Back to Journals » Research and Reports in Urology » Volume 15
Therapeutic Approaches to Penile Cancer: Standards of Care and Recent Developments
Authors White J, Mason R, Lawen T, Spooner J, Faria KVM, Rahman F, Ramasamy R
Received 6 March 2023
Accepted for publication 24 May 2023
Published 2 June 2023 Volume 2023:15 Pages 165—174
DOI https://doi.org/10.2147/RRU.S387228
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Panagiotis J Vlachostergios
Joshua White,1 Ross Mason,2 Tarek Lawen,2 Jesse Spooner,2 Kauy VM Faria,3 Farah Rahman,1 Ranjith Ramasamy1
1Department of Urology, University of Miami Miller School of Medicine, Miami, FL, USA; 2Department of Urology, Dalhousie University, Halifax, NS, Canada; 3Department of Urology, Institute of Cancer of São Paulo, University of São Paulo School of Medicine, Sao Paulo, Brazil
Correspondence: Ranjith Ramasamy, University of Miami, Miller School of Medicine, 1120 NW 14th Street, 15th Floor, Miami, FL, 33136, USA, Tel +1 201 388-6644, Email [email protected]
Abstract: Penile cancer is a rare malignancy, most commonly diagnosed in older men, associated with poor outcomes, dramatic decline in quality of life and sexual function. Squamous cell carcinoma is the most common histopathology of penile cancer, accounting for 95% of all cases. Localized, early-stage penile cancer can be effectively managed through penile-sparing techniques in many cases, though advanced stages of penile cancer carry a poor prognosis. Current innovative treatments are exploring the role of targeted therapy, HPV-directed therapy, immune checkpoint inhibitors and adoptive T-cell therapies in treatment and prevention of relapse of penile cancer. Clinical trials are investigating the potential of targeted therapies and immune checkpoint inhibitors in advanced penile cancer. This review examines the current management of penile cancer and highlights future directions in research and treatment.
Keywords: penile cancer, inguinal lymph node dissection, immunotherapy, targeted therapy, HPV, adoptive T-cell therapy, sexual function
Introduction
Penile cancer is an uncommon form of malignancy, with an estimated incidence of 0.8 cases per 100,000 men globally in 2020.1 It is typically diagnosed in older men, with the majority of cases occurring in men over the age of 60.2 Squamous cell carcinoma (SCC) is the most common histopathology and is responsible for 95% of all cases.2 Histological diagnosis may be made by punch, excisional, or incisional biopsy. Human papilloma virus (HPV) subtypes 16 and 18, infection, smoking and phimosis are the greatest risk factors for penile cancer and are more common in low socio-economic groups.2
If metastasis occurs, the inguinal lymph nodes will be involved first, and up to 50% of patients will present with inguinal adenopathy.3 The most important prognosticator in penile cancer is lymph node involvement.3 In cases of more advanced disease, multimodal therapy may be required, though such cases tend to have a dismal prognosis.4 Clinical trials specifically assessing advanced penile cancer outcomes are limited, in part due to the rarity of the disease. Therapeutic advances have emerged in recent years with promising results.5,6 In this review, an analysis of the current standard of care, as well as contemporary and investigative therapies, including immunotherapy, targeted therapy, and sequencing approaches will be performed.
Surgical Approaches – Localized Disease
With early detection of localized disease, penile cancer is highly curable with a good prognosis.7 There are a number of important determinants which must be considered when selecting the most appropriate treatment strategy, including the size, location, depth of invasion and stage of the tumor. Localized disease may be managed through penile-sparing techniques in appropriately selected patients. MRI can be considered to evaluate the depth of invasion of primary tumor which may be helpful in surgical planning.
