Back to Journals » Therapeutics and Clinical Risk Management » Volume 15
Therapeutic Approaches to Gastric Hepatoid Adenocarcinoma: Current Perspectives
Authors Søreide JA
Received 8 October 2019
Accepted for publication 13 December 2019
Published 23 December 2019 Volume 2019:15 Pages 1469—1477
DOI https://doi.org/10.2147/TCRM.S204303
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Jon Arne Søreide1,2
1Department of Gastrointestinal Surgery, Stavanger University Hospital, Stavanger, Norway; 2Department of Clinical Medicine, University of Bergen, Bergen, Norway
Correspondence: Jon Arne Søreide
Department of Gastrointestinal Surgery, Stavanger University Hospital, POB 8100, Stavanger N-4022, Norway
Tel +47 905 31770
Fax +47 51519919
Email [email protected]
Abstract: Hepatoid adenocarcinoma of the stomach (HAS) is a rare subgroup of gastric cancer (GC). Morphologically, this tumor exhibits both adenocarcinomatous and hepatocellular differentiation, and most tumors show immunohistochemical staining for alpha-fetoprotein (AFP) or elevated AFP serum levels. The diagnosis of HAS is frequently delayed, and at least half of patients have advanced disease at the time of diagnosis. Despite a lack of evidence, treatment approaches have mostly followed principles for the treatment of common gastric cancer (CGC), including radical surgery in eligible patients with curative intent. The indications for and the type of adjuvant systemic treatments remain unclear. Additionally, there is a lack of evidence allowing any firm conclusions to be drawn regarding the best treatment for patients with metastatic HAS (mHAS). Chemotherapy regimens, including cisplatin-based chemotherapy, are considered the most efficient first-line systemic treatment in advanced situations. Their combination with targeted therapy (i.e., trastuzumab) in HER2-positive tumors seems promising. The rarity of these patients and the scarce and heterogeneous literature on this particular subgroup of GC make it difficult to provide any robust evidence for the clinical management of patients with HAS.
Keywords: hepatoid adenocarcinoma, stomach, gastric, alpha-fetoprotein, therapy, prognosis
Introduction
The seminal case report by Bourreille et al1 in 1970 entertained for the first time the distinct entity of an alpha-fetoprotein (AFP)-producing malignant tumor of the stomach. However, the term “hepatoid carcinoma of the stomach” (HAS) was coined by Ishikura et al2 in 1985. This rare subtype of primary gastric cancer (GC) exhibits both adenocarcinomatous and hepatocellular differentiation. HAS is prone to early metastasis, specifically to lymph nodes, the liver and lung, and the prognosis is regarded as dismal.3–5 This tumor may also arise in other extrahepatic organs, including the esophagus, biliary tract, pancreas, colon and lungs.4,6–11
Due to its rarity, a timely and proper identification and a correct diagnosis may be delayed and sometimes challenging.7,12,13 This may further jeopardize the prognosis of these patients.
The scientific literature on this topic comprises mostly single case reports and some small single-institution patient series. Accordingly, scientific evidence for proper clinical decision-making and for the evaluation of various treatment outcomes is limited. Nevertheless, efforts have been made to extract some core knowledge.3–5,7,14–17
In this article, we want to provide pertinent and novel knowledge from the recently available literature to enable a better understanding of the appropriate clinical management of patients with HAS.
Materials and Methods
A literature search in PubMed was performed, and articles published until July 2019 were included. Various terms, including “hepatoid adenocarcinoma”, “stomach”, “gastric”, “gastric cancer”, “adenocarcinoma”, “alpha-fetoprotein”, “treatment”, “prognosis”, and “surgery”, were included. We performed a rather extensive review of the recent, pertinent literature.7 The current search was not a true systematic review. However, emphasis was placed on articles that could add useful clinical information with regard to a correct diagnosis and timely and proper treatment decision-making for patients with HAS. Specifically, we considered publications from the last few years that could provide novel information on treatments and prognostication. Only articles written in English were evaluated.
