Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Therapeutic Dose of Amitriptyline for Older Patients with Burning Mouth Syndrome
Authors Suga T , Takenoshita M, Watanabe T , Tu TTH , Mikuzuki L , Hong C, Miura K , Yoshikawa T, Nagamine T , Toyofuku A
Received 22 October 2019
Accepted for publication 13 December 2019
Published 30 December 2019 Volume 2019:15 Pages 3599—3607
DOI https://doi.org/10.2147/NDT.S235669
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Taro Kishi
Takayuki Suga,1 Miho Takenoshita,1 Takeshi Watanabe,1 Trang TH Tu,1 Lou Mikuzuki,1 Chaoli Hong,1 Kazuhito Miura,2 Tatsuya Yoshikawa,1 Takahiko Nagamine,3 Akira Toyofuku1
1Department of Psychosomatic Dentistry, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo, Japan; 2Department of Gerodontology, Division of Oral Health Science, Graduate School of Dental Medicine, Hokkaido University, Hokkaido, Japan; 3Department of Psychiatric Internal Medicine, Sunlight Brain Research Center, Yamaguchi, Japan
Correspondence: Akira Toyofuku
Department of Psychosomatic Dentistry, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, 1-5-45 Yushima, Bunkyo-Ku, Tokyo 113-8510, Japan
Tel/Fax +81 3 5803 5898
Email [email protected]
Objective: To assess the therapeutic dose and safety of amitriptyline and the outcome following treatment with amitriptyline among older patients with burning mouth syndrome (BMS).
Methods: 187 consecutive patients were prescribed amitriptyline as a first-line medication from April 2016 to September 2018 and followed-up for >1 month. Patients were divided into 3 groups: group 1, 113 patients aged <65 years; group 2, 52 patients aged between 65 and 74 years; and group 3, 22 patients aged 75 years or older. The visual analog scale (VAS), Pain Catastrophizing Scale (PCS), Somatic Symptom Scale-8 (SSS-8), Patient Global
Impression of Change (PGIC), and Short-form McGill Pain Questionnaire (SF-MPQ) were used for analysis.
Results: Thirty-two patients (17 in group 1, 10 in group 2, and 5 in group 3) stopped taking amitriptyline due to side effects. There were no differences among the groups with respect to sex; scores of VAS, PCS, and SSS-8; and drop-out ratio. There were no significant differences in the VAS, PCS, and PGIC scores among the groups after 1 month. The mean daily dose after 1 month was 20.4 ± 8.6 mg in group 1, 17.3 ± 8.7 mg in group 2, and 13.2 ± 5.8 mg in group 3; this difference was significant (p value = 0.003). About 76% of patients showed improvements in their symptoms (PGIC ≥ 3). About 90% of patients reported side effects. No serious side effects occurred.
Conclusion: The therapeutic dose of amitriptyline may be lower for older BMS patients than for younger patients.
Keywords: amitriptyline, burning mouth syndrome, aged, chronic pain
Introduction
Burning mouth syndrome (BMS) or glossodynia involves chronic oral pain without any organic cause.1,2 BMS mainly affects middle-aged women. However, we previously reported that the ratio of older patients with BMS is gradually increasing.3 BMS has a negative effect on the quality of life of patients, and BMS management costs the society and individuals up to £3000 per year.4,5
Antidepressants are widely used for chronic pain.6 Amitriptyline is one of the most commonly used drugs for treating BMS.7 It is to be prescribed to older patients using the Screening Tool for Older Persons’ Appropriate Prescriptions for Japanese (STOPP-J).8 Generally, the required dose for analgesic treatment is lower than that required for depression treatment.9 While a high dose of amitriptyline is recommended for the treatment of depression, the appropriate dose of amitriptyline (lower than 40 mg/day) to control pain during BMS treatment has not been well investigated. A number of dosage regimens for amitriptyline have been proposed. In most studies, 10–40 mg/day of amitriptyline is recommended for adult patients.2,10 However, older patients need a lower dose of antidepressants than younger patients to achieve an effective blood level.11 Nishtala et al12 reported that the dose of antidepressants, especially tricyclic antidepressants (TCAs), for treating depression in older patients in care homes is less than the manufacturer’s recommended minimum effective dose. At the same dose, the side effects of antidepressants seem to be more severe in older patients than in younger patients, based on our clinical experience with thousands of BMS patients. However, there is no report on the therapeutic dose of amitriptyline for older BMS patients. In clinical practice, amitriptyline is commonly prescribed for geriatric patients in the treatment of chronic pain.13 Other than TCA, selective serotonin reuptake inhibitors (SSRIs) and serotonin–norepinephrine reuptake inhibitors (SNRIs) are recommended for the treatment of BMS.14 Some patients, especially older patients, do not benefit from using SSRIs and SNRIs and may even experience severe side effects due to drug-drug interaction.7 Nevertheless, some older BMS patients need amitriptyline because of severe oral pain. For these reasons, it is imperative to identify a safe and effective therapeutic dose of amitriptyline for older BMS patients. The aim of this study is to determine the therapeutic dose of amitriptyline for older patients with BMS.
