Back to Journals » Infection and Drug Resistance » Volume 14
The Value of the inhA Mutation Detection in Predicting Ethionamide Resistance Using Melting Curve Technology
Authors Song Y, Wang G, Li Q, Liu R, Ma L, Li Q , Gao M
Received 16 September 2020
Accepted for publication 30 November 2020
Published 29 January 2021 Volume 2021:14 Pages 329—334
DOI https://doi.org/10.2147/IDR.S268799
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Yanhua Song,1 Guirong Wang,2 Qiang Li,1 Rongmei Liu,1 Liping Ma,1 Qi Li,3 Mengqiu Gao1
1Department of Tuberculosis, Beijing Chest Hospital, Capital Medical University, Beijing 101149, People’s Republic of China; 2National Clinical Laboratory on Tuberculosis, Beijing Key Laboratory on Drug-Resistant Tuberculosis Research, Beijing Chest Hospital, Capital Medical University, Beijing 101149, People’s Republic of China; 3Clinical Center on Tuberculosis, Beijing Chest Hospital, Capital Medical University, Beijing 101149, People’s Republic of China
Correspondence: Qi Li
Clinical Center on Tuberculosis, Beijing Chest Hospital, Capital Medical University, Beijing 101149, People’s Republic of China
Tel +86 10 89509322
Fax +86 1069546790
Email [email protected]
Mengqiu Gao
Department of Tuberculosis, Beijing Chest Hospital, Capital Medical University, Beijing 101149, People’s Republic of China
Tel +86 10 89509322
Fax +86 1069546790
Email [email protected]
Objective: This study aims to analyze the correlation between gene inhA mutations by melting curve technology and phenotypic drug susceptibility (DST) results of ethionamide (ETH), and evaluate whether gene inhA mutations detection can serve as a molecular marker in predicting ETH resistance.
Methods: A retrospective analysis was conducted on 382 strains of Mycobacterium tuberculosis (MTB) with the anti-tuberculosis drugs isoniazid (INH), rifampicin (RIF), ETH, and others. Phenotypic drug susceptibility and the results of inhA and katG genotypes (mutation and no mutation) were obtained using the melting curve technology MeltPro TB assay.
Results: Of the 382 clinical strains of MTB tested, 118 (30.9%) were resistant to INH, and 28 (7.3%) were resistant to ETH. Among the 28 phenotypically ETH-resistant strains, inhA mutations accounted for 42.9% (12/28). These ETH-resistant strains comprise 35.3% (12/34) of the 34 inhA mutant strains. Of 8 single inhA mutation strains (without katG or rpoB mutation), 4(50%) were resistant to INH; however, all of these 8 strains were sensitive to ETH.
Conclusion: The inhA mutation test may not be a reliable predictor of ETH resistance. Mutant inhA strains are not necessarily resistant to ETH. The strains with single inhA mutation (without katG or rpoB mutation) may be effective for ETH treatment. The use of ETH in clinical medicine should be guided by gene (other than inhA alone) detection and phenotypic drug susceptibility testing.
Keywords: melting curve technique, Mycobacterium tuberculosis, drug resistance, inhA, gene mutation, prothionamide, ethionamide
Introduction
The arrival and prevalence of drug-resistant tuberculosis has become a major problem in global tuberculosis (TB) control. In 2019, it was estimated that there were 500,000 cases of rifampicin-resistant TB (RR-TB) worldwide, of which 78% were multidrug-resistant TB (MDR-TB), resistant to both isoniazid (INH) and rifampicin (RIF). China has a high burden of TB and RR/MDR-TB and accounts for 14% of global RR/MDR-TB cases.1 INH and RIF are the core first-line drugs in the treatment of TB, but treatment of RR/MDR-TB with these first-line regimens will have poor effect. It is important to quickly identify the results of drug susceptibility tests (DST) in patients, especially tests relating to INH and RIF, to enable appropriate drugs to be chosen based on DST profiles.