Results
The Literature
A variety of papers were identified, most comprising single case reports and small single-institution series. Articles other than single case reports, including some selected small patient series and reviews of collected single case reports, are displayed in Table 1. In 2003, Adachi et al14 reported on 270 cases described in the Japanese literature, and in 2016, a systematic review of case reports and series from China was published by Qu et al16 Recently, Zeng et al3 described 34 patients from their institution and included 294 cases reported in the literature in their review. Of note is that the majority of papers beyond single case reports typically comprised between 10 and 30 patients from each institution,14 mostly originating from Japan,14,18–20 or China.5,15,21,22
 |
Table 1 Characteristics of Patients with Hepatoid Adenocarcinoma of the Stomach (HAS) |
HAS as a Rare Disease
HAS is a rare GC subtype and accounts for 0.38–1.6% of all GCs,5,20 although others have suggested an incidence between 1.3–15% of all gastric carcinomas.24 The estimated annual incidence is reported to be between 0.58–0.83 cases per million inhabitants.25,26 There is a male predominance (between 2–3-fold), and most patients are in their 60s or 70s at diagnosis (Table 1). Moreover, the majority of patients (i.e., at least 70–80%) have elevated serum alpha-fetoprotein (AFP) levels. At the time of diagnosis, clinical work-up, including imaging, will reveal advanced disease, including distant metastases (predominately in the liver and lung), in many patients and is confirmed in at least half of the patients. Accordingly, the prognosis for patients with HAS is generally dismal.3–5,15–17
Clinical Diagnosis
Clinical symptoms and signs are unspecific. Hence, the diagnosis of a gastric tumor is mostly made at gastroscopy with biopsy after referral due to abdominal pain, epigastric discomfort, hematemesis/melena, loss of weight, or clinical manifestations related to liver metastases.3,4 In particular, if elevated serum AFP levels have been encountered as a single sign, the diagnosis can be delayed or misdiagnosed as hepatocellular carcinoma (HCC).3,7,12,13 No correlation between preoperative AFP levels and tumor size has been found.20
Imaging
Computed tomography (CT) is now readily available and remains a cornerstone in the staging of patients with malignant solid tumors. However, with obvious lesions encountered on imaging, it is not always easy to define the true nature of this imaging feature. A spectrum of imaging features of HAS liver metastasis has been reported.27,28 On dynamic CT of the liver, an arterial hyperattenuation followed by a late wash is common. Notably, this pattern resembles a pattern similar to that of HCC. However, if an isolated portal vein thrombosis and a tendency for tumor necrosis are encountered related to liver nodules, a diagnosis of HAS is more likely.27,28 Magnetic resonance imaging (MRI) adds to the tools, and MRI diffusion-weighted imaging was recently introduced for the identification and diagnosis of primary HAS.29
With scarce pooled information in imaging aspects,3 the exact usefulness of PET/CT in the management of HAS patients remains to be shown.30
Pathology
HAS belongs to the indeterminate type of GC according to Lauren´s classification.31,32 The pathological diagnosis is based on morphological characteristics irrespective of the serum AFP levels or tissue AFP staining by immunohistochemistry (IHC).13,19,33 The primary HAS lesion comprises tubular as well as hepatoid components.17 For poorly differentiated adenocarcinoma of the solid type and small cell neuroendocrine cell carcinoma, the growth pattern resembles that of HAS, except for the lack of positive staining for AFP by IHC. Of note, Liu et al17 claimed that the diagnosis of HAS should be strictly based on a combination of hepatoid features and the secretion of AFP. Thus, while evaluating the incidence of HAS and making a consistent diagnosis, it is important to distinguish between HAS, AFP-producing GC without hepatoid features (AFPPGC) and common gastric cancer (CGC).17 The same authors also reported that vascular invasion is more often found in HAS compared to AFPPGC and CGC, which is likely associated with the observation that liver metastasis is more often encountered at the time of diagnosis, and patients with HAS have a shorter time until metachronous liver metastasis is diagnosed compared to patients diagnosed with AFPPGC or CGC.17
Human epithelial growth factor receptor 2 (HER2) is a member of the human epidermal growth factor receptor (EGFR) family. Amplification of this receptor has been observed in approximately 20% of GCs31 and has also been introduced as a marker for targeted therapy in advanced GC.34 HER2 overexpression has been frequently observed in the subgroup of patients with HAS.12,17,18 In contrast, no EGFR, KRAS or BRAF overexpression has been observed in the same subgroup.18