Methods
In this retrospective study, we divided BMS patients into 3 groups according to their age and compared the daily doses, clinical efficacy, and safety of amitriptyline.
Patients
A retrospective chart review was performed by including consecutive BMS patients. Figure 1 shows the flowchart of this study. A total of 668 consecutive patients with BMS who first visited Psychosomatic Dentistry Clinic in Tokyo Medical and Dental University Dental Hospital, Tokyo, Japan, between April 2016 and September 2018 were included. Patients met the criteria of BMS according to the International Classification of Headache Disorders (ICHD)-3 (Category 13.11 Burning mouth syndrome).1 Only adult patients (>18 years) who complained of pain or burning sensation for more than 3 months, despite having healthy oral mucosa,1,15 were included in the study. Patients younger than 18 years, patients with obvious delusional disorders, and patients with dementia, minor cognitive impairment, narrow-angle glaucoma, or acute myocardial infarction were excluded from the study. Among these patients, 494 received prescriptions from our clinic, while the remaining patients did not receive any prescriptions but were followed-up. 187 patients (males: n=23, females: n = 164, mean age: 60.4 ± 12.4 years) who were prescribed amitriptyline as a first medication and were followed-up for more than 1 month were enrolled in the study. Patients were divided into 3 groups according to their age: group 1 (113 patients aged <65 years; mean age: 52.5 ± 9.1 years), group 2 (52 patients aged between 65 and 74 years; mean age: 69.7 ± 2.8 years), and group 3 (22 patients aged 75 years or older; mean age: 78.4 ± 3.3 years). Thirty-two patients (17 patients in group 1, 10 in group 2, and 5 in group 3; no statistical significance among groups for number of patients) stopped taking amitriptyline within 1 month because of side effects. One hundred and fifty-five patients continued taking amitriptyline for more than 1 month.
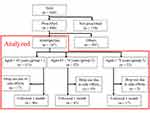 |
Figure 1 Flow chart of the study design. |
Assessment
The intensity and characteristic of pain were assessed using the Short-form McGill Pain Questionnaire (SF-MPQ),16 in which severity was estimated using the visual analog scale (VAS: from 0 to 100) and Present Pain Intensity (PPI) index. SF-MPQ consists of 15 descriptors, which are used to evaluate qualities of pain through sensory and affective perspectives. The Pain Catastrophizing Scale (PCS),17 a 13-item self-report questionnaire, was used to evaluate pain catastrophizing in patients at every visit. The Somatic Symptom Scale-8 (SSS-8)18 was used at first visit. SSS-8 is an abbreviated version of the Patient Health Questionnaire-15. It assesses somatic symptom burden. The Patient Global Impression of Change (PGIC)19 was used to evaluate the overall status and improvement in the clinical status of patients. In this study, a PGIC score ≥ 3 indicates improvement in clinical status. Demographic information (age and sex) were obtained from the medical charts of patients. At every visit, all patients reported any side effects they had experienced.
Prescription
The starting dose of amitriptyline in this study was 5 or 10 mg, depending on the age, comorbidities, and tolerability of side effect. The dose was increased by 5 or 10 mg at every visit, as long as the efficacy is insufficient and side effects are acceptable. The patients were followed-up every week or every other week.
Comorbidity
We required all patients to bring referral letters from their family physician. Information on psychiatric comorbidities were obtained from referral letters from the patients’ attending psychiatrists. For patients who did not provide referral letters, we sent an inquiry form to their psychiatrists to verify their diagnosis. Psychiatric comorbidities were categorized according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).20 Similarly, physical comorbidities were examined by reviewing referral letters from attending physicians and by interviewing patients.