Detection of drug-resistant target gene mutation can help to detect drug resistance earlier than phenotypic DST. Molecular DST in MTB has been widely used in clinical work to evaluate resistance to INH and RIF. KatG and inhA gene mutations are the main mechanism of INH resistance in MTB.2 Gene inhA (including promoter and coding areas) is one of important molecular markers of INH resistance, and inhA is also the molecular basis of cross resistance to ethionamide (ETH) prothionamide (PTH),2–4 a group C drug recommended by the WHO for the treatment of MDR-TB.5 ETH/PTH and INH are activated by monooxygenase EthA and catalase-peroxidase KatG. The activated forms of the two drugs act on a common target—the NADH-dependent enoyl-ACP reductase inhA (Rv1484) binding—with a bactericidal effect that affects cell wall synthesis.2 ETH/PTH has obvious adverse reactions such as nausea and drug-induced liver injury,6 and care is needed when choosing this drug. However, due to the convenience and ease of oral administration, ETH/PTH is still recommended in MDR-TB treatment in China.6 Based on the correlation between ETH/PTH resistance and inhA, clinicians may refer to inhA gene detection to guide the use of ETH/PTH.3,4,7
A variety of reports suggest that mutations in the inhA gene in TB strains can predict ETH/PTH resistance, although some studies have also shown that clinical strains with inhA mutations are sensitive to ETH.3,4,7 MeltPro TB assay utilizes the real-time polymerase chain reaction (PCR) probe-based melting curve analysis technique8 to detect the common drug-resistant mutation sites of katG, inhA and rpoB genes in MTB and rapidly diagnose INH and RIF resistance. This technique is widely used in clinical work.9,10 In this study, we analyze the correlation between inhA mutation test results and phenotypic ETH susceptibility through MeltPro TB assay and evaluate whether the inhA test can be used to guide the clinical application of ETH where phenotypic DST results are unavailable.
Materials and Methods
Study Subjects
In this retrospective study, patients undergoing treatment at Beijing Chest Hospital, Capital Medical University with positive MeltPro TB assay results for inhA and katG genes (mutated or not mutated) were screened from February 2015 to February 2016. Samples were tested for katG and inhA genes with culture and phenotypic DST and the dissociation curve method. Patients who met the following conditions were subsequently included in the analysis: cultured clinical specimens were positive for MTB; DST results were available for INH, RIF, Levofloxacin (Lfx), Amikacin (Am), Capreomycin (Cm), and ETH; and test results were positive for katG and inhA mutations. If two or more samples from the same patient were positive, the first sample was recorded. The basic information collected for each patient included their age, gender, disease diagnosis, initial treatment, and subsequent treatment.
The study was conducted in accordance with the Declaration of Helsinki (revised 2013). The study was approved by Beijing Chest Hospital, Capital Medical University (No.2019–86) and informed consent was obtained from all the patients.
Clinical Samples
Sample processing, culture, and drug susceptibility detection were conducted in accordance with the Laboratory Inspection Procedure of Tuberculosis Diagnosis.11 The clinical samples were treated and cultured on a modified Lowenstein–Jensen culture medium (Zhuhai intkr Co. Ltd., China). Positive colonies were cultured for DST and strain identification using the Lowenstein–Jensen proportion method. The critical concentration references were as follows: low-concentration INH 0.2 μg/mL, high-concentration INH 1.0 μg/mL, RIF 40 μg/mL, Levofloxacin2 μg/mL, Amikacin 30μg/mL, Capreomycin 40 μg/mL, and ETH 40μg/mL. Growth (cultivation) at this concentration was defined as indicating drug resistance.3
Genetic Testing of Samples
An automatic DNA extraction machine (Zeesan Biotech, Xiamen, China) and a paramagnetic particle method were used to extract crude DNA (1 mL) from the decontaminated samples according to the MeltPro TB assay instructions. The amplification program was used to analyze the melting. The fluorescence signal intensity was collected on the LightCycler 480 System (Indianapolis Roche Group) in the FAM and TET channels, and the melting temperature TM value was obtained by identifying the peak of the melting.7,12 The detection sites of INH resistance included inhA94, inhA promoter region −17 ~ −8 mutation, and katG315 codon mutation. The katG and inhA mutation results were recorded.