Recently, other molecular characteristics of HAS have also been revealed, showing that TP53, as the most frequently encountered mutation, was found in 30% of the tumors.32 The same authors reported that other genes, including CEBPA, RPTOR, WISP3, MARK1, and CD3EAP, were identified with rather high mutation rates (10–20%), and copy number gains (CNGs) at 20q11.21–13.12 were the most frequent genetic alteration in HAS patients.32
A more extensive morphological work-up using hepatocellular markers has been recommended.35 Researchers have found that AFP, SALL4, Hep-Par-1 and glypican 3 were significantly more positive in HAS than in nonhepatoid gastric adenocarcinoma, and the frequency of distant metastasis was significantly higher in SALL4-negative cases compared to SALL4-positive cases. Hep-Par-1 positivity was associated with liver metastases, and palate, lung, and nasal epithelium clone (PLUNC) positivity was correlated with lymph node metastases. In addition, the combination of PLUNC, Hep-Par-1 and SALL4 could serve as a reliable prognostic factor in HAS.35
Treatments
Primary Treatment
Surgery is a cornerstone for treatment with curative intent. Gastric resection is sometimes also indicated for palliation, and surgery is generally employed in most patients (90%).3,7,15,20 Operative principles in line with surgery for CGC are recommended.36,37 Whether neoadjuvant or adjuvant therapy should be a part of the primary treatment of this particular subgroup of GC patients is not clear. In a single-institution study from Japan during a ten-year time period, adjuvant chemotherapy including 5-fluorouracil, cisplatin, irinotecan, docetaxel, paclitaxel, methotrexate, mitomycin C or tegafur/uracil was offered to 28 (52.3%) of 53 patients at the discretion of each attending doctor, but details on the doses, frequencies, treatment durations and outcomes related to chemotherapy were not given.20 Moreover, Zhang et al15 described 14 patients who were surgically treated for a cure, including 8 patients who were given postoperative adjuvant chemotherapy. Without providing details on the specific adjuvant chemotherapy treatment employed, they claimed that adjuvant chemotherapy was an independent factor for improved survival.
Treatment of Metastatic Disease
The treatment of metastatic HAS (mHAS) remains even less elucidated. While a number of various drugs (e.g., 5-fluorouracil, cisplatin, irinotecan, docetaxel, paclitaxel, methotrexate, and mitomycin C) and various treatment schedules have been used for the treatment of mHAS, the scarce literature provides only fragmental information. Nevertheless, in a recent systematic review based on 18 case reports on the treatment of metastatic hepatoid gastric adenocarcinomas (n=11) or other primary locations (n=7), the authors concluded that cisplatin-based chemotherapy is the most efficient first-line systemic treatment in advanced situations, with a clinical response observed in 75% of the patients.38
The introduction of molecular targeted therapy has expanded the therapeutic tool box and improved the survival of patients with certain types of solid tumors, including GC.31,39 HER2 is overexpressed in 25% of patients with HAS,18 and trastuzumab in combination with chemotherapy has been offered to patients with HER2-positive CGC.34 Moreover, ramucirumab, a monoclonal antibody for VEGF receptor-2, has been approved as the first antiangiogenic drug for the treatment of advanced CGC. Arakawa et al40 recently reported a significant clinical response to ramucirumab monotherapy in an HAS patient with chemotherapy-resistant recurrent disease.
Anecdotally, Wang et al41 recently described a 56-year-old male with progressive therapy-resistant metachronous liver metastases, as judged by increased biomarker levels (CEA and AFP), despite transcatheter arterial chemoembolization (TACE) and radiofrequency ablation. Sorafenib had to be stopped due to early adverse reactions. The patient underwent ex vivo hepatectomy and a partial liver autotransplantation, and 20 months after the liver autotransplantation, he was reported to be relapse-free and still alive.41
Prognosis and Prognostication
The prognosis of HAS is regarded as poor and is predominantly related to the stage of the disease.15,20 As a distinct entity, the particular dismal prognosis of HAS patients is emphasized when comparing the prognosis of patients with AFP-producing GC without hepatoid features (AFPPGC) to those with CGC17 (Figure 1). Neither age, sex nor preoperative serum AFP levels are associated with prognosis.20 Nevertheless, elevated preoperative serum AFP levels ≥500 ng/mL are significantly associated with poor overall survival and tend to be associated with poor disease-free survival.32
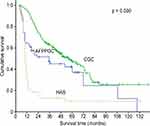 |
Figure 1 Differences in survival among the three groups (AFPPGC, HAS and CGC). Notes: Copyright © 2012 Wiley Periodicals, Inc. Reproduced from Liu X, Sheng W, Wang Y. An analysis of clinicopathological features and prognosis by comparing hepatoid adenocarcinoma of the stomach with AFP-producing gastric cancer. J Surg Oncol. 2012;106(3):299–303.17 Abbreviations: AFPPGC, AFP producing gastric cancer; HAS, hepatoid adenocarcinoma of the stomach; CGC, common gastric cancer. |
