Back to Journals » International Journal of General Medicine » Volume 15
The Value of Serum CHI3L1 for the Diagnosis of Chronic Liver Diseases
Received 7 March 2022
Accepted for publication 11 May 2022
Published 28 June 2022 Volume 2022:15 Pages 5835—5841
DOI https://doi.org/10.2147/IJGM.S364602
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Hanyu Qiu, Xiaomei Zhang
Department of Gastroenterology, Xiangya Hospital, Central South University, Changsha, Hunan, 410008, People’s Republic of China
Correspondence: Xiaomei Zhang, Department of Gastroenterology, Xiangya Hospital, Central South University, 87 Xiangya Road, Changsha, Hunan, 410008, People’s Republic of China, Tel: +86 731 8432 7331, Email [email protected]
Objective: To explore the value of chitinase-3-like protein 1 (CHI3L1) for the diagnosis of patients with chronic liver diseases such as chronic viral hepatitis B, liver cirrhosis and hepatocellular carcinoma (HCC), and analyze the correlation between serum CHI3L1 level and Child–Pugh grading of chronic liver diseases.
Methods: The serum CHI3L1 levels of 154 cases of chronic viral hepatitis B, 132 cases of liver cirrhosis and 88 cases of HCC were detected by ELISA, and 92 healthy subjects were taken as the control.
Results: The –serum CHI3L1 levels in HCC group, liver cirrhosis group and chronic viral hepatitis B group were higher than in healthy control group (P < 0.001). Serum CHI3L1 level showed a trend of increase in patients with chronic hepatitis to liver cirrhosis and to HCC. The diagnostic efficacy of serum CHI3L1 level on liver cirrhosis showed the sensitivity of 64.4% and the specificity of 96.7%. The diagnostic efficacy of serum CHI3L1 level on HCC showed the sensitivity of 86.8% and the specificity of 97.8%. Serum CHI3L1 level was higher in Child–Pugh grade B and C patients than in Child–Pugh grade A patients, and was positively correlated with Child–Pugh grading of liver function (rs = 0.301, P < 0.001).
Conclusion: Serum CHI3L1 level increased in chronic liver diseases and showed an increase trend with the progression of liver diseases. Serum CHI3L1 could be a biomarker for the auxiliary diagnosis of chronic liver diseases.
Keywords: chitinase-3-like protein 1, chronic hepatitis B, liver cirrhosis, hepatocellular carcinoma, Child–Pugh grading
Introduction
Chitinase-3-like protein 1 (CHI3L1), also known as YKL-40 and human cartilage glycoprotein-39 (HC-gp39), is a highly conserved glycoprotein that can bind to heparin, chitin and collagen.1,2 CHI3L1 plays an important role in tissue inflammation, tissue remodeling, fibrosis and cancer.3–5 Current studies have reported that serum CHI3L1 levels are elevated in liver inflammation, liver fibrosis and hepatocellular carcinoma (HCC), and CHI3L1 may be related to the prognosis of patients with chronic liver disease. However, there are few reports on the changes in serum CHI3L1 levels in different types of chronic liver diseases such as chronic viral hepatitis B, liver cirrhosis and HCC, the diagnostic value of CHI3L1 for liver cirrhosis and HCC, and the correlation between CHI3L1 and Child-Pugh grading. Therefore, in this study, we detected the levels of serum CHI3L1 in healthy subjects, patients with chronic viral hepatitis B, patients with liver cirrhosis and patients with HCC, and evaluated the value of serum CHI3L1 for the diagnosis of chronic liver diseases.
Subjects and Methods
Subjects
This study enrolled patients with different chronic liver diseases, including 88 cases of liver cancer, 132 cases of liver cirrhosis, and 154 cases of chronic hepatitis B, who were treated at Xiangya Hospital from August 2018 to December 2019. In addition, 92 healthy subjects visiting Physical Examination Center of Xiangya Hospital were selected as the control. This study was approved by the Ethics Committee of Xiangya Hospital and followed the Declaration of Helsinki. All participants signed informed consent.
Inclusion Criteria
(1) Inclusion criteria for chronic viral hepatitis B: had stage 4 hepatitis B, HBeAg positive, HBsAg positive, hepatitis B virus (HBV) DNA positive for more than 6 months, had antiviral therapy, no hepatitis D virus infection, and had chronic inflammation of the liver.
(2) Inclusion criteria for liver cirrhosis: including compensatory and decompensated liver cirrhosis, which met the diagnostic criteria for liver cirrhosis in the Guidelines for Diagnosis and Treatment of Liver Cirrhosis.