Statistical Methods
Data collection was carried out using Excel 2007 and the statistical analysis employed SPSS 17.0 software. The count data were represented by “rate (%)”, Χ2 TEST and Fisher’s exact test to compare the differences between the groups. The parameters of the continuous measurements were expressed as mean ± standard deviation and compared using a t-test. P < 0.05 indicated that a result was statistically significant.
Results
Basic Information of the Patients Enrolled
A total of 704 clinical specimens were tested using the dissociation curve method and found to be positive for inhA and katG genes. Specimens of MeltPro TB assay detect negative, specimens without phenotypic DST results and repeated samples were removed. Following this, 382 patients were enrolled in the study. These patients included 283 (74.1%) cases of sputum, 58 (15.2%) cases of bronchial lavage fluid, 1(0.3%) case of cerebrospinal fluid, and 40 (10.5%) cases of sanious; 292 were initial treatment patients and 90 were re-treatment patients. And 28 (7.3%) cases were resistant to ETH. The proportion of re-treatment patients with resistance to ETH was higher than the proportion of initial treatment patients (P < 0.001; Table 1). In addition, 11.0% (42/382) were MDR-TB, 10.2% (39/382) were pre-extensively drug resistant tuberculosis (pre-XDR-TB); 4.2% (16/382) were extensively drug resistant tuberculosis (XDR-TB).
 |
Table 1 Demographic and Clinical Characteristics of Patients |
Overall Isoniazid and Ethionamide Resistance
Of the 382 bacterial strains, 118 strains (30.9%) were resistant to INH. Among these INH resistant strains, 22.9% (27/118) were also resistant to ETH, and all of these strains were MDR-TB. Of the 118 INH-resistant strains, katG mutation accounted for 52.5% (62/118), inhA mutation accounted for 20.3% (24/118), and inhA+katG mutation accounted for 4.2% (5/118); strains with no mutation accounted for 22.9% (27/118). Of the 28 phenotypic ETH-resistant strains, 27 (96.4%) resistant to INH resistance. Of the same 28 strains, inhA mutation accounted for 42.9% (12/28). Of the 34 inhA mutant strains, 85.3% (29/34) had an inhA mutation without a katG mutation; among this group, 34.5% (10/29) showed low resistance to INH, 48.3% (14/29) showed high resistance to INH, and 13.8% (5/29) were sensitive to INH. The rates of ETH-resistance in low- and high-level INH-resistant strains showed no statistical differences (χ2 = 2.264; P = 0.132; Fisher’s test). The rate of single inhA mutations (without katG mutation) in strains with low INH-resistance was higher than the rate in strains with high INH-resistance (χ2 = 13.076; P < 0.001; Fisher’s test). All of 21 INH-resistant but non-MDR-TB strains were sensitive to ETH; four of these were inhA mutant strains. In addition, eight strains (four INH-resistant and four INH-sensitive) with single inhA mutation (without katG and rpoB mutations) were sensitive to ETH, and the patients carrying those eight strains were not initially treated with anti-TB drugs (Figure 1 and Table 2).