The presence of distant metastases, including synchronous or metachronous liver metastases, is significantly associated with poor survival.16,20 Improved survival is observed in patients surgically treated for a cure.3,20 However, information on the true importance of other employed treatments with regard to overall survival is conflicting.15
A more extensive morphological work-up using hepatocellular markers seems warranted.35 Researchers have found that AFP, Sal-like protein 4 (SALL4), Hepatocyte paraffin 1 (Hep-Par-1) and glypican 3 are significantly more positive in HAS than in nonhepatoid adenocarcinoma, and the frequency of distant metastasis was significantly higher in SALL4-negative cases compared to SALL4-positive cases. Hep-Par-1 positivity was associated with liver metastases, and PLUNC positivity was correlated with lymph node metastases. The combination of PLUNC, Hep-Par-1 and SALL4 could serve as a reliable prognostic factor in HAS.35
Discussion
HAS, as a particular subgroup of gastric carcinomas, is rare, with a suggested incidence between 1.3–15% of all gastric carcinomas.24 This wide incidence range reported in the literature likely mirrors some uncertainty or discrepancy with regard to criteria for the diagnosis of HAS.20 Others have reported the incidence to be between 1–6%,42 and from a Japanese single-institution series of 3374 GC patients, only 1.6% met the given criteria to be diagnosed with HAS.20
While a large proportion of the literature on HAS originates from authors from the East (i.e., Japan or China), this is most likely explained by the much higher incidence of GC, in general, in this part of the world. If the percentage of HAS differs between the East and the West (i.e., Europe and the US), the total number of patients with GC worldwide remains to be further evaluated.
HAS should be diagnosed by the characteristic histologic features of a gastric tumor resembling hepatocellular carcinoma.13,21,22,31 A distinction between HAS and AFP-positive carcinoma without hepatoid features is necessary.33 While Liu et al21 claim that the diagnosis of HAS should be based strictly on the combination of hepatoid morphological features and the secretion of AFP, the summarized results displayed in Table 1 indicate that a strict and consistent definition has not always been used by all researchers. Moreover, it remains uncertain which cut-off levels have been used in various studies for the definition of increased AFP levels.
A main obstacle when reviewing the pertinent literature on HAS is the heterogeneous reports available for data extraction. Important information on the definitions used, details on employed treatments and follow-up is frequently missing. Thus, incomplete data make it difficult to arrive at any firm conclusion and to provide evidence-based recommendations for treatment decision-making.
Nevertheless, the daily routine clinical challenge of suspecting the presence of HAS in a patient with unspecific symptoms or in a biopsy to confirm “endoscopic suspicious GC” remains.7,16 Another clinical scenario is faced by the clinician who encounters a patient with elevated AFP levels and is diagnosed with hepatic lesions on imaging. Thus, distinguishing a patient with metastatic HAS from a patient with HCC may not always be straightforward.17,27,35
The prognosis of HAS is regarded as dismal. Whether this is attributed to a more aggressive biology only or whether clinical challenges, including a delayed diagnosis and misinterpretation, play a role remains uncertain. As shown by Liu et al17 patients with HAS more often have liver metastases, and the time to the diagnosis of liver metastasis is shorter in HAS patients than in non-HAS AFP-producing GC patients and in those with CGC. A number of reports support this finding. However, in contrast, a recent report found a 5-year overall survival rate of 34%, and the authors suggested that the prognosis of HAS is not as poor as previously believed.20 Moreover, they found no correlation between preoperative AFP levels and prognosis, and long-time survivors had a primary tumor <10 cm in size, no peritoneal disease and had undergone R0/1 resection.20
As for most solid tumors, the confirmation of advanced disease with distant metastases is of great prognostic importance. While metastases to lymph nodes, the liver and lungs are the most frequently reported locations, cerebral metastasis has also been reported.43
Nevertheless, clinical evidence for the management of these patients is limited. Attention to various challenges, including the proper and timely identification of the patients, is of importance.7 If or how novel technologies, including artificial intelligence (AI)44 and liquid biopsies,45 may add to the clinical tools in this context remain to be shown. The diagnostic criteria and classifications should be discussed and acknowledged. In this regard, scrutinizing the radiomic signatures of the primary gastric tumor to predict occult peritoneal metastases may have important clinical implications.46 Treatments should be applied according to the stage of the disease, and the therapeutic principles in line with those for CGC should be followed.