Penile intraepithelial neoplasia (PeIN) is a precancerous lesion that warrants treatment.3 Topical chemotherapy (5-fluorouracil/Imiquimod) may provide complete response in up to 57% of patients.8 The safety profile of these is excellent with limited serious adverse events being reported.8 Patients with PeIN or superficial pT1a disease may also be managed by laser ablation therapy which is performed with either ND:YAG or carbon dioxide.9 Circumcision may be curative in patients with PeIN or pT1 disease confined to the foreskin.10 Circumcision in childhood or adolescence substantially reduces the risk of penile cancer.11
Moh’s micrographic surgery optimizes aesthetic and functional results, and involves sequential removal of tissue layers until negative margins are achieved.12 Recurrence rates are 20–30%, which is much higher than tumors managed with partial penectomy (5%); nonetheless cancer specific and survival outcomes are similar between the two modalities.13 Glans resurfacing may also be offered to select patients with PeIN and pT1 tumors. Glans resurfacing (Figures 1–3)14 is the removal of both the epithelial and subepithelial glans, followed by placement of a partial thickness skin graft.15 Sexual function has been demonstrated to improve with glans resurfacing, and oncological outcomes are satisfactory with up to 100% overall survival at one year.16
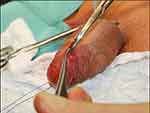 |
Figure 1 Initial step in glans resurfacing where the epithelium and subepithelial tissue is removed from the spongiosum by sharp dissection. Notes: Reproduced with permission from Sosnowski R, Kuligowski M, Kuczkiewicz O, et al. Primary penile cancer organ sparing treatment. Cent European J Urol. 2016;69(4):377–383. Creative Commons.14 |
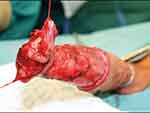 |
Figure 2 Developing the plane to allow separation of the glans from corpora cavernosa during the initial step of glansectomy. Notes: Reproduced with permission from Sosnowski R, Kuligowski M, Kuczkiewicz O, et al. Primary penile cancer organ sparing treatment. Cent European J Urol. 2016;69(4):377–383. Creative Commons.14 |
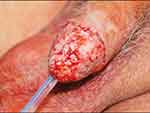 |
Figure 3 Completed glansectomy with split-skin graft. Notes: Reproduced with permission from Sosnowski R, Kuligowski M, Kuczkiewicz O, et al. Primary penile cancer organ sparing treatment. Cent European J Urol. 2016;69(4):377–383. Creative Commons.14 |
For tumors involving under 50% of the glans, partial glansectomy may be performed.17 Glansectomy may also be indicated in patients with smaller tumors and pT1-T2 disease, though local recurrence is greater with organ-sparing surgery compared with penectomy in patients with risk factors.17 Risk factors for local recurrence following glansectomy include perineural invasion, PeIN, lymphovascular invasion (LVI), high grade disease and positive margins.18
External beam radiotherapy and brachytherapy have both been evaluated as penile-sparing alternatives to surgical resection in patients with early-stage disease. Prospective randomized control trials comparing the two modalities are lacking, and shared decision-making is an important element of any oncological discussion in such patients. Indeed, meta-analysis data have shown no statistically significant differences in overall survival (OS) or local recurrence (LR) between patients managed with radiotherapy and surgery.19
In patients with more extensive disease where penile-sparing surgeries are not feasible, partial penectomy can provide a functional penile stump in many patients. Two centimeters (cm) has previously been advocated as the required surgical margin, however this dogma has been challenged in recent years with as low as 1 millimeter being shown to offer excellent oncological control.20 A standard excision ought to include a margin of clinically normal-appearing skin around the tumour and surrounding erythema.21 Intraoperative frozen section analysis is performed to evaluate tumor margins and may be used to identify adverse pathological features that may increase the risk of LR and/or metastatic disease.22 Total penectomy is usually performed in patients with T3 or T4 disease in whom a functional penile stump cannot be achieved. Radical penectomy, which is rarely performed, involves complete removal of the corpora to the level of the pubis. Total and radical penectomy are typically accompanied by creation of a perineal urethrostomy.15
There are established techniques to reconstruct the glans or the remaining penile stump; with the most common being split-thickness skin graft (STSG), the use of buccal mucosal graft (BMG), and the use of an inverted urethral flap (IUF). Patient selection is critical and must incorporate patient and tumor characteristics, including location, stage and grade.23
Sexual Function
All treatments for penile SCC may result in genital disfiguration, which may have a substantial impact on quality of life (QoL) and sexual function.24 Indeed, the majority of patients with penile cancer express anxiety regarding the potential impact on their sexual functioning.25 Extirpative penile surgery affects many QoL metrics including sexual function, sexual confidence, and positive male self-image.26 As survivorship continues to improve, sexual dysfunction has become an increasingly recognized adverse effect of treatment.27 Despite the involvement of the primary male sexual organ, evidence examining the sexual impacts of penile cancer is lacking.
Penectomy, despite being the gold-standard treatment for penile cancer, has significant QOL and sexual impacts.14 Organ-sparing techniques may have similar oncologic outcomes in appropriately selected patients with the added benefit of preservation of sexual function.14,26 The studies on sexual function and penile cancer tend to be small and retrospective in nature. Almost unanimously, these studies use the International Index of Erectile Function (IIEF) and Social Functioning Questionnaire (SFQ) to assess sexual function during penile cancer treatment.28
According to observational data, laser ablation tends to have minimal sexual side effects, with studies citing between 0% and 22% of patients having altered erectile function after treatment.26,29 A small study from the UK demonstrated that glans resurfacing had high patient sexual satisfaction, with a large proportion even reporting improved sex life after their treatment.30 Moreover, patients in this study reported no new erectile dysfunction and normal penile sensation after treatment.30 A 2011 study found that radical circumcision with or without wide local excision preserved sexual and erectile function.31 Wide local excision alone also does not affect erectile function or penetrative intercourse.32
Glansectomy, on the other hand, tends to have more profound effects on sexual function compared with other organ-sparing techniques. A Cleveland Clinic study found poor post-operative erectile function in 50% and reports of no sexual activity in 67% of patients treated with glansectomy alone.33 Despite this, these patients overall reported satisfaction with their procedure and good QOL.33 Encouragingly, glansectomy with neoglans reconstruction seems to better preserve orgasm, ejaculatory function and ability to engage in penetrative sex.34 This was despite the expected decreased sensation in the neoglans. Fortunately, contemporary data shows improved erectile function with more complex reconstructive plastic procedures.35,36
