Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 14
The Whitening Properties of the Mixture Composed of Pomegranate, Osmanthus and Olive and the Protective Effects Against Ultraviolet Deleterious Effects
Authors Wang X, Heraud S, Thepot A, Dos Santos M, Luo Z
Received 20 January 2021
Accepted for publication 12 May 2021
Published 27 May 2021 Volume 2021:14 Pages 561—573
DOI https://doi.org/10.2147/CCID.S302997
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Xiaoling Wang, 1 Sandrine Heraud, 2 Amelie Thepot, 2 Morgan Dos Santos, 2 Zhen Luo 1
1Infinitus Company Ltd, Guangzhou, People’s Republic of China; 2LabSkin Creations, Edouard Herriot Hospital, Lyon, France
Correspondence: Xiaoling Wang Tel +86 13631351533
Email [email protected]
Background: Ultraviolet (UV) rays are the major environmental factor that damage skin physiology causing deleterious effects such as oxidation, photoaging and pigmentation. There has been considerable interest in using botanicals to prevent skin damages caused by UV irradiation.
Aim: In this study, three plant extracts were tested either individually or combined together (mixture) as well as their corresponding main active compound: pomegranate/punicalagin, osmanthus/verbascoside and olive/hydroxytyrosol. We evaluated the whitening and anti-photoaging properties of the nutritional mixture using 2D human culture model and a 3D full-thickness pigmented skin model exposed to UVB and UVA.
Methods: For exploring skin pigmentation, oxidation and aging, we performed cell viability, tyrosinase activity and melanin content assays as well as histology analysis (Whartin–Starry staining), immunodetection (PMEL, MDA, collagen type I and elastin) and carbonylated proteins analysis by electrophoresis separation.
Results: Results showed that the pomegranate extract and the active molecule punicalagin could reduce the tyrosinase activity and melanin content in melanocytes (P < 0.05). The mixture, pomegranate extract and punicalagin inhibited the melanin production and pre-melanosomal protein (PMEL) expression in the 3D skin pigmented model (P < 0.001). Furthermore, the mixture treatment repaired the expressions of collagen I and elastin decrease by UV exposure (P < 0.01). The mixture also significantly decreased lipid peroxidation (P < 0.001) and carbonylated proteins (P < 0.05) in the skin model compared to the UV-exposed condition.
Conclusion: To conclude, the mixture composed of pomegranate, osmanthus and olive extracts protects human skin from UV rays deleterious effects and exhibits antioxidative, anti-aging and skin whitening properties. Our data suggested pomegranate contributed to the whitening properties of the mixture notably through its main active compound, punicalagin. The mixture might be a good candidates for further development as natural antioxidant and skin care product.
Keywords: ultraviolet radiation, plants extracts, antioxidation, punicalagin, pigmentation
Introduction
Ultraviolet (UV) rays are the main component of solar radiation that our skin exposed to daily. The major acute effects of UV irradiation on normal human skin are sunburn inflammation, pigmentation and immunosuppression.1 The UV spectrum is divided into UVA (320–400 nm), UVB (280–320 nm) and UVC (200–280 nm) wavelengths. UVA radiation diffuses 3 to 4 mm deep into the skin and directly damages the cellular components, which can contribute to skin aging and carcinogenesis.2 Compared to UVA, UVB cannot penetrate deeper than 0.1 mm into skin and is more active in inducing skin carcinogenesis. UVB causes DNA damage and mutations and leads to induction of immunosuppressive cytokines in skin.3 UVC is effectively screened from the Earth’s surface by the ozone layer and has no real impact on our skins. The negative effects of UV radiation on human skin are usually related to UVA and UVB radiation. Studies showed that exposure of the skin to UV radiation leads to collagen damage and elastin degradation in photoaging.4,5 In addition, UV radiation induces reactive oxygen species (ROS) production and leads to cellular damage (proteins oxidation and DNA damage), apoptosis and cell death.6 Oxidative stress plays an important role in the aging process of skin.7 Furthermore, UV-induced ROS plays a critical role in pigmentation stimulation. Indeed, in response to ROS accumulation, keratinocytes secrete melanocyte activating factors, including αMSH and induce pigmentation.8 They also release nitric oxide, a free radical, which increases the dendricity and melanin synthesis in melanocytes by up-regulating the production of melanogenic enzymes.9 The potential of nutritional antioxidants to scavenge ROS would be a promising strategy to reduce melanogenesis and prevent skin aging caused by UV rays.