Statistics
Demographic information was presented as means (± standard deviation, SD) for continuous variables and percentages for categorical variables. Data were analyzed using one-way analysis of variance and the Dunnett’s T3 test to compare continuous variables among groups. The chi-square test was used to analyze categorical variables, while the Wilcoxon signed-rank test was used to compare the differences of VAS and PCS between baseline and 1 month. A p value <0.05 was considered statistically significant. The statistical software package, SPSS for Windows version 25 (SPSS IBM, Armonk, NY, USA) was used for the analysis.
Results
The major finding was that the efficacy and safety of amitriptyline were comparable between older patients receiving low daily doses of amitriptyline and younger patients.
Patients
There was no difference with respect to sex and the VAS, PCS, and SSS-8 scores. The drop-out ratio was not different among the groups. When the responses of patients in the SF-MPQ were evaluated, no descriptors were significantly different among the groups (Tables 1 and 2). The initial daily dose at baseline was different among the groups (p value < 0.001).
 |
Table 1 Patient Demographics |
 |
Table 2 Mental and Physical Comorbidities |
Comorbidity
The mental and physical comorbidities of patients are presented in Table 2. Though there was no difference in mental comorbidities among the groups, cancer history, hyperlipidemia, and the absence of physical comorbidities were significantly different. The frequency of mental comorbidities in the initial cohort, 668 consecutive patients with BMS who first visited our clinic, was not different from the prevalence reported in the previous study.2 However, for the 187 patients analyzed in this study, the frequency of mental comorbidities was lower than that of BMS patients previously reported.2
Sites affected by cancer included the lung (1 patient), stomach (4 patients), esophagus (1 patient), urinary bladder (1 patient), colon (3 patients), kidney (2 patients), and uterine cervix (1 patient). The most common cancer was breast cancer (10 patients). Ariyawardana et al21 reported that patients with Parkinson’s disease, autoimmune diseases, and diabetes were often excluded from BMS studies. In our study, there were no patients with Parkinson’s disease or autoimmune diseases. However, 3 patients had diabetes.
One-Month Outcome
There were no statistically significant differences in VAS, PCS, and PGIC scores after 1 month among groups. However, the average VAS score increased slightly after 1 month. The mean daily dose after 1 month was 20.4 ± 8.6 mg in group 1, 17.3 ± 8.7 mg in group 2, and 13.2 ± 5.8 mg in group 3. The mean daily dose after 1 month was significantly different among the groups (p value = 0.003) and between groups 1 and 3, following post hoc analysis using Dunnett’s T3 test (p value = 0.001) (Table 3). The proportion of patients who showed improvement in symptoms (PGIC ≥ 3) was 76.0% in group 1, 76.2% in group 2, and 76.5% in group 3. The VAS and PCS scores after 1 month were significantly improved from the first visit.
 |
Table 3 Comparison of the 3 Groups After 1 Month |
Side Effects
About 90% of patients reported side effects due to the use of amitriptyline. The side effects reported during treatment are summarized in Table 4. The most common side effect was drowsiness (57.5% in group 1, 48.1% in group 2, 36.4% in group 3), followed by dry mouth (38.1% in group 1, 50.0% in group 2, 36.4% in group 3) and constipation (26.5% in group 1, 25.0% in group 2, 22.7% in group 3). Other side effects included weight gain (3.5% in group 1, 3.8% in group 2, 4.5% in group 3), nausea (4.4% in group 1, 3.8% in group 2, 0.0% in group 3), headache (8.0% in group 1, 0.0% in group 2, 4.5% in group 3) and trouble urinating (3.5% in group 1, 1.9% in group 2, 9.1% in group 3).
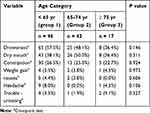 |
Table 4 Side Effects of Amitriptyline During Burning Mouth Syndrome Treatment |
The incidence of each side effect was not different among groups. Thirty-two patients stopped taking amitriptyline within 1 month because of side effects, including drowsiness and dry mouth. However, no serious side effects, such as falls, QT prolongation, and cognitive impairment, occurred in the patients. Whenever cognitive impairment was suspected, we performed the Mini-Mental State Examination (MMSE). However, the scores were >24 in all cases.