Conclusions
HAS, as a rare disease, poses distinct diagnostic and therapeutic challenges for clinicians. The diagnosis is often delayed, and advanced disease is frequently found at the time of diagnosis. Accordingly, the prognosis is severe for many of these patients. Despite the fact that therapeutic approaches in line with principles for the treatment of CGC have been applied in single patients, true evidence for the indication and type of adjuvant chemotherapy and for the choice of drugs or a combination of drugs for the systemic chemotherapy treatment of patients with advanced disease is flawed.
Abbreviations
AFP, alpha-fetoprotein; AFPPGC, AFP-producing gastric cancer; AI, artificial intelligence; BRAF, proto-oncogene B-raf; CNG, copy number gain; CT, computer tomography; CGC, common gastric cancer; EGFR, epidermal growth factor receptor; GC, gastric cancer; HAS, hepatoid adenocarcinoma of the stomach; HCC, hepatocellular carcinoma; Hep-Par-1, hepatocyte paraffin 1; HER2, human epithelial growth receptor 2; IHC, immunohistochemistry; KRAS, K-ras gene; MRI, magnetic resonance imaging; PET, positron emission tomography; PLUNC, palate, lung, and nasal epithelium clone; SALL4, sal-like protein 4; TACE, transcatheter arterial chemoembolization; VEGF, vascular endothelial growth receptor.
Disclosure
Professor Jon Arne Søreide, MD, PhD, FACS, FISS is an approved specialist in general- and gastrointestinal surgery. The author reports no conflicts of interest in this work.
References
1. Bourreille J, Metayer P, Sauger F, Fondimare A. Existence d`alpha fetoprotein au cours d`un cancer secondaire du foie d`origine gastrique. Press Med. 1970;78(28):1277–1278.
2. Ishikura H, Fukasawa Y, Ogasawara K, Natori T, Tsukada Y, Aizawa M. An AFP-producing gastric carcinoma with features of hepatic differentiation. A case report. Cancer. 1985;56(4):840–848. doi:10.1002/(ISSN)1097-0142
3. Zeng XY, Yin YP, Xiao H, et al. Clinicopathological characteristics and prognosis of hepatoid adenocarcinoma of the stomach: evaluation of a pooled case series. Curr Med Sci. 2018;38(6):1054–1061. doi:10.1007/s11596-018-1983-1
4. Zeng X, Zhang P, Xiao H, et al. Clinicopathological features and prognosis of intestinal hepatoid adenocarcinoma: evaluation of a pooled case series. Oncotarget. 2018;9(2):2715–2725. doi:10.18632/oncotarget.v9i2
5. Yang J, Wang R, Zhang W, Zhuang W, Wang M, Tang C. Clinicopathological and prognostic characteristics of hepatoid adenocarcinoma of the stomach. Gastroenterol Res Pract. 2014;2014:140587. doi:10.1155/2014/140587
6. Hu M, Liu W, Yin F, Zhang D, Liu X, Lai J. Liver metastasis of hepatoid colonic adenocarcinoma: a rare and unusual entity with poor prognosis and review of the literature. Gastroenterol Res. 2018;11(6):430–435. doi:10.14740/gr1097
7. Søreide JA, Greve OJ, Gudlaugsson E, Størset S. Hepatoid adenocarcinoma of the stomach–proper identification and treatment remain a challenge. Scand J Gastroenterol. 2016;51(6):646–653. doi:10.3109/00365521.2015.1124286
8. Grossman K, Beasley MB, Braman SS. Hepatoid adenocarcinoma of the lung: review of a rare form of lung cancer. Respir Med. 2016;119:175–179. doi:10.1016/j.rmed.2016.09.003
9. Devi NR, Sathyalakshmi R, Devi J, Lilly SM. Hepatoid adenocarcinoma of the gall bladder-a rare variant. J Clin Diagn Res. 2015;9(8):ED09–ED10. doi:10.7860/JCDR/2015/10799.6324
10. Marchegiani G, Gareer H, Parisi A, Capelli P, Bassi C, Salvia R. Pancreatic hepatoid carcinoma: a review of the literature. Dig Surg. 2013;30(4–6):425–433. doi:10.1159/000355442
11. Kuroda N, Onishi K, Lee GH. Combined tubular adenocarcinoma and hepatoid adenocarcinoma arising in barrett esophagus. Ann Diagn Pathol. 2011;15(6):450–453. doi:10.1016/j.anndiagpath.2010.06.006
12. Fakhruddin N, Bahmad HF, Aridi T, et al. Hepatoid adenocarcinoma of the stomach: a challenging diagnostic and therapeutic disease through a case report and review of the literature. Front Med. 2017;4:164. doi:10.3389/fmed.2017.00164
13. Xie Y, Zhao Z, Li P, et al. Hepatoid adenocarcinoma of the stomach is a special and easily misdiagnosed or missed diagnosed subtype of gastric cancer with poor prognosis but curative for patients of pN0/1: the experience of a single center. Int J Clin Exp Med. 2015;8(5):6762–6772.