As for partial or total penectomy, the data is limited again by small studies. Interestingly, a 2018 single-institution study found that close to 70% of penectomy patients were satisfied with their sex life with over 80% reporting satisfaction with their operation.37 Studies comparing partial penectomy patients to age-matched controls have found only 40–50% lower erectile function, orgasmic function and sexual function on IIEF scores.37,38 In terms of stump length after partial penectomy, there is no clear cut-off, as successful intercourse post-operatively is multifactorial. That being said, in one small cohort study including 18 patients, the majority reported erectile function with a median 4 cm phallus suitable for penetrative intercourse.39 Of not those not engaging in intercourse, a large proportion reported the reason being “shame” for small phallus size than true erectile rigidity.39
Unsurprisingly, studies find that total penectomy patients, despite maintaining their libido, have decreased sexual satisfaction compared to those managed with partial penectomy.40 Nonetheless, total penectomy patients do report some sexual satisfaction, suggesting forms of intimacy without penetrative intercourse still confer benefit in this population.37 Although not well studied, novel reconstructive techniques may aid in improving sexual satisfaction in these patients. After total penectomy, performing neophalloplasty is a possibility. Both semi-rigid and inflatable prosthesis can be placed within the neophallus to aid in penetrative intercourse.41 These cases are surgically and oncologically complex given the risk of tumor seeding and paucity of tunica or corpora that would ideally protect the phallus from erosion, infection, and extrusion.41
Inguinal Lymphadenectomy
Early treatment of lymph node involvement can improve survival, though this observation is limited to patients without bulky lymph node involvement or metastatic disease.42,43 High-risk patients (ie pathological stage T1b or greater) require nodal staging regardless of the clinical node status. Inguinal lymphadenectomy is associated with significant surgical morbidity with contemporary series reporting up to a 57% complication rate.44 Infection, skin necrosis, deep vein thrombosis (DVT) and lymphedema are common adverse events associated with inguinal lymphadenectomy.
Not all patients with palpable lymph nodes at diagnosis will require an immediate inguinal lymph node dissection (ILND), as 30% to 50% of inguinal lymphadenopathy may be secondary to inflammation.45 In the past, a prolonged course of antibiotics following penile resection was used to distinguish between reactive lymphadenopathy and metastatic disease. Antibiotics may be used in cases of overlying infection, though this should not delay percutaneous biopsy in most cases.42 Penile cancer experts now favor more expeditious evaluation of the palpable lymph nodes by performing a percutaneous lymph node biopsy, with the sensitivity and specificity of fine needle aspiration (FNA) among men with clinically palpable nodes are up 90%.46,47 Dynamic sentinel node biopsy (DSNB) is a reasonable option for patients with non-palpable or non-bulky inguinal nodes, with contemporary results demonstrating sensitivity ranging from 88% to 93%.48 DSNB should only be performed in high-volume centers with extensive experience in the procedure.48
Robotic ILND has been evaluated as a more novel surgical approach that uses a robot-assisted technique to remove lymph nodes from the inguinal region. The evidence for robotic ILND is limited in penile cancer, though currently the literature supports the approach as safe and effective, with a more favorable risk profile compared with open surgery.49 However, patient selection is critical for the procedure, particularly those with non-palpable or non-bulky inguinal nodes49 and should only be offered within a clinical trial.21
Imaging for Nodal and Metastatic Disease
Cross-sectional imaging is critical in the evaluation of patients with penile cancer and is used to determine the size, location and proximity of ILNs in patients with suspected penile cancer. Imaging can also help to identify metastatic disease or other affected lymph nodes in the chest, abdomen, or pelvis.50 It is important to note that imaging is a useful adjunct to physical examination. Indeed, as much as 16% of patients without palpable lymphadenopathy may harbor occult metastatic disease, and 20–40% of patients with palpable lymphadenopathy are found to be non-metastatic.42,51 Magnetic resonance imaging (MRI) is considered the best imaging modality in patients where physical exam is clearly limited, such as in those who have had previous chemotherapy or radiation therapy, and in the setting of obesity.42,52
The current National Comprehensive Cancer Network (NCCN) recommendations for 18F fluorodeoxyglucose (FDG) PET/CT are that they should only be used as a diagnostic modality in patients with clinically node-positive disease or in the setting of a clinical trial.48 This recommendation is supported by the findings of a systematic review and meta-analysis that found that FDG PET had a relatively low pooled sensitivity for detecting inguinal metastasis in patients with clinically node-negative disease (56.5%), but a higher pooled sensitivity for patients with clinically node-positive disease (96.4%).53
Advanced Disease
Multimodal therapy involving chemotherapy, radiation and/or extirpative surgical resection are mainstays in the management of locally advanced and metastatic penile cancer. Lymph node status is the single most important predictor of survival in penile cancer. Adverse lymph node characteristics include biopsy confirmed metastatic disease, fixed, bulky (>4cm), bilateral, or positive pelvic lymph nodes. Neoadjuvant chemotherapy (NAC) is recommended by NCCN and European Association of Urology (EAU) in such cases prior to attempting surgical resection.48,54 NAC regimens typically consist of four cycles of combination paclitaxel, ifosfamide and cisplatin (TIP). These recommendations are based on the findings of a single-arm prospective Phase II trial that evaluated TIP regimens in the neoadjuvant setting.55 In a pooled meta-analysis including 10 studies evaluating taxane-based NAC, an objective response of only 53% was identified.56 Adjuvant multimodal therapy can also be considered for patients with high-risk features, namely pelvic lymph node metastases, extranodal extension, bilateral inguinal lymph node involvement and tumor size >4 cm.48
The International Penile Advanced Cancer Trial (InPACT) is the first Phase III trial evaluating histologically confirmed penile SCC with regional lymph node metastasis.57 Patients were randomly assigned to one of three arms: ILND alone, NAC prior to ILND, or neoadjuvant chemoradiation prior to ILND in patients with regional lymph node metastasis. InPACT was also designed to provide insight into the management of patients with a high risk of recurrence based on ILN histology. The value of prophylactic pelvic lymph node dissection combined with chemoradiation to the inguinal and pelvic lymph nodes will be compared with chemoradiation to evaluate if prophylactic inguinal and pelvic lymph node dissection provides any survival benefit.58 Patient accrual has been very poor, and the results of the trial must be interpreted with caution.