Discussion
Even though the daily dose in group 3 was about two-thirds of that for group 1, the clinical efficacy of amitriptyline, assessed using the VAS, PCS, and PGIC scores after 1 month, was not significant. These results suggest that the efficacy of amitriptyline for BMS treatment was not different among the groups. In addition, the drop-out rate due to side effects and the incidence of side effects were not significantly different among the groups. The tolerability of amitriptyline is low because of its side effects.22 Thus, the dose of amitriptyline should be as low as possible for older patients. Currently, many drugs have been proposed for the treatment of BMS.2 These drugs include amitriptyline, clonazepam, SSRIs, and SNRIs.10,14 SSRIs and SNRIs are featured by drug-drug interaction via cytochrome P450. The risk of adverse reactions is especially high in older patients with several comorbidities.23 When prescribed with diuretics, particularly thiazide diuretics, the risk of hyponatremia increases.24 However, the use of benzodiazepines associates with withdrawal symptoms and dependency.25 In addition to that, there is a concern that gabapentinoids are often prescribed without enough efficacy.26
Amitriptyline is a type of TCA. It causes anti-histaminic and anti-cholinergic side effects.22 The risk of severe side effects due to amitriptyline is lower than that of other antidepressants used for treating depression in older patients.27 Furthermore, another study reported that the side effects of low-dose antidepressants differ from those of high-dose antidepressants used for treating depression.9 Kroenke et al reported that the side effects may be less when a low dose is prescribed for analgesia.28 The most common side effects of low-dose amitriptyline are daytime fatigue, weight gain, dry mouth, and constipation.9,29 However, there is little information in the literature on low-dose antidepressant treatment for older patients with BMS. In this study, the most common side effects were drowsiness, dry mouth, and constipation, which are similar to those reported in other studies.9,29 (Table 4).
SSRIs and SNRIs seem to have a low risk for severe adverse effects.30 While the relative risk of adverse effects for amitriptyline is high, the relative risk of withdrawals due to side effects is low compared to SSRIs and SNRIs.9 SNRIs and SSRIs are not always effective. Some patients need amitriptyline instead of SSRI and SNRI for their pain.7 Nevertheless, no study has been conducted to investigate the therapeutic dose of amitriptyline for older BMS patients. Older patients become sensitive to medication probably because of the reduction in numbers of neurons and receptors, age-related change in blood-brain barrier.25 In addition to that, low liver and kidney function, weight, body fat/water distribution, pharmacodynamics changes and pharmacokinetics are involved in vulnerability to medications.31
In this study, we demonstrated that the therapeutic dose of amitriptyline for older patients is lower than that for younger patients. With careful side effect monitoring and dose adjustment, low-dose amitriptyline seemed to be effective in older BMS patients.
A previous study reported the SF-MPQ scores of BMS patients.15 In the aforementioned study, the mean pain severity and the characteristics of pain were not different from that reported in other studies.15 There were no significant differences in the descriptors among the groups. Severity and characteristics of pain might not change according to the age of patients. This means that the target symptoms were similar in all groups.
The socioeconomic burden of chronic pain is huge for individuals and the society.7 Safer and more effective treatments are needed for chronic pain to reduce this burden. One of the advantages of amitriptyline is its low cost.28 Hens et al reported that the most cost-effective therapy for BMS is topical clonazepam. However, amitriptyline was not included in their analysis.4 Based on cost, TCAs are the least expensive among the pain medications.32 In addition, TCA treatment yields extremely good outcomes compared to other antidepressants for neuropathic pain.33 Generally, low-dose amitriptyline is well tolerated.9 However, another study reported that TCAs are poorly tolerated by older patients.33 In our study, there was no difference in the safety and treatment outcome among the 3 groups, even though the therapeutic doses for each group were different. Therefore, the therapeutic dose of amitriptyline for older BMS patients may be lower than that for younger patients. The world population is ageing and the number of older BMS patients is increasing.3 Amitriptyline would be useful for treating older BMS patients.
This study suggests that older BMS patients can experience clinical improvements with a low dose of amitriptyline compared to younger patients. Low-dose amitriptyline may be tolerable and effective in older patients with BMS. The elderly patients are vulnerable to medication and at higher risk for side effects.31 Thus, low dose of amitriptyline would reduce the risk of side effects. Further research is necessary to investigate the long-term safety and outcome of amitriptyline for BMS treatment in older patients. Careful evaluation of patients, careful prescription of drugs, and careful monitoring are critical for the treatment of older BMS patients.
Limitation
Our study has some limitations. Because of the short-term study design, we could not assess the long-term side effects of amitriptyline. Additionally, we did not include a control group in this study. Moreover, the side effects were not systematically monitored and recorded, which may have led to an underestimation of the occurrence rate of these side effects. Regarding cognitive impairment, we assessed cognitive ability based on our clinical impression and by using the MMSE. Cognitive impairment might be assessed differently by a specialist. Regarding the dosage of amitriptyline, there may be a bias in deciding the dose of amitriptyline because each attending dentist might have a preference in prescribing a smaller dose for older patients to avoid side effects.