Market demands increasingly oriented towards natural antioxidants and innovative cosmeceutical ingredients of plant origin. As one of the oldest known edible fruits, pomegranate is a rich source of natural bioactive constituents. Various studies showed that pomegranate and its products exhibited potent antioxidative, antimicrobial and anticarcinogenic properties.10 Osmanthus is widely cultivated in Asia, especially in southern and central China. Osmanthus is well-known for its fragrance and is suitable for food additive.11 In addition, osmanthus plant extract had been showed the protective effects including free radical scavenging, antioxidative and anti-aging effects.12 Olive plant extract and its associated active molecule hydroxytyrosol were proven to have antioxidant and anti-atherogenic effects.13 Because osmanthus, pomegranate and olive plant extracts had the antioxidant activity, the mixture of these three plant extracts may exhibit favorable anti-oxidation and anti-aging effects against UV-induced skin damage. Furthermore, ROS induced by UV could promote the development of pigmentation the mixture might also have the whitening properties. The evaluation of UV-induced damages and the development of skincare that prevent photoaging and pigmentation are important in skin science research.14 However, the protective effects of the mixture composed of pomegranate, osmanthus and olive extracts on UV-induced photoaging and pigmentation remain to be explored.
In this study, we evaluated the properties of whitening and anti-photoaging of the nutritional mixture composed of pomegranate, osmanthus and olive extracts using 2D culture model and 3D full-thickness reconstructed human skin exposed to UV rays. The most abundant antioxidant molecule, punicalagin, verbascoside and hydroxytyrosol in pomegranate, osmanthus and olive, respectively, were also evaluated to explain the underlying mechanisms of the mixture.
Materials and Methods
Ethical Considerations and Human Cells Isolation
This study was approved by the ethical research committee “Comité de Protection des Personnes Sud-Est II” and declared to the French Research Ministry (declaration no. DC-2014-2281 delivered to LabSkin Creations, Lyon, France). Infant foreskins were collected according to the Declaration of Helsinki Principles. A written informed consent was obtained from infants’ parents according to the French bioethical law of 2014 (loi 94–954 du 29 Juillet 1994). Primary cultures of human fibroblasts, melanocytes and keratinocytes were established from healthy foreskin biopsies obtained from young male patients (under 3 years old). Epidermal keratinocytes and melanocytes and dermal fibroblasts were isolated from human foreskin as previously described.15 For melanocytes, skin samples with a phototype IV were used.
Cell Culture
Primary human melanocytes between passages 2 to 4 whose density was 20,000 cells/well were used for the 2D tests and 3D reconstructed skin. For the 3D skin models reconstruction, fibroblasts and keratinocytes were used in passages 6 and 4, respectively. Fibroblasts were cultured in a medium consisted in DMEM Medium with Glutamax (Gibco Life Technologies, USA) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich, Saint-Quentin-Fallavier, France) and 1% penicillin/streptomycin (Gibco Life Technologies, USA) at 37°C in 5% CO2. Keratinocytes were grown on irradiated human fibroblasts, using a medium containing DMEM and Ham’s F12 at a ratio of 3:1 (Gibco Life Technologies, USA) supplemented with adenine, 10ng/mL human epidermal growth factor (Austral Biologicals), hydrocortisone (Sigma-Aldrich, Saint-Quentin-Fallavier, France), insulin (Sigma-Aldrich, Saint-Quentin-Fallavier, France), with 10% FBS and 1% penicillin/streptomycin (Gibco Life Technologies, USA). Melanocytes were culture in a dedicated defined medium (Gibco Life Technologies, USA).
Reagents and Antibodies
The plant extracts from pomegranate (Punica granatum L.), osmanthus (Osmanthus fragrans Lour.) and olive (Olea europaea L.) were prepared using the dry plants powders dissolved in an aqueous solution under stirring and filtered. The active molecules, punicalagin, verbascoside and hydroxytyrosol corresponding to pomegranate, osmanthus and olive, respectively, were detected using high performance liquid chromatography (HPLC) analysis according to the Pharmacopoeia of the People’s Republic of China (version 2010). The standard substances of punicalagin, verbascoside and hydroxytyrosol were dissolved in dimethyl sulfoxide (DMSO) and then diluted into the cell culture medium.
Rabbit polyclonal anti-Collagen I (Novotec, France), Rabbit polyclonal anti-Elastin antibody (Abcam, UK), Mouse polyclonal anti-Melan A (Novotec, France), Rabbit polyclonal anti-PMEL (Sigma, USA), Rabbit polyclonal anti-Malondialdehyde (Abcam, UK) and Alexa-Fluor 568 anti-rabbit secondary antibody (Thermo Fisher Scientific, USA) were used for immunofluorescence staining.