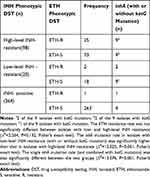 |
Table 2 Frequency of Ethionamide Resistance in Low-and High-Level Isoniazid Resistant and Isoniazid Susceptible Mycobacterium tuberculosis Isolates |
 |
Figure 1 Phenotypic DST results of ETH in MTB with inhA gene mutation enrolled in this study. |
Discussion
China has a high burden of TB and one of the highest incidences of MDR-TB in the world.13 Particularly in recent years, the incidence of DR-TB has been on the rise in China. Rifampicin (RIF) and isoniazid (INH) are the leading first-line anti-TB drugs, playing an important role in the treatment of TB. MDR-TB is widely regarded as an important factor in the failure of chemotherapy in treating TB. The resistance of genes to INH is more complicated, and is mainly caused by mutations in genes such as katG and inhA. Each mutation site has a certain correlation with drug resistance. ETH/PTH is a second-line drug treatment for TB, used mostly in MDR-TB and XDR-TB. According to data from domestic and overseas research, the majority of ETH/PTH-resistant strains also show INH resistance.10,14,15 In the present study, almost all ETH-resistant strains were also found to be resistant to both INH and RIF (96.4%), and the ETH-resistance rate in MDR-TB was 27.8%, which is consistent with our previous studies and similar data (20%–24.8%) from TB treatment institutions in China.16–19
The main molecular mechanisms underlying INH resistance are inhA and katG mutations, reported to account for 8%–43% and 50%–95% of drug-resistant strains, respectively.20 Tests for these two genetic mutations are used to diagnose the majority of instances of MTB resistance to INH. Mutations in the inhA gene are the molecular basis of cross resistance to ETH/PTH and INH. Therefore, the inhA gene can also aid in diagnosing ETH resistance. This study analyzed inhA and katG mutations and phenotypic INH and ETH susceptibility in clinical strains. Of the 118 INH-resistant strains analyzed, 56.7% (67/118) were katG mutations, and 24.5% (29/118) were inhA mutations. Of the 28 phenotypically ETH-resistant strains, inhA mutations accounted for 42.9%, which is consistent with previous reports.10,20 However, in this study, only 35.3% of the 34 inhA mutant strains were resistant to ETH, and only 42.9% of ETH-resistant strains had inhA mutations. A recent study in South Korea found that only 23 (67%) of 34 PTH-resistant strains had an inhA mutation, while data from a study in Guangzhou, China indicates that, of 46 PTH-resistant strains, 43.2% had an inhA promoter (12 strains were c-15t and 4 strains t-8c), and 6.2% had a coding gene mutation (all were S94A).10,21 The results of the present study show an inhA mutation rate in ETH-resistant strains (42.9%) similar to that in the study in China referenced above.
The question of whether inhA mutations can indicate phenotypic ETH resistance remains. Most previous research has focused on inhA mutations in INH- or ETH/PTH-resistant strains (mainly MDR-TB), while inhA mutations in sensitive strains have rarely been studied. There is a moderate level of evidence for an association between c-15t inhA promoter mutations and low-to-moderate INH resistance.22 This study shows that only 35.3% of 34 inhA mutant strains were resistant to ETH. The possible reasons for the inconsistency between inhA mutation and the ETH-resistant phenotype include the dissociation curve detection of mutant codons on inhA94 from −17 to −8 in the inhA promoter region. In addition, some positive mutations may be synonymous mutations and will not cause protein changes or ETH resistance.15,17,23 The ETH-resistant phenotype may have other regulatory mechanisms that cause strains with inhA mutations not to generate ETH resistance. This study also found that all strains with single inhA mutations (without katG or rpoB mutations) were sensitive to ETH. Taking the results of the present study in combination with those of other domestic studies, it can be argued that inhA mutations are not a reliable indicator of ETH resistance in China;17,24 the detection of inhA mutations is not necessarily a sign of resistance to ETH, and genotypic and phenotypic drug susceptibility must be detected simultaneously to guide clinical use of ETH.