Conclusion
This study suggests that the therapeutic dose of amitriptyline may be lower for older BMS patients than for younger patients. Low-dose amitriptyline may be tolerable and effective in older patients with BMS.
Abbreviations
BMS, Burning mouth syndrome; TCA, Tricyclic Antidepressants; SF-MPQ, Short-form McGill Pain Questionnaire; PPI, Present pain intensity; VAS, Visual analog scale; PCS, Pain catastrophizing scale; SSS-8, Somatic Symptom Scale-8; PGIC, Patient global impression of change; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; ICHD, International Classification of Headache Disorders; SSRI, Selective serotonin reuptake inhibitor; SNRI, Serotonin–norepinephrine reuptake inhibitor; MMSE, Mini-Mental State Examination.
Ethics Approval and Informed Consent
The study protocol was approved by the Ethical Committee of Tokyo Medical and Dental University (D2018-089).
Consent for Publication
Written informed consent was obtained from all patients.
Data Sharing Statement
The dataset supporting the conclusions of this article is available in the Department of Psychosomatic Dentistry, Graduate School of Tokyo Medical and Dental University.
Author Contributions
The roles of the authors were: AT confirmed dental diagnosis. TS, TY, LM, MT, TW and AT treated the patients. TS, TN, and MT have been involved in drafting the manuscript. TTHT, KM, CH and TW revised it critically. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
This work was supported in part by the JSPS KAKENHI (Grant Number 19K10328) awarded to Dr. Toyofuku. The authors report no other conflicts of interest in this work.
References
1. Urquhart DM, Wluka AE, van Tulder M, et al. Efficacy of Low-dose amitriptyline for chronic low back pain: a randomized clinical trial. JAMA Intern Med. 2018;178(11):1474–1481. doi:10.1001/jamainternmed.2018.4222
2. Jaaskelainen SK, Woda A. Burning mouth syndrome. Cephalalgia. 2017;37(7):627–647. doi:10.1177/0333102417694883
3. Suga T, Watanabe T, Aota Y, Nagamine T, Toyofuku A. Burning mouth syndrome: the challenge of an aging population. Geriatr Gerontol Int. 2018;18(12):1649–1650. doi:10.1111/ggi.2018.18.issue-12
4. Hens MJ, Alonso-Ferreira V, Villaverde-Hueso A, Abaitua I, Posada de la Paz M. Cost-effectiveness analysis of burning mouth syndrome therapy. Community Dent Oral Epidemiol. 2012;40(2):185–192. doi:10.1111/j.1600-0528.2011.00645.x
5. Breckons M, Shen J, Bunga J, Vale L, Durham J. DEEP study: indirect and out-of-pocket costs of persistent orofacial pain. J Dent Res. 2018;97:22034518773310.
6. Cooper TE, Heathcote LC, Clinch J, et al. Antidepressants for chronic non-cancer pain in children and adolescents. Cochrane Database Syst Rev. 2017;8:CD012535.