(3) Inclusion criteria for primary liver cancer: liver cancer can be diagnosed if any one of the following three items is met: (i) two typical imaging manifestations of liver cancer (ultrasound, enhanced CT, MRI or selective hepatic arteriography), with the focus >2 cm; (ii) a typical imaging manifestation of liver cancer, with the focus >2 cm and AFP >400 ng/mL; (iii) positive liver biopsy.
Exclusion Criteria
Pregnant, lactating women and persons with allergic constitution, severe diseases of important organs, other malignant tumors of the system, autoimmune diseases, and acute and chronic infections.
Elisa
Blood samples were collected from all participants, and serum CHI3L1 level was detected by using CHI3L1 ELISA kit (BioteK ELx50, BioTek Instruments, USA) following the manufacturer’s protocol.
Statistical Analysis
SPSS26.0 software was used for statistical analysis. Count data were expressed as constituent ratio, and measurement data were expressed as quartile or mean ± standard deviation. The Chi-square test was used to compare constituent ratios among multiple groups, and the Kruskal–Wallis H-test was used for comparison among multiple groups. The Nemenyi test was used for pairwise comparison between multiple groups of samples. The Mann–Whitney U-test was used for comparison between two groups, and P < 0.05 was considered statistically significant. Spearman correlation analysis was used to study the correlation of each indicator, and ROC curve was used to analyze the diagnostic efficacy of the indicator.
Results
General Data of the Subjects
A total of 88 patients with liver cancer were enrolled in this study, including 70 males and 18 females, with an average age of 56.9 ± 11.0 years. Total 51 patients with liver cancer were complicated with liver cirrhosis, and one patient was complicated with HCV infection. All patients with liver cancer had hepatocellular carcinoma except one case with cholangiocarcinoma.
According to Barcelona Clinic Liver Cancer Staging, 20 patients were in Stage 0 (Very Early), they had no vascular invasion, distant metastasis or lymph node invasion, all were single tumor and the largest tumor lesion was 1.9 cm; 21 patients were in Stage A (Early), they had no vascular invasion, distant metastasis or lymph node invasion, while one patient had 2 tumors the rest had single tumor, and the largest tumor lesion was 5.4 cm; 17 patients were in Stage B (Intermediate), they had no vascular invasion, lymph node metastasis or distant metastasis, the number of tumor in each patient was more than 3, and the largest tumor lesion was 6.6 cm; 18 patients were in Stage C (Advanced), 15 patients had portal vein invasion and 3 patients had distant metastasis, 7 patients had single tumor, while other 11 patients had more than 3 tumors, and the largest tumor lesion was 8.3 cm; and 12 patients were in Stage D (End-stage), 2 cases had no distant metastasis, while the other cases had distant metastasis, the number of tumors was more than 3 in all 12 patients and the largest tumor lesion was 8.2 cm.
In addition, this study enrolled 132 cases of liver cirrhosis, including 85 male and 47 female, with an average age of 57.0 ± 11.9 years; 154 cases of chronic viral hepatitis B, including 103 male and 51 female, with an average age of 54.0 ± 10.2 years; 92 healthy controls, including 56 male and 36 female, with an average age of 54.4 ± 9.7 years. The general information of the four groups of subjects is listed in Table 1. There was no significant difference in age and gender among the four groups of subjects.
 |
Table 1 General Information of the Subjects |
Comparison of Serum CHI3LI Level Between Patients with Chronic Liver Diseases and Healthy Controls
The serum CHI3LI levels in patients with different chronic liver diseases and healthy controls are shown in Table 2. The serum CHI3L1 levels of chronic hepatitis group, liver cirrhosis group and HCC group were higher than that of healthy control group. From chronic hepatitis to liver cirrhosis and then to HCC, serum CHI3L1 level showed a trend of increase. The Kruskal-Wall H-test showed that the difference in CHI3L1 level among the groups was statistically significant (χ2=122.194, P < 0.001).
 |
Table 2 Comparison of Serum CHI3L1 Levels in Patients with Chronic Liver Diseases |
ROC Curve Analysis of the Diagnostic Efficacy of Serum CHI3L1 on Liver Cirrhosis
Liver cirrhosis group was compared to healthy control group, and ROC curve was used to analyze the diagnostic efficacy of serum CHI3L1 level on liver cirrhosis. The AUC was 0.782 (95% confidence interval, 0.721–0.844). When the cutoff value was 63 ng/mL, the sensitivity was 64.4% and the specificity was 96.7% (Figure 1).