Cell Viability Assay
To test the cytotoxicity of plant extracts and active molecules on cells, we performed colorimetric 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays on human keratinocytes, fibroblasts and melanocytes cultured in monolayer (2D culture) for 7 days. Briefly, human keratinocytes, melanocytes or fibroblasts were seeded into 96-well plates at the density of 10,000 cells/well and cultured until confluency. Cells were treated with the plant extract or the active molecule in the culture medium every day for 7 days. For the plant extracts, the tested concentrations varied from 0 to 1%. Active molecules concentrations were determined in function of their concentrations in their corresponding plant extract. For the mixture composed of osmanthus, pomegranate and olive, the tested concentrations varied from 0 to 1.5%. Cells treated with 0.05% sodium dodecyl sulfate (SDS) were used as positive control. At the end of the treatments, MTT solution (0.5 mg/mL) was added to cells for incubation at 37°C for 3 h. The formazan crystals were dissolved with DMSO for 30 minutes under stirring and the absorbance was then measured by spectrometry at 550 nm (Bio-Tek, Winooski, VT, USA). The percentage of viability for each condition was then calculated after normalization of the values to the untreated control, representing 100% viability.
Tyrosinase Activity Assay
Primary human melanocytes at passage 3 were cultured to form monolayers. Then cells were treated every day with the plant extract or the active molecule or the mixture for 3 days. At the end of the treatments, the tyrosinase activity was determined based on the oxidation of L-dopa in accordance with the method previously described with slight modifications.16 Cells were lysed with 0.1% Triton X-100 and a solution with 10 mM L-DOPA and 20mM MBTH was added. The mixtures were incubated for 3 h at 37°C and the absorbance at 490 nm was measured. OD values were normalized with the total protein content measured via a Bradford test. The percentage of tyrosinase activity for each condition was then calculated after normalization of the values in relation to the untreated control, representing 100% of tyrosinase activity. The assay was validated by a positive control with arbutin.
Melanin Content Assay
Primary human melanocytes at passage 3 were cultured to form monolayers. Cells were treated every day with the plant extract or the active molecule or the mixture for 4 days. The melanin content of the cultured melanocytes was evaluated in accordance with the method described previously with slight modifications.17 Cells were lysed the cells with 1M NaOH at 80°C for 2 hours and the absorbance was then measured by spectrometry at 505 nm. Absorbance values were compared with a standard range of synthetic melanin between 0 and 400 μg/mL. The assay is validated by a positive control with arbutin. The melanin content is normalized with the metabolic activity measured via an MTT test.
Full-Thickness Human Skin Models Exposed to UV
Full-thickness human 3D skin models were produced using a dermal scaffold made of chitosan-cross-linked collagen–GAG matrix prepared as previously described.18 For dermal reconstruction, human primary fibroblasts were cultured in DMEM medium supplemented with FBS, L-ascorbic acid and antibiotics. These dermal equivalent samples were cultured for 3 weeks at 37°C in 5% CO2. On the 21st day, human primary keratinocytes and melanocytes were seeded on the surface of the dermis at a density of 250,000 cells/cm2 to establish a pigmented skin equivalent. After 7 days of culture in immersion, the samples were raised at the air-liquid interface and continued to culture for two weeks. After treatment with the plant extracts or active molecules, the human 3D skin models were exposed to 100 mJ/cm2 of UVB and 12 J/cm2 of UVA for 24 h. After 24 hours of incubation, the samples were collected for histology and immunofluorescence staining.
Treatment
The mixture or pomegranate plant extract or punicalagin was added to the culture medium of the 3D skin models from day 15 to 21 and day 28 to 45. The mixture of the three plant extracts was composed of 0.01% osmanthus, 0.01% pomegranate and 0.001% olive.
Histological Staining
The full-thickness human 3D skin samples were fixed in formalin and embedded in paraffin. The 5-µm paraffin-embedded sections were deparaffinized and stained with Haematoxylin-Phloxin-Saffron (HPS) to evaluate the global cutaneous structure of samples. Furthermore, Whartin–Starry silver-plating kit (Merck, France) was used to evaluate melanin synthesis according to the manufacturer’s instructions. The images were observed with the system optical microscope Axio Observer D1/high resolution camera Axiocam (Zeiss, Le Pecq, France). Image processing and analysis were performed using the software Image J.
Immunofluorescence Staining
The immunofluorescence staining of frozen sections was performed to detect Elastin and Collagen I. The full-thickness human 3D skin samples fixed with ice-cold acetone for 10 minutes and then incubated for one hour with 4% bovine serum albumin (BSA) solution. The sections were then incubated overnight with primary antibodies at 4 °C overnight, followed by secondary antibodies for 1 h and finally mounted with the Hoechst nuclear dye (Thermo Fisher Scientific, MA, USA).