7. Tu TTH, Takenoshita M, Matsuoka H, et al. Current management strategies for the pain of elderly patients with burning mouth syndrome: a critical review. Biopsychosoc Med. 2019;13:1. doi:10.1186/s13030-019-0142-7
8. Kojima T, Mizukami K, Tomita N, et al. Screening tool for older persons’ appropriate prescriptions for Japanese: report of the Japan Geriatrics Society Working Group on “Guidelines for medical treatment and its safety in the elderly”. Geriatr Gerontol Int. 2016;16(9):983–1001. doi:10.1111/ggi.12890
9. Riediger C, Schuster T, Barlinn K, Maier S, Weitz J, Siepmann T. Adverse effects of antidepressants for chronic pain: a systematic review and meta-analysis. Front Neurol. 2017;8:307. doi:10.3389/fneur.2017.00307
10. Fenelon M, Quinque E, Arrive E, Catros S, Fricain JC. Pain-relieving effects of clonazepam and amitriptyline in burning mouth syndrome: a retrospective study. Int J Oral Maxillofac Surg. 2017. doi:10.1016/j.ijom.2017.03.032
11. Association AP. Practice guideline for the treatment of patients with major depressive disorder (3rd); 2009. Available from: http://psychiatryonlineorg/guidelines aspx
12. Nishtala PS, McLachlan AJ, Bell JS, Chen TF. Determinants of antidepressant medication prescribing in elderly residents of aged care homes in Australia: a retrospective study. Am J Geriatr Pharmacother. 2009;7(4):210–219. doi:10.1016/j.amjopharm.2009.07.001
13. Katz P, Pegoraro V, Liedgens H. Characteristics, resource utilization and safety profile of patients prescribed with neuropathic pain treatments: a real-world evidence study on general practices in Europe - the role of the lidocaine 5% medicated plaster. Curr Med Res Opin. 2017;33(8):1481–1489. doi:10.1080/03007995.2017.1335191
14. Kato Y, Sato T, Katagiri A, et al. Milnacipran dose-effect study in patients with burning mouth syndrome. Clin Neuropharmacol. 2011;34(4):166–169. doi:10.1097/WNF.0b013e318227f100
15. Takenoshita M, Sato T, Kato Y, et al. Psychiatric diagnoses in patients with burning mouth syndrome and atypical odontalgia referred from psychiatric to dental facilities. Neuropsychiatr Dis Treat. 2010;6:699–705. doi:10.2147/NDT.S12605
16. Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30(2):191–197. doi:10.1016/0304-3959(87)91074-8
17. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524–532. doi:10.1037/1040-3590.7.4.524
18. Gierk B, Kohlmann S, Kroenke K, et al. The somatic symptom scale-8 (SSS-8): a brief measure of somatic symptom burden. JAMA Intern Med. 2014;174(3):399–407. doi:10.1001/jamainternmed.2013.12179
19. Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulative Physiol Ther. 2004;27(1):26–35. doi:10.1016/j.jmpt.2003.11.003
20. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; Washington, DC: American Psychiatric Association; 2013.
21. Ariyawardana A, Chmieliauskaite M, Farag AM, et al. World workshop on oral medicine VII: burning mouth syndrome: a systematic review of disease definitions and diagnostic criteria utilized in randomized clinical trials. Oral Dis. 2019. doi:10.1111/odi.13067
22. Rico-Villademoros F, Slim M, Calandre EP. Amitriptyline for the treatment of fibromyalgia: a comprehensive review. Expert Rev Neurother. 2015;15(10):1123–1150. doi:10.1586/14737175.2015.1091726
23. Lotrich FE, Pollock BG. Aging and clinical pharmacology: implications for antidepressants. J Clin Pharmacol. 2005;45(10):1106–1122. doi:10.1177/0091270005280297
24. Mori M, Koide T, Imanishi Y, Matsui Y, Matsuda T. Duloxetine-induced hyponatremia in an elderly patient treated with thiazide diuretics. Indian J Pharmacol. 2014;46(6):657–659. doi:10.4103/0253-7613.144947
25. Lader M. Benzodiazepine harm: how can it be reduced? Br J Clin Pharmacol. 2014;77(2):295–301. doi:10.1111/bcp.2014.77.issue-2
26. Goodman CW, Brett AS. Gabapentin and pregabalin for pain - is increased prescribing a cause for concern? N Engl J Med. 2017;377(5):411–414. doi:10.1056/NEJMp1704633
27. Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, Hippisley-Cox J. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ. 2011;343:d4551. doi:10.1136/bmj.d4551
28. Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31(3):206–219. doi:10.1016/j.genhosppsych.2008.12.006
29. Doyle Strauss L, Weizenbaum E, Loder EW, Rizzoli PB. Amitriptyline dose and treatment outcomes in specialty headache practice: a retrospective cohort study. Headache. 2016;56(10):1626–1634. doi:10.1111/head.2016.56.issue-10
30. Rochester MP, Kane AM, Linnebur SA, Fixen DR. Evaluating the risk of QTc prolongation associated with antidepressant use in older adults: a review of the evidence. Ther Adv Drug Saf. 2018;9(6):297–308. doi:10.1177/2042098618772979
31. Tobias DE. Start low and go slow. Hosp Pharm. 2003;38(7):634–636. doi:10.1177/001857870303800707
32. Rasu RS, Vouthy K, Crowl AN, et al. Cost of pain medication to treat adult patients with nonmalignant chronic pain in the United States. J Managed Care Specialty Pharm. 2014;20(9):921–928. doi:10.18553/jmcp.2014.20.9.921
33. Moulin D, Boulanger A, Clark AJ, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19(6):328–335. doi:10.1155/2014/754693
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