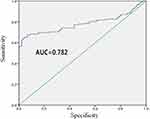 |
Figure 1 ROC curve analysis of diagnostic efficacy of serum CHI3L1 on healthy control group and liver cirrhosis group. |
ROC Curve Analysis of the Diagnostic Efficacy of Serum CHI3L1 on HCC
HCC group without liver cirrhosis was compared to healthy control group, and ROC curve analysis showed that AUC was 0.911 (95% confidence interval, 0.831–0.991). When the cutoff value was 67 ng/mL, the sensitivity was 86.8% and the specificity was 97.8% (Figure 2). HCC group combined with liver cirrhosis was compared to liver cirrhosis group, and ROC curve analysis showed that AUC was 0.644 (95% confidence interval, 0.562–0.726). When the cutoff value was 60 ng/mL, the sensitivity was 92% and the specificity was 35.6% (Figure 3).
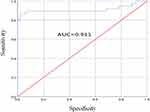 |
Figure 2 ROC curve analysis of diagnostic efficacy of serum CHI3L1 on healthy control group and HCC group. |
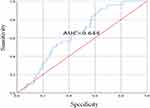 |
Figure 3 ROC curve analysis of diagnostic efficacy of serum CHI3L1 on liver cirrhosis group and HCC group. |
The Correlation Between Serum CHI3L1 Level and Child-Pugh Grade
According to the Child-Pugh scoring standard, 88 cases of liver cancer and 132 cases of liver cirrhosis were classified into Child-Pugh grades as follows: 95 Child-Pugh grade A patients, 75 Child-Pugh grade B patients, and 50 Child-Pugh grade C patients. Comparison of CHI3L1 levels of different Child-Pugh graded patients showed that serum CHI3L1 levels of Child-Pugh grade B patients (χ2=−31.561, P = 0.001) and Child-Pugh grade C patients (χ2=−46.031, P < 0.001) were significantly higher than those of Child-Pugh grade A patients, and there was no significant difference in serum CHI3L1 levels between Child-Pugh grade B patients and Child-Pugh grade C patients (χ2=−14.47, P = 0.639) (Table 3). In addition, serum CHI3L1 level was positively correlated with Child-Pugh grading of liver function (rs = 0.301, P < 0.001) (Table 4).
 |
Table 3 Comparison of Serum CHI3L1 Levels in Patients with Different Child–Pugh Grades |
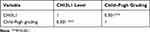 |
Table 4 Correlation Between Serum CHI3L1 Level and Child–Pugh Grading |
Discussion
CHI3L1 is a member of the glycosyl hydrolase family. The expression of CHI3L1 shows tissue specificity.6 Normal liver tissues only express CHI3L1 in the mesenchymal structure of the portal area, and hepatocytes do not express CHI3L1. Therefore, CHI3L1 is highly expressed in the liver and secreted into the extracellular matrix. The accumulation of extracellular matrix protein is the cause of liver fibrosis. Serum CHI3L1 level is closely related to tissue damage, such as various types of inflammation, tissue remodeling and cancer, and is usually related to the severity of the disease.
Johansen et al showed that serum CHI3L1 level in patients with various types of liver cirrhosis was higher than that in patients with liver fibrosis and chronic hepatitis, and serum CHI3L1 level in chronic hepatitis group was higher than that in the normal control group.7 Jiang et al found that serum CHI3L1 level gradually increased from chronic hepatitis B to liver cirrhosis and then to liver cancer.8 Kamal et al showed that serum CHI3L1 level was related to chronic hepatitis C virus (HCV) infection and liver fibrosis, the dynamic change of CHI3L1 could indicate the progression of fibrosis.9 These studies indicate that serum CHI3L1 is a new non-invasive indicator for the diagnosis and staging of liver fibrosis and cirrhosis, and can be used as a serum marker of liver fibrosis. Indeed, CHI3L1 is abundant in the liver and has been proposed as a biomarker of liver fibrosis in patients with chronic liver diseases of any etiologies such as HBV, HCV, non-alcoholic fatty liver diseases (NAFLD) and alcoholic liver diseases (ALD).10–12 Kumagai et al reported that serum YKL-40 was a fibrotic marker in NAFLD patients with a specificity of 0.70 and a sensitivity of 0.768.13 Tran et al found that YKL40 could be a fibrosis marker for ALD to distinguish cirrhosis or severe fibrosis (F3-F4) from F0-F2 with a sensitivity of 88.5 and a specificity of 50.8.14
However, Yang et al found that there was no significant difference in serum CHI3L1 level between patients with HCC and patients with end-stage liver disease (ESLD).15 Moreover, Malik et al observed that CHI3L1 level could not distinguish simple fatty liver disease from non-alcoholic steatohepatitis.16 Therefore, further studies are needed to verify the expression of CHI3L1 in different stages of chronic liver disease and clarify clinical value of serum CHI3L1 level in the diagnosis of liver fibrosis.