Development of Novel Therapeutic Approaches
HPV-Directed Therapy
HPV status is an intriguing area requiring further research as up to 50% of penile cancer is HPV-positive.59,60 Indeed, HPV-positive and HPV-negative tumors have discrete molecular features, highlighting the importance of HPV status in tumor profiling.61 This information can help guide the development of novel immune-based treatments, including vaccines designed to target HPV-positive tumors. This approach may improve treatment outcomes and reduce the risk of side effects associated with traditional treatment modalities. HPV positivity may predict superior locoregional control in patients with node-positive disease who received adjuvant chemoradiation, compared to HPV-negative patients.59
Given the high prevalence of HPV in patients with penile cancer, there has been a growing interest in evaluating targeted therapies to HPV proteins, specifically proteins E6 and E7.62 These are believed to play a role in the development of penile cancer. Prophylactic HPV vaccines for primary prevention in HPV-associated precancerous lesions have demonstrated promise, particularly in cervical cancer.63 The long-term impact of these vaccines on penile cancer prevalence is yet to be elucidated. Clinical trials are currently under way which evaluate combination therapy with HPV vaccines and immunotherapies such as anti-PD-1, anti-PD-L1 and adoptive T-cell therapy.3
Targeted Therapy
Given the poor response to traditional first-line, platinum-based chemotherapy, different targeted treatment modalities have been explored as a potential alternative. EGFR protein is highly expressed in penile SCC, and represents an area of contemporary research in advanced disease.64 Anti-EGFR agents such as panitumumab have been investigated in the setting of unresectable and/or metastatic disease, with a median OS of 9.5 months.65 The efficacy of cetuximab (anti-EGFR) was evaluated retrospectively in patients with advanced disease, comparing the efficacy of cetuximab given alone versus taxane therapy alone versus combination cetuximab and taxane therapy. The overall risk reduction (ORR) associated with cetuximab was 27%, though these results did not reach statistical significance.66 Anti-EGFR agents may be reasonably effective, well-tolerated treatments on patients who are not eligible for standard platinum-based chemotherapy, though further trials are required to draw definitive conclusions. A number of other targetable pathways, including mTOR and DDR, are currently under investigation.67,68
Immunotherapy
Immunotherapies include immune checkpoint inhibitors and adoptive T-cell therapies. These represent a contemporary modality which show some promise in the management of advanced or refractory penile cancer. The available literature on these therapies is currently limited to small case-series, with some reports of sustained responses in those with high microsatellite instability or positive PD-L1 expression.69–71 Combination therapies have also shown some promising preliminary results, although data is limited.69 The NCCN guidelines currently support pembrolizumab as a second-line systemic therapy in patients with metastatic or refractory disease in patients with high microsatellite instability, as well as other molecular characteristics (ie dMMR, TMB-H).48
A number of malignancies are currently treated with immune checkpoint inhibitors regardless of these molecular characteristics, and as such these markers may not be reliable predictors of response to treatment.72,73 Identifying novel therapies which may be used in the neoadjuvant setting is critical, as the current standard of care does not provide a durable response in large proportion of men. Clinical trials are currently evaluating combination therapy with pembrolizumab and platinum-based chemotherapy as first-line treatment in the metastatic disease setting.74 Avelumab is also being evaluated as maintenance therapy in patients with stable disease following TIP chemotherapy, both in the locally advanced and metastatic.75 Given the influx of clinical trials evaluating the management of advanced disease, clinicians should remain hopeful for more effective therapeutic options to be elucidated.
Immune checkpoint inhibitors can be administered in an outpatient setting, which can be more convenient for patients. TIP is the current standard of care chemotherapy regimen in patients with advanced disease. TIP administration, however, must be performed in the inpatient setting, ultimately limiting its availability to patients in more rural locations.3 Administration also requires expertise due to its potential for significant toxicities, including bone marrow suppression, kidney damage, and neuropathy. Expert monitoring and handling are necessary to ensure patient safety and minimize the risk of adverse reactions, which may be barriers to providing care in some settings.3 Immune checkpoint inhibitors are a promising therapeutic option which may increase access to care for patients with advanced or metastatic disease.