The immunofluorescence staining of paraffin sections was performed to detect Melan A, pre-melanosomal protein (PMEL) and Malondialdehyde (MDA). The 5-µm paraffin-embedded sections were deparaffinized and rehydrated. After heat antigen-retrieval, the sections were incubated with a solution of 4% BSA-5% H2O2 to inactivate the endogenous peroxidases. Unspecific sites were saturated with 4% BSA solution. The sections were then incubated overnight with the primary antibodies at 4 °C overnight, followed by secondary antibodies for 1 h and finally mounted with the Hoechst nuclear dye. Immunofluorescences were observed using an Axio observer D1 optical microscope (Zeiss, Le Pecq, France). Image analysis was performed with software Image J.
Carbonylated Proteins Analysis
The proteins were extracted from 3D human skin samples and quantified total proteins were assessed by the Bradford method.13 Carbonylated proteins were labelled with specific functionalized fluorescent probe and samples were resolved by high-resolution electrophoresis separation (polyacrylamide gradient 4–20%). Total proteins were post-stained with SYPRO Ruby Protein Gel Stain (Thermo Fisher Scientific, MA, USA). Gel image acquisition for carbonylated and total proteins by differential fluorescence was performed using the Ettan DIGE imager (GE Healthcare). The signal obtained for carbonylated proteins was quantified and normalized in relation with total proteins level resulting in Carbonyl Score values. Image processing and analysis were performed using Image J.
Statistical Analysis
All statistical analyses were performed using SPSS v.21.0 software (IBM, Armonk, NY, USA). Statistical differences between experimental groups were evaluated by one-way analysis of variance (ANOVA) with the least significant difference (LSD) test. All data are expressed as the mean ± SD. Statistical differences were considered significant at a P value of ≤0.05.
Results
Cytotoxicity of Plants Extracts in Primary Keratinocytes, Fibroblasts and Melanocytes
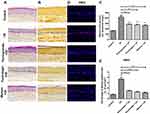
The cytotoxicity of plants extracts and their active molecules in the human cells was assessed by MTT assays. We considered a molecule concentration as cytotoxic when cells viability is fewer than 90% of the untreated control. There was no significant cytotoxicity of osmanthus extract (0.01–1%) in fibroblasts and keratinocytes. Osmanthus extract induced toxicity in melanocytes starting from the concentration 0.05%. Verbascoside, the active molecule of osmanthus extract, showed cytotoxicity in melanocytes and fibroblasts at the concentrations of 10 μM and 50 μM, respectively. For keratinocytes, any concentration of verbascoside showed a significant cytotoxicity (Figure 1A). Pomegranate extract showed cytotoxicity in fibroblasts and melanocytes starting at the concentration 0.05% and 0.01%, respectively. There was no significant cytotoxicity of pomegranate extract (0.01–1%) in keratinocytes. Punicalagin, the active molecule of pomegranate extract, showed no cytotoxicity in keratinocytes at the concentrations from 0.1 μM to 20 μM (Figure 1B). Punicalagin concentrations higher than 5 μM induced toxicity in fibroblasts and melanocytes. Olive extract showed the cytotoxicity in fibroblasts and keratinocytes starting from the concentration 1% and in melanocytes at 0.005%. For all cells types, the active molecule hydroxytyrosol induced cytotoxicity at the higher concentrations (>50 μM) (Figure 1C). The mixture composed of 0.15% osmanthus, 0.05% pomegranate and 0.005% olive extracts showed no significant cytotoxicity on fibroblasts and keratinocytes. In addition, the mixture composed of 0.01% osmanthus, 0.0033% pomegranate and 0.0003% olive extracts showed no cytotoxicity on melanocytes (Figure 1D). Based on these results, the appropriate concentrations of the mixture used for the following experiments were composed of 0.01% osmanthus, 0.01% pomegranate and 0.005% olive extracts.
Pomegranate Extracts and the Active Molecule Punicalagin Reduced Tyrosinase Activity and Melanin in Melanocytes
To test the whitening effects of plant extract on melanocytes, the tyrosinase activity and melanin content assays were performed. Arbutin, the positive control whitening agent, significantly reduced tyrosinase activity (P < 0.001) and melanin synthesis (P < 0.01) compared to the untreated control. Osmanthus extract and verbascoside reduced tyrosinase activity (P < 0.05) but not the melanin content in melanocytes (Figure 2A and B). Both pomegranate extract and punicalagin could inhibit tyrosinase activity (P < 0.05) and melanin synthesis (P < 0.001) with a dose–response relationship (Figure 2C and D). Olive extract and hydroxytyrosol reduced the melanin synthesis (P < 0.01) but not the tyrosinase activity in melanocytes (Figure 2E and F). These results suggested that the pomegranate extract and the active molecule punicalagin exhibited whitening properties. The optimal concentrations of pomegranate extract and punicalagin were 0.01% and 0.5 μM, respectively, which could suppress the tyrosinase activity and melanin synthesis but not impact the cell viability.