Although inhA mutations have been shown to be associated with low INH resistance, this study shows that, of 29 inhA mutant strains, 48.3% (14/29), mainly INH- and RIF-resistant strains, had high INH resistance.2 InhA mutations were not a good indicator of low INH resistance. Other studies have also shown that inhA mutant (non-katG mutation) strains are highly resistant to INH, as are some strains combined with furA, oxyr-ahpc, or inhA double (c-15t combined with S94A or I194T) mutations.25 The data in this study were derived from clinical data, and no particular type of inhA mutation was specified. In addition, apart from inhA and katG315, no other INH-resistant genes were examined. Hence, the specific mechanism underlying the high drug resistance of nearly half of inhA remains unclear. Nonetheless, we found that the single inhA mutant strain (without katG and rpoB mutation) showed low-level resistance and susceptibility to INH, and these strains were sensitive to ETH. Hence, ETH and high-dose INH treatment may be effective for the majority of single inhA mutated INH-resistant strains.
However, this study has certain limitations. First, the present study is a summary of clinical data. Laboratory tests only reported whether or not the isolates had katG and inhA mutations; they did not provide detailed descriptions including the mutation sites. In addition, we did not discuss the mechanism of drug resistance in isolates where the inhA genotype and ETH-resistant phenotype were inconsistent. This will be the focus of further study, and we will evaluate the feasibility of using the targets identified by the melting curve analysis in the Chinese population. Third, the sample size was small, and all included patients came from the hospital where the author worked. The research findings may therefore contain some bias; however, they are still encouraging.
Conclusion
Although inhA mutations are associated with mechanisms of joint INH and ETH resistance, they may not be a reliable indicator of ETH resistance. In particular, TB strains with single inhA mutations (without katG or rpoB mutations) may remain sensitive to ETH. This is a preliminary study,future work is required to explore the mechanism of ETH resistance, to look for the reasons of inconsistency of phenotype and gene mutation.
Acknowledgments
We are particularly grateful to all the people who have given us help on our article.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. World Health Organization. Global tuberculosis report. 2020.
2. Vilchèze C, Jacobs JR. WR. Resistance to isoniazid and ethionamide in Mycobacterium tuberculosis: genes, mutations, and causalities. Microbiol Spectr. 2014;2(4):MGM2–2013. doi:10.1128/microbiolspec.MGM2-0014-2013
3. Vadwai V, Ajbani K, Jose M, et al. Can inhA mutation predict ethionamide resistance? Int J Tuberc Lung Dis. 2013;17(1):129–130. doi:10.5588/ijtld.12.0511
4. Niehaus AJ, Mlisana K, Gandhi NR, Mathema B, Brust JC. High prevalence of inhA promoter mutations among patients with drug-resistant tuberculosis in KwaZulu-Natal, South Africa. PLoS One. 2015;10(9):e0135003. doi:10.1371/journal.pone.0135003
5. World Health Organization. WHO consolidated guidelines on tuberculosis. Module 4: treatment - drug-resistant tuberculosis treatment. 2020. Availble from: https://www.who.int/tb/publications/global_report/TB20_Exec_Sum_20201014.pdf.
6. Tuberculosis society of Chinese Medical Association. Chinese expert consensus on multidrug-resistant tuberculosis and Rifampicin-resistant tuberculosis treatment. Chin J Tuberc Respir. 2019;42(10):733–749.
7. Lee JH, Jo KW, Shim TS. Correlation between genoType MTBDRplus assay and phenotypic susceptibility test for prothionamide in patients with genotypic isoniazid resistance. Tuberc Respir Dis (Seoul). 2019;82(2):143–150. doi:10.4046/trd.2018.0027
8. Pang Y, Dong H, Tan Y, et al. Rapid diagnosis of MDR and XDR tuberculosis with the MeltPro TB assay in China. Sci Rep. 2016;6:25330. doi:10.1038/srep25330
9. Haeili M, Fooladi AI, Bostanabad SZ, Sarokhalil DD, Siavoshi F, Feizabadi MM. Rapid screening of rpoB and katG mutations in Mycobacterium tuberculosis isolates by high-resolution melting curve analysis. Indian J Med Microbiol. 2014;32(4):398–403. doi:10.4103/0255-0857.142245
10. Darban-Sarokhalil D, Nasiri MJ, Fooladi AA, Heidarieh P, Feizabadi MM. Rapid detection of rifampicin- and isoniazid-resistant Mycobacterium tuberculosis using TaqMan allelic discrimination. Osong Public Health Res Perspect. 2016;7(2):127–130. doi:10.1016/j.phrp.2016.01.003
11. Basic Professional Committee of China National Defense Tuberculosis Association. TB diagnostic laboratory test procedures. Beijing: China Education Press; 2006.