It is important to note that while immune-checkpoint inhibitors (ICI) and other novel therapies have shown promising results in the relapsed or refractory setting, most of the data on these therapies has been limited to this stage of the disease. Therefore, it is critical to continue researching the use of these therapies earlier in the disease course, such as in the neoadjuvant or adjuvant settings. Additionally, it is also worth noting that combining ICI with other therapies such as radiotherapy, chemotherapy or surgery is also being studied as a means of achieving better outcomes.3
Adoptive T-Cell Therapy
Adoptive T-cell therapy is an emerging modality that has growing interest due to its novel approach. Adoptive T-cell therapy is a type of immunotherapy that involves the ex-vivo expansion of tumor-infiltrating lymphocytes (TIL), which are then infused back into the patient to target and destroy cancer cells.76 Tumor cells may also be targeted through T-cell antigens engineered towards cancerous cells.
The first trial to evaluate the efficacy of adoptive t-cell therapy was published in 2021. In this small pilot feasibility trial, the authors found that TIL expansion was independent of prior infection with HPV and NAC exposure.76 A phase I/II clinical trial in patients with refractory penile cancer evaluated the role of genetically engineered T-cells therapy. In this trial, patients received autologous T-cell therapy that was genetically engineered with a T-cell receptor directed against HPV16 E6.77 No dose-limiting toxicities were observed in the trial, with two patients experiencing objective tumor responses.77 These findings suggest that adoptive T-cell therapy may be a promising approach for the treatment of penile cancer and other HPV-associated malignancies.
Conclusion
Penile cancer is an uncommon form of malignancy with limited effective therapeutic options in advanced stages. Localized disease can be managed with penile-sparing techniques in appropriately selected patients, though patients must be counselled regarding the risk of sexual dysfunction and changes in quality of life. In advanced scenarios, ILN involvement remains the most important prognosticator in penile cancer, and the morbidity may be reduced with minimally invasive approaches such as robotic ILND compared with the traditional open dissection. There is a number of clinical trials currently evaluating novel therapies for advanced or recurrent penile cancer, such as targeted therapy, HPV-directed therapy, immune checkpoint inhibitors and adoptive T-cell therapies. These therapies have shown promise in the treatment of penile cancer, particularly in the relapsed or refractory setting. Further research is required to continue appropriately guide clinical practice, particularly in those with advanced disease.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fu L, Tian T, Yao K, et al. Global pattern and trends in penile cancer incidence: population-based study. JMIR Public Health Surveill. 2022;8(7):e34874. doi:10.2196/34874
2. Giona S. The epidemiology of penile cancer. In: Barber N, Ali A, editors. Urologic Cancers. Exon Publications; 2022.
3. Chadha J, Chahoud J, Spiess PE. An update on treatment of penile cancer. Ther Adv Med Oncol. 2022;14:17588359221127254. doi:10.1177/17588359221127254
4. Hakenberg OW, Drager DL, Erbersdobler A, Naumann CM, Junemann KP, Protzel C. The diagnosis and treatment of penile cancer. Dtsch Arztebl Int. 2018;115(39):646–652. doi:10.3238/arztebl.2018.0646
5. Tang Y, Hu X, Wu K, Li X. Immune landscape and immunotherapy for penile cancer. Front Immunol. 2022;13:1055235. doi:10.3389/fimmu.2022.1055235
6. Joshi VB, Chadha J, Chahoud J. Penile cancer: updates in systemic therapy. Asian J Urol. 2022;9(4):374–388. doi:10.1016/j.ajur.2022.03.006
7. Board, PDQ Adult Treatment Editorial. Penile Cancer Treatment (PDQ(R)): health professional version. PDQ Cancer Information Summaries; 2002.
8. Manjunath A, Brenton T, Wylie S, Corbishley CM, Watkin NA. Topical therapy for non-invasive penile cancer (Tis)-updated results and toxicity. Transl Androl Urol. 2017;6(5):803–808. doi:10.21037/tau.2017.06.24
9. Tang DH, Yan S, Ottenhof SR, et al. Laser ablation as monotherapy for penile squamous cell carcinoma: a multi-center cohort analysis. Urol Oncol. 2018;36(4):147–152. doi:10.1016/j.urolonc.2017.09.028
10. Wikstrom A, Hedblad MA, Johansson B, et al. The acetic acid test in evaluation of subclinical genital papillomavirus infection: a comparative study on penoscopy, histopathology, virology and scanning electron microscopy findings. Genitourin Med. 1992;68(2):90–99. doi:10.1136/sti.68.2.90
11. Larke NL, Thomas SL, Dos Santos Silva I, Weiss HA. Male circumcision and penile cancer: a systematic review and meta-analysis. Cancer Causes Control. 2011;22(8):1097–1110. doi:10.1007/s10552-011-9785-9
12. Prickett KA, Ramsey ML. Mohs Micrographic Surgery. StatPearls; 2022.
13. Shindel AW, Mann MW, Lev RY, et al. Mohs micrographic surgery for penile cancer: management and long-term followup. J Urol. 2007;178(5):1980–1985. doi:10.1016/j.juro.2007.07.039
14. Sosnowski R, Kuligowski M, Kuczkiewicz O, et al. Primary penile cancer organ sparing treatment. Cent European J Urol. 2016;69(4):377–383. doi:10.5173/ceju.2016.890