14. Adachi Y, Tsuchihashi J, Shiraishi N, Yasuda K, Etoh T, Kitano S. AFP-producing gastric carcinoma: multivariate analysis of prognostic factors in 270 patients. Oncology. 2003;65(2):95–101. doi:10.1159/000072332
15. Zhang J, Shi S, Shao Y, Zhang H. Clinicopathological and prognostic features of hepatoid adenocrcinoma of the stomach. Chin Med J. 2011;124(10):1470–1476.
16. Qu BG, Bi WM, Qu BT, et al. PRISMA-compliant article: clinical characteristics and factors influencing prognosis of patients with hepatoid adenocarcinoma of the stomach in China. Medicine (Baltimore). 2016;95(15):e3399. doi:10.1097/MD.0000000000003399
17. Liu X, Sheng W, Wang Y. An analysis of clinicopathological features and prognosis by comparing hepatoid adenocarcinoma of the stomach with AFP-producing gastric cancer. J Surg Oncol. 2012;106(3):299–303. doi:10.1002/jso.v106.3
18. Fujimoto M, Matsuzaki I, Nishino M, et al. HER2 is frequently overexpressed in hepatoid adenocarcinoma and gastric carcinoma with enteroblastic differentiation: a comparison of 35 cases to 334 gastric carcinomas of other histological types. J Clin Pathol. 2018;71(7):600–607. doi:10.1136/jclinpath-2017-204928
19. Ishikura H, Kirimoto K, Shamoto M, et al. Hepatoid adenocarcinomas of the stomach. An analysis of seven cases. Cancer. 1986;58(1):119–126. doi:10.1002/1097-0142(19860701)58:1<119::AID-CNCR2820580121>3.0.CO;2-U
20. Inoue M, Sano T, Kuchiba A, Taniguchi H, Fukagawa T, Katai H. Long-term results of gastrectomy for alpha-fetoprotein-producing gastric cancer. Br J Surg. 2010;97(7):1056–1061. doi:10.1002/bjs.7081
21. Liu X, Cheng Y, Sheng W, et al. Analysis of clinicopathologic features and prognostic factors in hepatoid adenocarcinoma of the stomach. Am J Surg Pathol. 2010;34(10):1465–1471. doi:10.1097/PAS.0b013e3181f0a873
22. Lin CY, Yeh HC, Hsu CM, Lin WR, Chiu CT. Clinicopathologial features of gastric hepatoid adenocarcinoma. Biomed J. 2015;38(1):65–69. doi:10.4103/2319-4170.126860
23. Su JS, Chen YT, Wang RC, Wu CY, Lee SW, Lee TY. Clinicopathological characteristics in the differential diagnosis of hepatoid adenocarcinoma: a literature review. World J Gastroenterol. 2013;19(3):321–327. doi:10.3748/wjg.v19.i3.321
24. Chang YC, Nagasue N, Kohno H, et al. Clinicopathologic features and long-term results of alpha-fetoprotein-producing gastric cancer. Am J Gastroenterol. 1990;85(11):1480–1485.