The Mixture of Plants Extracts treatment Reduced UV-Induced Pigmentation and Damage
Because the pomegranate extract and punicalagin inhibited the tyrosinase activity and melanin synthesis in melanocytes, we speculated that the mixture treatment might have the ability to reduce UV-induced pigmentation. Thus, the mixture, pomegranate extract and punicalagin were chosen to test the whitening properties using the full-thickness human 3D skin models. The full-thickness human 3D skin models were exposed to UV rays and the histological results showed that UV radiations decreased the thickness of the epidermis with the apparition of typical damages like sunburn cells. Pomegranate extract and the mixture of plants could effectively repair the epidermis (Figure 3A). The untreated control demonstrated a pigmented skin model with melanin expressed in the basal layer of the epidermis. As expected, the melanin in the epidermis was significantly increased after UV rays exposure (P < 0.001). Pomegranate, punicalagin and the mixture treatments reduced significantly the melanin synthesis by 31% (P < 0.001), 35% (P < 0.001) and 37% (P < 0.001) compared to the UV-exposed group (Figure 3B and C). Next, we detected the expression of PMEL that is essential for melanosomes biogenesis. UV radiations obviously increased the expression of PMEL in epidermis. Pomegranate, punicalagin and the mixture treatments reduced significantly the PMEL expression by 60% (P < 0.001), 66% (P < 0.001) and 69% (P < 0.001) compared to the UV exposure group (Figure 3D and E). There were no significant differences among the melanin synthesis and PMEL of these three treatments, which suggested that punicalagin played a critical role in the whitening effect of the mixture. Finally, we investigated Melan A that is a specific marker for melanocytes. Melan A positive cells number quantification showed no statistical difference in the basal layer of the epidermis, demonstrating the homogeneity of melanocytes density for all treatments in the pigmented skin samples (data not shown). These results indicate that the whitening effects induced by these treatments were not a result of melanocytes density diminution, but attributed to the melanin synthesis suppression.
The Mixture of Plants Extracts Treatment Inhibited UV-Induced Photoaging
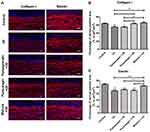
Except for preventing pigmentation, the anti-photoaging effects of skincare are also important in skin science research. The anti-photoaging effects of the mixture were evaluated using the 3D human full-thickness skin models. Collagen type I protein and Elastin fibres are the main components of the dermal extracellular matrix. As showed in Figure 4A, Collagen I and Elastin were highly expressed in the dermal extracellular matrix in the untreated control, indicating a physiological dermal morphology. UV exposure significantly disrupted the expressions of Collagen I (P < 0.01) and Elastin (P < 0.001), indicating the photoaging induced by UV rays. Both mixture treatment (P < 0.01) and punicalagin treatment (P < 0.05) repaired the reduction of Collagen I caused by UV exposure. However, the pomegranate extract showed no effect to repair the Collagen I reduction. Only the mixture treatment restored the expression of Elastin decreased by UV (P < 0.001) (Figure 4B and C). In brief, the mixture of plants extracts treatment could repaired the expressions of Collagen I and Elastin dermis as same as the untreated control, suggesting that the mixture could inhibit UV-induced photoaging.
The Mixture of Plants Extracts Treatment Inhibited UV-Induced Oxidation
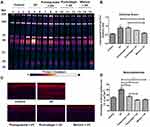
UV exposure is responsible for oxidative stress in skin and damages on cellular components.19 We have found that the mixture treatment could inhibit UV-induced pigmentation and photoaging, which suggested that the mixture have the ability to reduced UV-induced oxidation. The protein carbonylation rate, also named Carbonyl Score was assessed. Carbonylated proteins quantification showed that UV rays significantly increased the Carbonyl Score compared to the untreated control (P < 0.05), demonstrating the strong photooxidative damages in proteins caused by UV. The Carbonyl Scores of the pomegranate extract treatment showed no significantly different with the UV exposure group. Both the mixture treatment and punicalagin treatment could reduce protein carbonylation rate induced by UV exposure (P < 0.05). The mixture treatment significantly decreased Carbonyl Scores by 34% compared to the UV-exposed condition (P < 0.05) (Figure 5A and B). Furthermore, malondialdehyde (MDA) was detected to evaluate the level of lipid peroxidation in skin. As showed in the untreated control, MDA was slightly expressed in the epidermis as well as in fibroblasts in the dermis, indicating no cellular lipid damages. UV rays strongly increased the MDA expression in the epidermis and the dermis (P < 0.001), which suggested that UV-induced lipid peroxidation in the skin samples and consistent with the oxidative damages observed previously on proteins. Pomegranate extract, punicalagin and the mixture treatment groups demonstrated significant decrease of MDA expression in the skin model compared to the UV exposure group (P < 0.001) (Figure 5C and D). These results showed that the mixture treatment and punicalagin treatment could inhibit UV-induced oxidation.