15. Spiess PE, Necchi A; SpringerLink. Penile Carcinoma: Therapeutic Principles and Advances.
16. Falcone M, Preto M, Oderda M, et al. Total glans resurfacing for the management of superficial penile cancer: a retrospective cohort analysis in a tertiary referral center. Urology. 2020;145:281–286. doi:10.1016/j.urology.2020.06.066
17. Lont AP, Gallee MP, Meinhardt W, van Tinteren H, Horenblas S. Penis conserving treatment for T1 and T2 penile carcinoma: clinical implications of a local recurrence. J Urol. 2006;176(2):575–80; discussion 580. doi:10.1016/j.juro.2006.03.063
18. Roussel E, Peeters E, Vanthoor J, et al. Predictors of local recurrence and its impact on survival after glansectomy for penile cancer: time to challenge the dogma? BJU Int. 2021;127(5):606–613. doi:10.1111/bju.15297
19. Hasan S, Francis A, Hagenauer A, et al. The role of brachytherapy in organ preservation for penile cancer: a meta-analysis and review of the literature. Brachytherapy. 2015;14(4):517–524. doi:10.1016/j.brachy.2015.03.008
20. Minhas S, Kayes O, Hegarty P, Kumar P, Freeman A, Ralph D. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96(7):1040–1043. doi:10.1111/j.1464-410X.2005.05769.x
21. Brouwer OR, Albersen M, Parnham A, et al. European Association of Urology-American Society of Clinical Oncology Collaborative Guideline on Penile Cancer: 2023 update. Eur Urol. 2023;83(6):548–560. doi:10.1016/j.eururo.2023.02.027
22. Coddington ND, Redger KD, Higuchi TT. Surgical principles of penile cancer for penectomy and inguinal lymph node dissection: a narrative review. AME Med J. 2021;6:29. doi:10.21037/amj-20-159
23. Kamel MH, Bissada N, Warford R, Farias J, Davis R. Organ sparing surgery for penile cancer: a systematic review. J Urol. 2017;198(4):770–779. doi:10.1016/j.juro.2017.01.088
24. Maddineni SB, Lau MM, Sangar VK. Identifying the needs of penile cancer sufferers: a systematic review of the quality of life, psychosexual and psychosocial literature in penile cancer. BMC Urol. 2009;9:8. doi:10.1186/1471-2490-9-8
25. Schover LR, von Eschenbach AC, Smith DB, Gonzalez J. Sexual rehabilitation of urologic cancer patients: a practical approach. CA Cancer J Clin. 1984;34(2):66–74. doi:10.3322/canjclin.34.2.66
26. Hegarty PK, Shabbir M, Hughes B, et al. Penile preserving surgery and surgical strategies to maximize penile form and function in penile cancer: recommendations from the United Kingdom experience. World J Urol. 2009;27(2):179–187. doi:10.1007/s00345-008-0312-x
27. Pizzocaro G, Algaba F, Horenblas S, et al. EAU penile cancer guidelines 2009. Eur Urol. 2010;57(6):1002–1012. doi:10.1016/j.eururo.2010.01.039
28. Stroie FA, Houlihan MD, Kohler TS. Sexual function in the penile cancer survivor: a narrative review. Transl Androl Urol. 2021;10(6):2544–2553. doi:10.21037/tau-20-1228
29. Bandieramonte G, Colecchia M, Mariani L, et al. Peniscopically controlled CO2 laser excision for conservative treatment of in situ and T1 penile carcinoma: report on 224 patients. Eur Urol. 2008;54(4):875–882. doi:10.1016/j.eururo.2008.01.019
30. Windahl T, Skeppner E, Andersson SO, Fugl-Meyer KS. Sexual function and satisfaction in men after laser treatment for penile carcinoma. J Urol. 2004;172(2):648–651. doi:10.1097/01.ju.0000132891.68094.87
31. Hadway P, Corbishley CM, Watkin NA. Total glans resurfacing for premalignant lesions of the penis: initial outcome data. BJU Int. 2006;98(3):532–536. doi:10.1111/j.1464-410X.2006.06368.x
32. Sedigh O, Falcone M, Ceruti C, et al. Sexual function after surgical treatment for penile cancer: which organ-sparing approach gives the best results? Can Urol Assoc J. 2015;9(7–8):E423–E427. doi:10.5489/cuaj.2801
33. Scarberry K, Angermeier KW, Montague D, Campbell S, Wood HM. Outcomes for organ-preserving surgery for penile cancer. Sex Med. 2015;3(2):62–66. doi:10.1002/sm2.56
34. Morelli G, Pagni R, Mariani C, et al. Glansectomy with split-thickness skin graft for the treatment of penile carcinoma. Int J Impot Res. 2009;21(5):311–314. doi:10.1038/ijir.2009.17
35. Perez J, Chavarriaga J, Ortiz A, et al. Oncological and functional outcomes after organ-sparing plastic reconstructive surgery for penile cancer. Urology. 2020;142:161–165 e1. doi:10.1016/j.urology.2020.03.058