In this study, the diagnostic efficacy of CHI3L1 on liver cirrhosis was explored. The results showed that CHI3L1 had strong specificity in the diagnosis of liver cirrhosis, but the sensitivity was not good, suggesting that serum CHI3L1 detection has a certain diagnostic efficacy for patients with liver cirrhosis.
Primary liver cancer is one of the most common types of malignant tumors of the digestive system in China.17 Chronic hepatitis virus infection is the most important cause of primary liver cancer. Primary liver cancer is highly malignant and lacks typical symptoms in the early stage, and it is easy to miss and misdiagnose. Once obvious clinical symptoms appear, most of the patients have entered the middle and late stages, and the prognosis is not good. Currently, laboratories mainly use serum alpha-fetoprotein for HCC screening. However, it has been reported that about 30% of HCC patients have no elevated alpha fetoprotein level.18 Interestingly, CHI3L1 is located in human chromosome 1q31-q32, which is a region where HCC-related genes are often amplified.15 The expression of CHI3L1 protein in HCC tumor tissue is higher than that in adjacent normal tissues, and its level in serum of HCC patients is higher than that in healthy persons. Therefore, in this study, we explored the diagnostic efficacy of CHI3L1 on HCC. Since only some cases of HCC develop from liver cirrhosis, we compared serum CHI3L1 levels in patients with HCC combined with or without liver cirrhosis. The results showed that the diagnostic sensitivity of CHI3L1 for HCC combined with liver cirrhosis was 92%, significantly higher than that of AFP. The combination of serum CHI3L1 and AFP is expected to improve the sensitivity of early diagnosis of HCC.
Child-Pugh grading is composed of five indicators: prothrombin time, total bilirubin, serum albumin, ascites, and hepatic encephalopathy, and is a common indicator for clinical evaluation of liver function.19–21 According to the Child-Pugh scores of patients, liver function can be divided into three grades: A, B, and C. The mortalities related to liver disease in the 3 Child-Pugh grade patients within 1 year were <5%, 20% and 55%, respectively. However, at present, there are few studies on the correlation between CHI3L1 and Child-Pugh grading of liver function. Therefore, in this study, we analyzed the correlation between serum CHI3L1 levels in liver cirrhosis group and HCC group and Child-Pugh grading. The results showed that serum CHI3L1 levels of Child-Pugh grade B patients and Child-Pugh grade C patients were significantly higher compared to Child-Pugh grade A patients. In addition, serum CHI3L1 level was positively correlated with Child-Pugh grading. Notably, recent studies have reported the potential of CHI3L1 for the diagnosis of liver fibrosis.22,23 These data indicate that serum CHI3L1 level may be used as an indicator to evaluate liver function of patients with liver diseases.
This study has some limitations. First, this study is a single-center clinical study. Second, the sample size is relatively small. Further multi-center studies with large sample size are needed to confirm the value of serum CHI3L1 level for the diagnosis of chronic liver diseases.
In conclusion, serum CHI3L1 level increased in chronic liver diseases, and showed a trend of increase with the progression of liver diseases. Serum CHI3L1 could be a biomarker for the auxiliary diagnosis of chronic liver diseases.
Data Sharing Statement
All data are included in this manuscript.
Acknowledgment
This study was supported by the Natural Science Foundation of China (No. 81873575).
Disclosure
The authors declare no conflict of interest.
References
1. Rosa MD, Tibullo D, Saccone S, et al. CHI3L1 Nuclear localization in monocyte derived dendritic cells. Immunobiology. 2016;221(2):347–356.
2. Nielsen KR, Steffensen R, Boegsted M, et al. Promoter polymorphisms in the chitinase 3-like 1 gene influence the serum concentration of YKL-40 in Danish patients with rheumatoid arthritis and in healthy subjects. Arthritis Res Ther. 2011;13(3):R109.
3. Prakash M, Bodas M, Prakash D, et al. Diverse pathological implications of YKL-40: answers may lie in ‘outside-in’ signaling. Cell Signal. 2013;25(7):1567–1573.