Discussion
UV rays are the major environmental factor that affected the skin physiology and caused oxidation, photoaging and pigmentation. Skin pigmentation results from the production of melanin by highly specialized cells, melanocytes, localized in the basal layer and the transfer of melanin to the surrounding keratinocytes.20 Within melanocytes, melanin is synthesized in specific organelles named melanosomes. Factors underlying the development of skin pigmentation are not yet fully understood. Variations in pigmentation are due to differences in size, amount, distribution and the melanin content within the epidermal melanin unit rather than to differences in melanocyte density.21,22 The melanosome-specific proteins, including tyrosinase, ocular albinism type 1 protein and PMEL are involved in the melanosome maturation process. PMEL is one of the most important because it is required for the ultimate production of pigment.23 Melan-A, a melanocyte-specific protein, plays a vital role in the PMEL expression, trafficking and processing.24 In this study, we found the pomegranate extract and punicalagin inhibited the tyrosinase activity and melanin in melanocytes, which suggested that pomegranate extract and punicalagin had the whitening effects. Osmanthus extract and verbascoside reduced tyrosinase activity but not the melanin content in melanocytes. This could be explained by differences in sensitivity of these both assays but also by the concentrations we tested for the melanin content test, corresponding to the concentration range in the plants extract Mixture. Indeed, the mixture composed partly by Osmanthus corresponds to 1–5 µM verbascoside, but literature highlighted a decrease effect on melanin starting at 10µM verbascoside.25
The treatment with pomegranate extract, punicalagin or the mixture demonstrated the same reduction in the melanin content and PMEL expression. It was indicated that the punicalagin was the main whitening factor in the mixture. Moreover, the mixture treatment did not reduce the Melan-A protein, which suggested that whitening effect of punicalagin was not a result of melanocytes density diminution. These results indicated that punicalagin inhibited melanin synthesis contributed to the whitening effect of the mixture. In a study on monocultured melanocytes, it was demonstrated that punicalagin decreased melanin synthesis through downregulating microphthalmia-associated transcription factor (MITF) and tyrosinase, which was regulated by extracellular signal-regulated kinase (ERK) and Akt phosphorylation.26 A study on B16F10 melanoma cells highlighted the inhibition of melanogenesis by pomegranate through the decrease in tyrosinase activity and melanin production.27 This is consistent with our finding of tyrosinase activity was inhibited by pomegranate or punicalagin in melanocytes. In brief, the mixture has the whitening effect by inhibiting the tyrosinase activity and melanin synthesis.
Although the high melanin content confers better photoprotection, UV-induced photodamage is common in pigmentary disorders and cutaneous diseases.28 The mixture suppressed the melanin synthesis, which indicated that the mixture could reduce UV-induced photodamage. It has been showed that many natural products including antioxidants have whitening and anti-aging effects and can prevent UV-induced skin damage.29 Skin aging is largely affected by the cumulative damage from UV exposure. The integrity of skin tissue structures is formed primarily by collagen found in the dermal connective tissue. Collagen provides the skin with strength and resilience. The skin photoaging is marked by elevated elastosis, collagen fragmentation and irregular epidermal thickness.30 In this study, we found that the mixture treatment effectively prevented collagen I and elastin damages caused by UV, suggesting that the mixture is promising for photoaging prevention. However, pomegranate extract showed no effect on preventing photoaging. A recent study found that hydroxytyrosol from olive could prevent photoaging in human keratinocytes and fibroblasts.31 These results indicated that hydroxytyrosol from olive extracts could be responsible for the protective effect against photoaging in the mixture.