36. Chavarriaga J, Becerra L, Camacho D, et al. Inverted urethral flap reconstruction after partial penectomy: long-term oncological and functional outcomes. Urol Oncol. 2022;40(4):169 e13–169 e20. doi:10.1016/j.urolonc.2022.02.006
37. Edward JPFS, Sarah PP, Patricia V, Courtney MPH. Sexual and voiding outcomes in post-penectomy penile cancer patients. JOJ Uro Nephron. 2018;6. doi: 10.19080/JOJUN.2018.06.555678
38. Kieffer JM, Djajadiningrat RS, van Muilekom EA, Graafland NM, Horenblas S, Aaronson NK. Quality of life for patients treated for penile cancer. J Urol. 2014;192(4):1105–1110. doi:10.1016/j.juro.2014.04.014
39. Romero FR, Romero KR, Mattos MA, Garcia CR, Fernandes Rde C, Perez MD. Sexual function after partial penectomy for penile cancer. Urology. 2005;66(6):1292–1295. doi:10.1016/j.urology.2005.06.081
40. Bhat GS, Nelivigi G, Barude V, Shastry A. Sexuality in Surgically Treated Carcinoma Penis Patients and Their Partners. Indian J Surg. 2018;80(1):19–23. doi:10.1007/s12262-016-1543-5
41. Bickell M, Beilan J, Wallen J, Wiegand L, Carrion R. Advances in surgical reconstructive techniques in the management of penile, urethral, and scrotal cancer. Urol Clin North Am. 2016;43(4):545–559. doi:10.1016/j.ucl.2016.06.015
42. Heyns CF, Fleshner N, Sangar V, Schlenker B, Yuvaraja TB, van Poppel H. Management of the lymph nodes in penile cancer. Urology. 2010;76(2 Suppl 1):S43–S57. doi:10.1016/j.urology.2010.03.001
43. McDougal WS. Preemptive lymphadenectomy markedly improves survival in patients with cancer of the penis who harbor occult metastases. J Urol. 2005;173(3):681. doi:10.1097/01.ju.0000153484.07200.f6
44. Teh J, Duncan C, Qu L, et al. Inguinal lymph node dissection for penile cancer: a contemporary review. Transl Androl Urol. 2020;9(6):3210–3218. doi:10.21037/tau.2019.08.37
45. Pizzocaro G, Piva L, Bandieramonte G, Tana S. Up-to-date management of carcinoma of the penis. Eur Urol. 1997;32(1):5–15. doi:10.1159/000480874
46. Horenblas S. Lymphadenectomy for squamous cell carcinoma of the penis. Part 2: the role and technique of lymph node dissection. BJU Int. 2001;88(5):473–483. doi:10.1046/j.1464-410x.2001.00379.x
47. Saisorn I, Lawrentschuk N, Leewansangtong S, Bolton DM. Fine-needle aspiration cytology predicts inguinal lymph node metastasis without antibiotic pretreatment in penile carcinoma. BJU Int. 2006;97(6):1225–1228. doi:10.1111/j.1464-410X.2006.06159.x
48. Network NCC. Penile cancer (Version 1.2023). Available from: https://www.nccn.org/professionals/physician_gls/pdf/penile.pdf.
49. Gkegkes ID, Minis EE, Iavazzo C. Robotic-assisted inguinal lymphadenectomy: a systematic review. J Robot Surg. 2019;13(1):1–8. doi:10.1007/s11701-018-0823-4
50. Ottenhof SR, Leone AR, Horenblas S, Spiess PE, Vegt E. Advancements in staging and imaging for penile cancer. Curr Opin Urol. 2017;27(6):612–620. doi:10.1097/MOU.0000000000000447
51. Hughes B, Leijte J, Shabbir M, Watkin N, Horenblas S. Non-invasive and minimally invasive staging of regional lymph nodes in penile cancer. World J Urol. 2009;27(2):197–203. doi:10.1007/s00345-008-0288-6
52. Mueller-Lisse UG, Scher B, Scherr MK, Seitz M. Functional imaging in penile cancer: PET/computed tomography, MRI, and sentinel lymph node biopsy. Curr Opin Urol. 2008;18(1):105–110. doi:10.1097/MOU.0b013e3282f151fd
53. Sadeghi R, Gholami H, Zakavi SR, Kakhki VR, Horenblas S. Accuracy of 18F-FDG PET/CT for diagnosing inguinal lymph node involvement in penile squamous cell carcinoma: systematic review and meta-analysis of the literature. Clin Nucl Med. 2012;37(5):436–441. doi:10.1097/RLU.0b013e318238f6ea
54. Hakenberg OW, Comperat EM, Minhas S, Necchi A, Protzel C, Watkin N. EAU guidelines on penile cancer: 2014 update. Eur Urol. 2015;67(1):142–150. doi:10.1016/j.eururo.2014.10.017
55. Pagliaro LC, Williams DL, Daliani D, et al. Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy for metastatic penile cancer: a phase II study. J Clin Oncol. 2010;28(24):3851–3857. doi:10.1200/JCO.2010.29.5477
56. Azizi M, Aydin AM, Hajiran A, et al. Systematic review and meta-analysis-is there a benefit in using neoadjuvant systemic chemotherapy for locally advanced penile squamous cell carcinoma? J Urol. 2020;203(6):1147–1155. doi:10.1097/JU.0000000000000746
57. Pettaway CA, Nicholson S, Spiess PE, et al. The international penile advanced cancer trial (InPACT): the first phase III trial for squamous carcinoma of the penis with regional lymph node metastases. J Clin Oncol. 2022;40(6_suppl):TPS7–TPS7. doi:10.1200/JCO.2022.40.6_suppl.TPS7