4. Areshkov PO, Avdieiev SS, Balynska OV, Leroith D, Kavsan VM. Two closely related human members of chitinase-like family, CHI3L1 and CHI3L2, activate ERK1/2 in 293 and U373 cells but have the different influence on cell proliferation. Int J Biol Sci. 2012;8:39–48.
5. Scully S, Yan W, Bentley B, Cao QJ, Shao R. Inhibitory Activity of YKL-40 in Mammary Epithelial Cell Differentiation and Polarization Induced by Lactogenic Hormones: a Role in Mammary Tissue Involution. PLoS One. 2011;6(10):e25819.
6. Huang H, Wu T, Mao J, et al. CHI3L1 Is a Liver-Enriched, Noninvasive Biomarker That Can Be Used to Stage and Diagnose Substantial Hepatic Fibrosis. OMICS. 2015;19(6):339–345.
7. Johansen JS, Christoffersen P, Møller S, et al. Serum YKL-40 is increased in patients with hepatic fibrosis. J Hepatol. 2000;32(6):911–920.
8. Jiang Z, Wang S, Jin J, et al. The clinical significance of serum chitinase 3-like 1 in hepatitis B-related chronic liver diseases. J Clin Lab Anal. 2020;34(5):e23200.
9. Kamal SM, Moustafa KN, Chen J, et al. Duration of peginterferon therapy in acute hepatitis C: a randomized trial. Hepatology. 2006;43(5):923–931.
10. Wang S, Hu M, Qian Y, et al. CHI3L1 in the pathophysiology and diagnosis of liver diseases. Biomed Pharmacother. 2020;131:110680.
11. Nishimura N, De Battista D, McGivern DR, et al. Chitinase 3-like 1 is a profibrogenic factor overexpressed in the aging liver and in patients with liver cirrhosis. Proc Natl Acad Sci U S A. 2021;118(17):e2019633118.
12. Lee CG, Da Silva CA, Dela Cruz CS, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73:479–501.
13. Kumagai E, Mano Y, Yoshio S, et al. Serum YKL-40 as a marker of liver fibrosis in patients with non-alcoholic fatty liver disease. Sci Rep. 2016;6:35282.
14. Tran A, Benzaken S, Saint-Paul MC, et al. Chondrex (YKL-40), a potential new serum fibrosis marker in patients with alcoholic liver disease. Eur J Gastroenterol Hepatol. 2000;12(9):989–993.
15. Yang JD, Kim E, Pedersen RA, et al. Utility of Serum YKL-40 as a Tumor-Specific Marker of Hepatobiliary Malignancies. Gut Liver. 2010;4(4):537–542.
16. Malik R, Chang M, Bhaskar K, et al. The clinical utility of biomarkers and the nonalcoholic steatohepatitis CRN liver biopsy scoring system in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2009;24(4):564–568.
17. Li J, Li H, Zeng H, et al. Trends in high-risk rates and screening rates for the population-based cancer screening program on esophageal, stomach and liver cancer in China, 2010-2016. J National Cancer Center. 2021;1(3):101–107.
18. Xu WJ, Guo BL, Han YG, et al. Diagnostic value of alpha-fetoprotein-L3 and Golgi protein 73 in hepatocellular carcinomas with low AFP levels. Tumour Biol. 2014;35(12):12069–12074.
19. Wu P, Gao M, Dong J, et al. The role of mTOR signaling pathway in regulating autophagy in liver injury of TX mice with Wilson’s disease. Biocell. 2021;45(1):109–117.
20. Wang Y, Duan Y, Chen K, Li H, Quan Y. Protective effects of docosahexaenoic acid against non-alcoholic hepatic steatosis through activating of JAK2/STAT3 signaling pathway. Biocell. 2021;45(2):307–316.
21. Rassam F, Olthof PB, Bennink RJ, van Gulik TM. Current Modalities for the Assessment of Future Remnant Liver Function. Visc Med. 2017;33(6):442–448.
22. Huang Q, Wu J, Huang C, Wang X, Xu Z. A noninvasive diagnostic model for significant liver fibrosis in patients with chronic hepatitis B based on CHI3L1 and routine clinical indicators. Ann Palliat Med. 2021;10(5):5509–5519.
23. Das A, Kamrul-Hasan A, Kabir MR, Das S, Zaki K, Al Mahtab M. Evaluation of Chitinase 3-like 1 (CHI3L1) as a noninvasive biomarker of hepatic fibrosis in patients with Hepatitis B virus-related compensated chronic liver disease. J Family Med Prim Care. 2021;10(4):1694–1698.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