25. Shen Z, Liu X, Lu B, Ye M. Hepatoid adenocarcinoma of the stomach: a case report of a rare type of gastric cancer. Oncol Lett. 2016;11(2):1077–1080. doi:10.3892/ol.2015.4023
26. Xiao C, Wu F, Jiang H, et al. Hepatoid adenocarcinoma of the stomach: nine case reports and treatment outcomes. Oncol Lett. 2015;10(3):1605–1609. doi:10.3892/ol.2015.3430
27. Chang MY, Kim HJ, Park SH, et al. CT features of hepatic metastases from hepatoid adenocarcinoma. Abdom Radiol (NY). 2017;42(10):2402–2409. doi:10.1007/s00261-017-1150-3
28. Lin YY, Chen CM, Huang YH, et al. Liver metastasis from hepatoid adenocarcinoma of the stomach mimicking hepatocellular carcinoma: dynamic computed tomography findings. World J Gastroenterol. 2015;21(48):13524–13531. doi:10.3748/wjg.v21.i48.13524
29. Velut G, Mary F, Aflalo V, Aparicio T. Magnetic resonance imaging diffusion-weighted imaging for diagnosis of a gastric hepatoid adenocarcinoma. Dig Liver Dis. 2015;47(2):174. doi:10.1016/j.dld.2014.08.044
30. Seo HJ, Chung JK, Go H, Cheon GJ, Lee DS. A hepatoid adenocarcinoma of the stomach evaluated with (18)F-FDG PET/CT: intense (18)F-FDG uptake contrary to the previous report. Clin Nucl Med. 2014;39(5):442–445. doi:10.1097/RLU.0000000000000275
31. Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3(3):251–261. doi:10.3978/j.issn.2078-6891.2012.021
32. Wang Y, Sun L, Li Z, et al. Hepatoid adenocarcinoma of the stomach: a unique subgroup with distinct clinicopathological and molecular features. Gastric Cancer. 2019;22(6):1–10. doi:10.1007/s10120-019-00965-5
33. Nagai E, Ueyama T, Yao T, Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach. A clinicopathologic and immunohistochemical analysis. Cancer. 1993;72(6):1827–1835. doi:10.1002/(ISSN)1097-0142
34. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a Phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi:10.1016/S0140-6736(10)61121-X
35. Osada M, Aishima S, Hirahashi M, et al. Combination of hepatocellular markers is useful for prognostication in gastric hepatoid adenocarcinoma. Hum Pathol. 2014;45(6):1243–1250. doi:10.1016/j.humpath.2014.02.003
36. Coburn N, Cosby R, Klein L, et al. Staging and surgical approaches in gastric cancer: a systematic review. Cancer Treat Rev. 2018;63:104–115. doi:10.1016/j.ctrv.2017.12.006
37. Schwarz RE. Current status of management of malignant disease: current management of gastric cancer. J Gastrointest Surg. 2015;19(4):782–788. doi:10.1007/s11605-014-2707-x
38. Simmet V, Noblecourt M, Lizee T, et al. Chemotherapy of metastatic hepatoid adenocarcinoma: literature review and two case reports with cisplatin etoposide. Oncol Lett. 2018;15(1):48–54. doi:10.3892/ol.2017.7263
39. Yuan DD, Zhu ZX, Zhang X, Liu J. Targeted therapy for gastric cancer: current status and future directions (review). Oncol Rep. 2016;35(3):1245–1254. doi:10.3892/or.2015.4528
40. Arakawa Y, Tamura M, Aiba K, et al. Significant response to ramucirumab monotherapy in chemotherapy-resistant recurrent alpha-fetoprotein-producing gastric cancer: a case report. Oncol Lett. 2017;14(3):3039–3042. doi:10.3892/ol.2017.6514
41. Wang FQ, Lu Q, Yan J, et al. Ex vivo hepatectomy and partial liver autotransplantation for hepatoid adenocarcinoma: a case report. Oncol Lett. 2015;9(5):2199–2204. doi:10.3892/ol.2015.3041
42. Okita K, Noda K, Kodama T, et al. Carcino-fetal proteins and gastric cancer: the site of alpha-fetoprotein synthesis in gastric cancer. Gastroenterol Jpn. 1977;12(5):400–406. doi:10.1007/BF02774538
43. Zhang S. Cerebral metastaseis from hepatoid adenocarcinoma of the stomach. World J Gastroenterol. 2007;13(43):5787–5793. doi:10.3748/wjg.v13.i43.5787
44. Bi WL, Hosny A, Schabath MB, et al. Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin. 2019;69(2):127–157. doi:10.3322/caac.21552
45. Siravegna G, Mussolin B, Venesio T, et al. How liquid biopsies can change clinical practice in oncology. Ann Oncol. 2019;30(10):1580–1590. doi:10.1093/annonc/mdz227
46. Dong D, Tang L, Li ZY, et al. Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer. Ann Oncol. 2019;30(3):431–438. doi:10.1093/annonc/mdz001
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