12. Wang G, Dong W, Lan T, et al. Diagnostic accuracy evaluation of the conventional and molecular tests for Spinal Tuberculosis in a cohort, head-to-head study. Emerg Microbes Infect. 2018;7(1):109. doi:10.1038/s41426-018-0114-1
13. Jou R, Lee WT, Kulagina EV, et al. Redefining MDR-TB: comparison of Mycobacterium tuberculosis clinical isolates from Russia and Taiwan. Infect Genet Evol. 2019;72:141–146. doi:10.1016/j.meegid.2018.12.031
14. Morlock GP, Metchock B, Sikes D, Crawford JT, Cooksey RC. ethA, inhA, and katG loci of ethionamide-resistant clinical Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother. 2003;47(12):3799–3805. doi:10.1128/AAC.47.12.3799-3805.2003
15. Rueda J, Realpe T, Mejia GI, et al. Genotypic analysis of genes associated with independent resistance and cross-resistance to isoniazid and ethionamide in Mycobacterium tuberculosis clinical isolates. Antimicrob Agents Chemother. 2015;59(12):7805–7810. doi:10.1128/AAC.01028-15
16. Song YH, Wang GR, Huo FM, et al. Correlation analysis of inhA gene mutation in MTB and propioniazid resistance. Chin J Def Consumpt. 2018;40(8):821–824.
17. Liu YP, Wang J, Zhang JX, et al. Detection of clinical isolates of Mycobacterium tuberculosis resistant to isoniazid and propioniazid and study on related gene mutation. Chin J Def Consumpt. 2016;38(9):718–721.
18. Chen HF, Huang QS, Gao AX, et al. Observation on the sensitivity of mDR-MYCObacterium tuberculosis to second-line anti-tuberculosis drugs. J Nanjing Med Univ. 2014;34(1):69–71.
19. Li XD. Analysis of resistance of 174 mDR-Mycobacterium tuberculosis strains to second-line anti-tuberculosis drugs. Int J Lab Med. 2014;13:1732–1733,1748.
20. Zhang Y, Yew WW. Mechanisms of drug resistance in Mycobacterium tuberculosis: update 2015. Int J Tuberc Lung Dis. 2015;19(11):1276–1289. doi:10.5588/ijtld.15.0389
21. Tan Y, Su B, Zheng H, Song Y, Wang Y, Pang Y. Molecular characterization of prothionamide-resistant mycobacterium tuberculosis isolates in Southern China. Front Microbiol. 2017;8:2358. doi:10.3389/fmicb.2017.02358
22. Organization W.H. The use of next-generation sequencing technologies for the detection of mutations associated with drug resistance in Mycobacterium tuberculosis complex: technical guide. 2018.
23. Malinga L, Brand J, Jansen van Rensburg C, Cassell G, van der Walt M. Investigation of isoniazid and ethionamide cross-resistance by whole genome sequencing and association with poor treatment outcomes of multidrug-resistant tuberculosis patients in South Africa. Int J Mycobacteriol. 2016;5(Suppl 1):S36–S37. doi:10.1016/j.ijmyco.2016.11.020
24. Jia LL, Gao F, Zhang S. Relationship between high isoniazid resistance and propioniazid resistance.Inner. Mongolia Medical J. 2015;47(12):64.
25. Machado D, Perdigão J, Ramos J, et al. High-level resistance to isoniazid and ethionamide in multidrug-resistant Mycobacterium tuberculosis of the Lisboa family is associated with inhA double mutations. J Antimicrob Chemother. 2013;68(8):1728–1732. doi:10.1093/jac/dkt090
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
