocnemius resection on knee flexion in a total knee arthroplasty model. Arch Orthop Trauma Surg. 2021;142(10):2503–2511. doi:10.1007/s00402-020-03695-x
10. Guingamp C, Gegout-Pottie P, Philippe L, et al. Mono-iodoacetate-induced experimental osteoarthritis: a dose-response study of loss of mobility, morphology, and biochemistry. Arthritis Rheum. 1997;40(9):1670–1679. doi:10.1002/art.1780400917
11. Lampropoulou-Adamidou K, Lelovas P, Karadimas EV, et al. Useful animal models for the research of osteoarthritis. Eur J Orthop Surg Traumatol. 2014;24(3):263–271. doi:10.1007/s00590-013-1205-2
12. Luo Y, Sinkeviciute D, He Y, et al. The minor collagens in articular cartilage. Protein Cell. 2017;8(8):560–572. doi:10.1007/s13238-017-0377-7
13. Hollander AP, Pidoux I, Reiner A, et al. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995;96(6):2859–2869. doi:10.1172/JCI118357
14. Li H, Wang D, Yuan Y, et al. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res Ther. 2017;19(1):248. doi:10.1186/s13075-017-1454-2
15. Knauper V, Lopez-Otin C, Smith B, et al. Biochemical characterization of human collagenase-3. J Biol Chem. 1996;271(3):1544–1550. doi:10.1074/jbc.271.3.1544
16. Loeser RF. Chondrocyte integrin expression and function. Biorheology. 2000;37(1–2):109–116.
17. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. doi:10.1016/S0092-8674(02)00971-6
18. Xie F, Thumboo J, Lo N-N, et al. Cross-cultural adaptation and validation of Singapore English and Chinese versions of the Lequesne Algofunctional Index of knee in Asians with knee osteoarthritis in Singapore. Osteoarthritis Cartilage. 2007;15(1):19–26. doi:10.1016/j.joca.2006.06.013
19. Mankin HJ, Lippiello L. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. J Bone Joint Surg Am. 1970;52(3):424–434. doi:10.2106/00004623-197052030-00002
20. Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376–387. doi:10.1016/S0140-6736(14)60802-3
21. Thorlund JB, Felson DT, Segal NA, et al. Effect of knee extensor strength on incident radiographic and symptomatic knee osteoarthritis in individuals with meniscal pathology: data from the multicenter osteoarthritis study. Arthritis Care Res. 2016;68(11):1640–1646. doi:10.1002/acr.22889
22. Booij MJ, Richards R, Harlaar J, et al. Effect of walking with a modified gait on activation patterns of the knee spanning muscles in people with medial knee osteoarthritis. Knee. 2020;27(1):198–206. doi:10.1016/j.knee.2019.10.006
23. Chen Z, Ye X, Shen Z, et al. Comparison of the asymmetries in foot posture and properties of gastrocnemius muscle and achilles tendon between patients with unilateral and bilateral knee osteoarthritis. Front Bioeng Biotechnol. 2021;9:636571. doi:10.3389/fbioe.2021.636571
24. Elbaz A, Magram-Flohr I, Segal G, et al. Association between knee osteoarthritis and functional changes in ankle joint and achilles tendon. J Foot Ankle Surg. 2017;56(2):238–241. doi:10.1053/j.jfas.2016.11.017
25. Goldschlager GB, Gillespie VL, Palme R, et al. Effects of multimodal analgesia with LowDose buprenorphine and meloxicam on fecal glucocorticoid metabolites after surgery in New Zealand white rabbits (Oryctolagus cuniculus). J Am Assoc Lab Anim Sci. 2013;52(5):571–576.
26. Divincenti L, Meirelles LA, Westcott RA. Safety and clinical effectiveness of a compounded sustained-release formulation of buprenorphine for postoperative analgesia in New Zealand White rabbits. J Am Vet Med Assoc. 2016;248(7):795–801. doi:10.2460/javma.248.7.795
27. Loeser RF, Carlson CS, Mcgee MP. Expression of beta 1 integrins by cultured articular chondrocytes and in osteoarthritic cartilage. Exp Cell Res. 1995;217(2):248–257. doi:10.1006/excr.1995.1084
28. Kurtis MS, Tu BP, Gaya OA, et al. Mechanisms of chondrocyte adhesion to cartilage: role of beta1-integrins, CD44, and annexin V. J Orthop Res. 2001;19(6):1122–1130. doi:10.1016/S0736-0266(01)00051-1
29. Kurtis MS, Schmidt TA, Bugbee WD, et al. Integrin-mediated adhesion of human articular chondrocytes to cartilage. Arthritis Rheum. 2003;48(1):110–118. doi:10.1002/art.10704
30. Lapadula G, Iannone F, Zuccaro C, et al. Chondrocyte phenotyping in human osteoarthritis. Clin Rheumatol. 1998;17(2):99–104. doi:10.1007/BF01452253
31. Chakraborty S, Banerjee S, Raina M, et al. Force-directed “mechanointeractome” of talin-integrin. Biochemistry. 2019;58(47):4677–4695. doi:10.1021/acs.biochem.9b00442
32. Sun Z, Guo SS, Fassler R. Integrin-mediated mechanotransduction. J Cell Biol. 2016;215(4):445–456. doi:10.1083/jcb.201609037
33. Lim H, Kim HP. Matrix metalloproteinase-13 expression in IL-1beta-treated chondrocytes by activation of the p38 MAPK/c-Fos/AP-1 and JAK/STAT pathways. Arch Pharm Res. 2011;34(1):109–117. doi:10.1007/s12272-011-0113-4
34. Fang T, Li Q, Zhou F, et al. Effect and safety of acupotomy in treatment of knee osteoarthritis: a systematic review and Meta-analysis. J Tradit Chin Med. 2020;40(3):355–364. doi:10.19852/j.cnki.jtcm.2020.03.002
35. Hua Z, Deng H, Tang H, et al. Clinical study of acupotomy for knee osteoarthritis based on the meridian-sinew theory: a randomized controlled clinical trial. Evid Based Complement Alternat Med. 2021;2021:3987002. doi:10.1155/2021/3987002
36. Campbell TM, Ramsay T, Trudel G. Knee flexion contractures are associated with worse pain, stiffness, and function in patients with knee osteoarthritis: data from the osteoarthritis initiative. PM R. 2021;13(9):954–961. doi:10.1002/pmrj.12497
37. Campbell TM, Mcgonagle D. Flexion contracture is a risk factor for knee osteoarthritis incidence, progression and earlier arthroplasty: data from the osteoarthritis initiative. Ann Phys Rehabil Med. 2021;64(2):101439. doi:10.1016/j.rehab.2020.09.005
38. Campbell TM, Trudel G. Knee flexion contracture associated with a contracture and worse function of the contralateral knee: data from the osteoarthritis initiative. Arch Phys Med Rehabil. 2020;101(4):624–632. doi:10.1016/j.apmr.2019.11.018
39. Shi GX, Tu JF, Wang TQ, et al. Effect of Electro-Acupuncture (EA) and Manual Acupuncture (MA) on markers of inflammation in knee osteoarthritis. J Pain Res. 2020;13:2171–2179. doi:10.2147/JPR.S256950
40. Russell W, Burch R. The Principles of Humane Experimental Technique. Hertfordshire: UFAW Publications; 1959.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.