58. Canter DJ, Nicholson S, Watkin N, Hall E, Pettaway C, In PEC. The International Penile Advanced Cancer Trial (InPACT): rationale and current status. Eur Urol Focus. 2019;5(5):706–709. doi:10.1016/j.euf.2019.05.010
59. Yuan Z, Naghavi AO, Tang D, et al. The relationship between HPV status and chemoradiotherapy in the locoregional control of penile cancer. World J Urol. 2018;36(9):1431–1440. doi:10.1007/s00345-018-2280-0
60. Miralles-Guri C, Bruni L, Cubilla AL, Castellsague X, Bosch FX, de Sanjose S. Human papillomavirus prevalence and type distribution in penile carcinoma. J Clin Pathol. 2009;62(10):870–878. doi:10.1136/jcp.2008.063149
61. Lohneis P, Boral S, Kaufmann AM, et al. Human papilloma virus status of penile squamous cell carcinoma is associated with differences in tumour-infiltrating T lymphocytes. Virchows Arch. 2015;466(3):323–331. doi:10.1007/s00428-014-1713-4
62. Yugawa T, Kiyono T. Molecular mechanisms of cervical carcinogenesis by high-risk human papillomaviruses: novel functions of E6 and E7 oncoproteins. Rev Med Virol. 2009;19(2):97–113. doi:10.1002/rmv.605
63. Trimble CL, Morrow MP, Kraynyak KA, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled Phase 2b trial. Lancet. 2015;386(10008):2078–2088. doi:10.1016/S0140-6736(15)00239-1
64. Chahoud J, Pham R, Sonpavde G. Innovative systemic therapies for penile cancer. Curr Opin Urol. 2022;32(1):8–16. doi:10.1097/MOU.0000000000000941
65. Necchi A, Giannatempo P, Lo Vullo S, et al. Panitumumab treatment for advanced penile squamous cell carcinoma when surgery and chemotherapy have failed. Clin Genitourin Cancer. 2016;14(3):231–236. doi:10.1016/j.clgc.2015.08.001
66. Buonerba C, Di Lorenzo G, Pond G, et al. Prognostic and predictive factors in patients with advanced penile cancer receiving salvage (2nd or Later Line) systemic treatment: a retrospective, multi-center study. Front Pharmacol. 2016;7:487. doi:10.3389/fphar.2016.00487
67. Chahoud J, Tamil M, Necchi A. Second line salvage systemic therapy for advanced penile cancer. Urol Oncol. 2022;40(6):229–234. doi:10.1016/j.urolonc.2020.08.001
68. Jacob JM, Ferry EK, Gay LM, et al. Comparative genomic profiling of refractory and metastatic penile and nonpenile cutaneous squamous cell carcinoma: implications for selection of systemic therapy. J Urol. 2019;201(3):541–548. doi:10.1016/j.juro.2018.09.056
69. Baweja A, Mar N. Metastatic penile squamous cell carcinoma with dramatic response to combined checkpoint blockade with ipilimumab and nivolumab. J Oncol Pharm Pract. 2021;27(1):212–215. doi:10.1177/1078155220922602
70. Hahn AW, Chahoud J, Campbell MT, et al. Pembrolizumab for advanced penile cancer: a case series from a phase II basket trial. Invest New Drugs. 2021;39(5):1405–1410. doi:10.1007/s10637-021-01100-x
71. Chahoud J, Skelton W, Spiess PE, et al. Case report: two cases of chemotherapy refractory metastatic penile squamous cell carcinoma with extreme durable response to pembrolizumab. Front Oncol. 2020;10:615298. doi:10.3389/fonc.2020.615298
72. Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the Phase II KEYNOTE-158 study. J Clin Oncol. 2020;38(1):1–10. doi:10.1200/JCO.19.02105
73. Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–1365. doi:10.1016/S1470-2045(20)30445-9
74. Group LACO. Pembrolizumab combined with cisplatin-based chemotherapy as first-line systemic therapy in advanced penile cancer; 2022. Available from: https://ClinicalTrials.gov/show/NCT04224740.
75. Gassian N, Frontczak A, Mouillet G, et al. Activity and tolerability of maintenance avelumab immunotherapy after first line polychemotherapy including platinum in patients with locally advanced or metastatic squamous cell penile carcinoma: PULSE. Bull Cancer. 2020;107(5S):eS16–eS21. doi:10.1016/S0007-4551(20)30282-4
76. Aydin AM, Hall M, Bunch BL, et al. Expansion of tumor-infiltrating lymphocytes (TIL) from penile cancer patients. Int Immunopharmacol. 2021;94:107481. doi:10.1016/j.intimp.2021.107481
77. Doran SL, Stevanovic S, Adhikary S, et al. T-cell receptor gene therapy for human papillomavirus-associated epithelial cancers: a first-in-human, Phase I/II study. J Clin Oncol. 2019;37(30):2759–2768. doi:10.1200/JCO.18.02424
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