UV rays exposure causes tremendous insult to the skin and mechanism involved the ROS accumulation. In response to ROS accumulation, keratinocytes secrete melanocyte activating factors to induce pigmentation.8 Both mixture treatment and punicalagin treatment protected the skin from oxidative damages caused by UV rays. It was indicated that the antioxidant properties of mixture and punicalagin also played an important role on suppressing the process of UV-induced pigmentation. Excessive ROS caused oxidative damage to skin cells, which resulted in collagen breakdown.32 Punicalagin exhibits considerable antioxidant properties by reducing ROS and NO generation and increasing superoxide dismutase (SOD) 1 mRNA expression.33 The mixture and punicalagin repaired the expression of Collagen I disrupted by UV, which was largely due to the antioxidant properties of the mixture and punicalagin. Osmanthus and olive extracts also have the antioxidant activity.34,35 As the mixture exhibited the better effect against photoaging than the punicalagin, it was suggested that the antioxidant activity of osmanthus and olive extracts also contributed to the anti-photoaging effect. Antioxidants have been regarded as functional ingredients for anti-aging preparations to prevent skin damage. The mixture composed of pomegranate, osmanthus and olive, effectively exhibited the antioxidative, anti-aging and skin whitening properties. Furthermore, the raw materials of mixture are safe and easy to be obtained. These advantages demonstrate the mixture might be good candidate for further development as natural antioxidants and skin care products.
Conclusion
The mixture composed of pomegranate, osmanthus and olive extracts protects human skin from UV rays deleterious effects and exhibits antioxidative, anti-aging and skin whitening properties. The mixture might be good candidate for further development as natural antioxidants and skin care products.
Acknowledgments
We are very grateful to Song Ximing for his help in work management.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195:298–308. doi:10.1016/j.taap.2003.08.019
2. Ridley AJ, Whiteside JR, McMillan TJ, Allinson SL. Cellular and sub-cellular responses to UVA in relation to carcinogenesis. Int J Radiat Biol. 2009;85:177–195. doi:10.1080/09553000902740150
3. Yarosh DB, Boumakis S, Brown AB, et al. Measurement of UVB-Induced DNA damage and its consequences in models of immunosuppression. Methods. 2002;28:55–62. doi:10.1016/S1046-2023(02)00209-8
4. Brennan M, Bhatti H, Nerusu KC, et al. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem Photobiol. 2003;78:43–48. doi:10.1562/0031-8655(2003)078<0043:MMITMC>2.0.CO;2
5. Weihermann AC, Lorencini M, Brohem CA, de Carvalho CM. Elastin structure and its involvement in skin photoageing. Int J Cosmet Sci. 2017;39:241–247. doi:10.1111/ics.12372
6. de Jager TL, Cockrell AE, Du Plessis SS. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv Exp Med Biol. 2017;996:15–23.
7. Rinnerthaler M, Bischof J, Streubel MK, Trost A, Richter K. Oxidative stress in aging human skin. Biomolecules. 2015;5:545–589. doi:10.3390/biom5020545
8. Chakraborty AK, Funasaka Y, Slominski A, et al. Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: regulation by ultraviolet B. Biochimica Et Biophysica Acta (BBA) Mol Cell Res. 1996;1313:130–138. doi:10.1016/0167-4889(96)00063-8
9. Roméro-Graillet C, Aberdam E, Biagoli N, Massabni W, Ortonne J-P, Ballotti R. Ultraviolet B radiation acts through the nitric oxide and cGMP signal transduction pathway to stimulate melanogenesis in human melanocytes*. J Biol Chem. 1996;271:28052–28056. doi:10.1074/jbc.271.45.28052
10. Wang D, Özen C, Abu-Reidah IM, et al. Vasculoprotective effects of pomegranate (Punica granatum L.). Front Pharmacol. 2018;9:544. doi:10.3389/fphar.2018.00544
11. Liao X, Hu F, Chen Z. Identification and quantitation of the bioactive components in osmanthus fragrans fruits by HPLC-ESI-MS/MS. J Agric Food Chem. 2018;66:359–367. doi:10.1021/acs.jafc.7b05560
12. Xiong L, Mao S, Lu B, et al. Osmanthus fragrans flower extract and acteoside protect against d-galactose-induced aging in an ICR mouse model. J Med Food. 2016;19:54–61.
13. Gorzynik-Debicka M, Przychodzen P, Cappello F, et al. Potential health benefits of olive oil and plant polyphenols. Int J Mol Sci. 2018;19:686. doi:10.3390/ijms19030686
14. Levi K. UV damage and sun care: deciphering mechanics of skin to develop next generation therapies. J Mech Behav Biomed Mater. 2013;28:471–473. doi:10.1016/j.jmbbm.2013.02.008
15. Germain L, Rouabhia M, Guignard R, Carrier L, Bouvard V, Auger FA. Improvement of human keratinocyte isolation and culture using thermolysin. Burns J Int Soc Burn Injuries. 1993;19:99–104.
16. Winder AJ, Harris H. New assays for the tyrosine hydroxylase and dopa oxidase activities of tyrosinase. Eur J Biochem. 1991;198:317–326. doi:10.1111/j.1432-1033.1991.tb16018.x
17. Hamid MA, Sarmidi MR, Park CS. Mangosteen leaf extract increases melanogenesis in B16F1 melanoma cells by stimulating tyrosinase activity in vitro and by up-regulating tyrosinase gene expression. Int J Mol Med. 2012;29:209–217. doi:10.3892/ijmm.2011.840
18. Shahabeddin L, Berthod F, Damour O, Collombel C. Characterization of skin reconstructed on a chitosan-cross-linked collagen-glycosaminoglycan matrix. Skin Pharmacol. 1990;3:107–114. doi:10.1159/000210857
19. Kammeyer A, Luiten RM. Oxidation events and skin aging. Ageing Res Rev. 2015;21:16–29. doi:10.1016/j.arr.2015.01.001
20. Del Bino S, Duval C, Bernerd F. Clinical and biological characterization of skin pigmentation diversity and its consequences on UV Impact. Int J Mol Sci. 2018;19(9):2668. doi:10.3390/ijms19092668
21. Whiteman DC, Parsons PG, Green AC. Determinants of melanocyte density in adult human skin. Arch Dermatol Res. 1999;291:511–516. doi:10.1007/s004030050446
22. Stierner U, Rosdahl I, Augustsson A, Kågedal B. UVB irradiation induces melanocyte increase in both exposed and shielded human skin. J Invest Dermatol. 1989;92:561–564. doi:10.1111/1523-1747.ep12709572
23. Sun L, Hu L, Zhang P, et al. Silencing of PMEL attenuates melanization via activating lysosomes and degradation of tyrosinase by lysosomes. Biochem Biophys Res Commun. 2018;503:2536–2542. doi:10.1016/j.bbrc.2018.07.012
24. Chen YT, Stockert E, Jungbluth A, et al. Serological analysis of Melan-A(MART-1), a melanocyte-specific protein homogeneously expressed in human melanomas. Proc Nat Acad Sci. 1996;93:5915–5919. doi:10.1073/pnas.93.12.5915
25. Song HS, Sim SS. Acteoside inhibits alpha-MSH-induced melanin production in B16 melanoma cells by inactivation of adenyl cyclase. J Pharm Pharmacol. 2009;61:1347–1351. doi:10.1211/jpp.61.10.0011
26. Shin JS, Cho JH, Lee H, et al. Dual hypopigmentary effects of punicalagin via the ERK and Akt pathways. Biomed Pharmacother. 2017;92:122–127. doi:10.1016/j.biopha.2017.05.070
27. Kang SJ, Choi BR, Lee EK, et al. Inhibitory effect of dried pomegranate concentration powder on melanogenesis in B16F10 melanoma cells; involvement of p38 and PKA Signaling Pathways. Int J Mol Sci. 2015;16:24219–24242. doi:10.3390/ijms161024219
28. Ho SG, Chan HH. The Asian dermatologic patient: review of common pigmentary disorders and cutaneous diseases. Am J Clin Dermatol. 2009;10:153–168. doi:10.2165/00128071-200910030-00002
29. Zaid AN, Al Ramahi R. Depigmentation and Anti-aging Treatment by Natural Molecules. Curr Pharm Des. 2019;25:2292–2312. doi:10.2174/1381612825666190703153730
30. Poon F, Kang S, Chien AL. Mechanisms and treatments of photoaging. Photodermatol Photoimmunol Photomed. 2015;31:65–74. doi:10.1111/phpp.12145
31. Avola R, Graziano ACE, Pannuzzo G, Bonina F, Cardile V. Hydroxytyrosol from olive fruits prevents blue-light-induced damage in human keratinocytes and fibroblasts. J Cell Physiol. 2019;234:9065–9076. doi:10.1002/jcp.27584
32. Fisher GJ, Kang S, Varani J, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–1470. doi:10.1001/archderm.138.11.1462
33. Xu X, Li H, Hou X, et al. Punicalagin Induces Nrf2/HO-1 Expression via Upregulation of PI3K/AKT Pathway and Inhibits LPS-induced oxidative stress in RAW264.7 Macrophages. Mediators Inflamm. 2015;2015:380218. doi:10.1155/2015/380218
34. Wang H, Gan D, Zhang X, Pan Y. Antioxidant capacity of the extracts from pulp of Osmanthus fragrans and its components. Lebenson Wiss Technol. 2010;43:319–325. doi:10.1016/j.lwt.2009.08.003
35. De Bruno A, Romeo R, Fedele FL, Sicari A, Piscopo A, Poiana M. Antioxidant activity shown by olive pomace extracts. J Environ Sci Health B. 2018;53:526–533. doi:10.1080/03601234.2018.1462928
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.