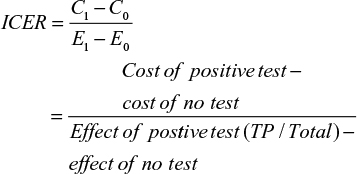Back to Journals » ClinicoEconomics and Outcomes Research » Volume 9
The value of corneoscleral rim cultures in keratoplasty: a systematic review and cost-effectiveness analysis
Authors Kiatos E, Armstrong JJ , Hutnik CML, Tsioros SM, Malvankar-Mehta MS , Hodge WG
Received 19 April 2017
Accepted for publication 28 June 2017
Published 9 August 2017 Volume 2017:9 Pages 459—474
DOI https://doi.org/10.2147/CEOR.S139949
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Efstathia Kiatos,1 James J Armstrong,2,3 Cindy ML Hutnik,3,4 Stephen M Tsioros,5 Monali S Malvankar-Mehta,1,4 William G Hodge1,4
1Department of Epidemiology and Biostatistics, 2Department of Pathology, 3Department of Ophthalmology, Schulich School of Medicine and Dentistry, Western University, 4Department of Ophthalmology, Ivey Eye Institute, St Joseph’s Health Care London, 5Department of Kinesiology, Western University, London, ON, Canada
Purpose: This study evaluated the performance of donor corneoscleral rim cultures for predicting infection after corneal transplantation, and determines if there is a correlation between positive corneoscleral rim cultures and postkeratoplasty infection.
Design and data sources: This was a systematic review, prognostic accuracy analysis, and cost-effectiveness analysis. Databases searched were: Medline (Ovid), Embase (Ovid), CINAHL, Cochrane Library, Web of Science, and BioSis Previews. Grey literature was also explored.
Materials and methods: A systematic review was conducted to locate published and unpublished studies. All studies examining corneal button contamination and its association with endophthalmitis and keratitis posttransplantation were included. Extracted data were used to calculate sensitivity, specificity, positive predictive value, and negative predictive value. Cost data from the London Laboratory Services Group in London, ON were used to calculate the cost-effectiveness of culturing donor rim cultures.
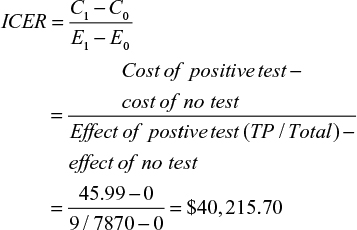
Results: Of 7,870 grafts, 954 had a positive rim culture (12.1%), with 12 patients going on to develop keratitis or endophthalmitis (1.3%). The prevalence of keratitis and endophthalmitis in this study was 0.15%, and the positive predictive value 1.5%. Of the 12 infections, nine were fungal and three bacterial. The estimated cost of a positive and negative test result was CAD$45.99 and $14.15, respectively. The cost to run all 7,870 tests was estimated to be $141,735.86, with an incremental cost-effectiveness ratio of $40,215.70.
Conclusion: There was a significant divergence between bacterial and fungal rim-culture results. Bacterial cultures predicted clinical infection poorly, did not change management, and were expensive. Fungal cultures predicted clinical infection in over 10% of patients, had the potential to change management, and were 40% less expensive than full rim culturing (bacterial and fungal tests). Fungal rim cultures may be considered in areas where fungal infection rates are high.
Keywords: corneoscleral rim cultures, eye infection, keratoplasty, culture techniques
Introduction
Keratoplasty (corneal transplantation) is the surgical procedure where damaged or diseased cornea is replaced with donated corneal tissue from a cadaver. The number of procedures conducted has been steadily rising over the past decade. In 2016, there were 82,994 grafts1 provided for transplants in the US, a 5% increase from the previous year.2 The aging population in North America may be partly responsible for this increase. In 2005, Canada’s seniors represented 13.2%3 of the population (4.2 million people). It is projected that this will increase to 24.5% (9.8 million) in 2036, and 27.2% (11.5 million) in 2056.3 A direct result of this population growth will be an increase in corneal transplant procedures (penetrating keratoplasty, anterior lamellar keratoplasty, and endothelial keratoplasty). This trend highlights the need to make evidence-based decisions on resource allocations in both publicly and privately funded environments to ensure corneal transplants are cost-effective as they continue to increase in number.
Corneoscleral rim cultures are performed to detect microorganisms.4 At the time of transplantation, the outer edge of the corneal tissue is removed and submitted for culture.5 The transplantation proceeds, and 24–72 hours later the lab will report the culture result to the surgeon. A preliminary and final culture result is often reported. This is a crucial period: if infection occurs, it may happen during this time window. Unfortunately, the results of the culture may not return in time to influence treatment decisions if infection does occur.
Keratitis and endophthalmitis are uncommon yet potentially devastating complications that may result in severe vision loss and even complete enucleation of the eye in cases of endophthalmitis. In the 1980s and early 1990s, there was a focus on the presence of contaminants in cultures and their role in postkeratoplasty infection.6–12 In 1983, Leveille et al found that eyes with positive donor-rim cultures had a 22-fold increased incidence of endophthalmitis.6 As a result of these articles, the procedure of culturing rims expanded to most settings. In the 1990s/2000s, many researchers began to recognize that the prognostic value of screening donor rims at the time of surgery remained unclear.4,13,14 In 2007, Wilhelmus and Hassan14 conducted a systematic review and meta-analysis reporting the discriminatory performance of donor corneoscleral rim cultures for predicting endophthalmitis after corneal transplantation. They found that from 1979 to 2006, 14% of corneoscleral rim cultures were positive, with only 0.2% of those positive results resulting in endophthalmitis. They concluded that endophthalmitis after penetrating keratoplasty is more likely with a culture-positive rim, but that better evidence is needed to determine the prognostic value and manner of routine microbiological screening. There has been a split in the literature: there have been studies recommending this practice for diagnosis and treatment of postoperative infection,15,16 yet several studies claim that there is no correlation between positive rim cultures and infection.13,14,17
The protocols put out by the overseeing associations fail to add clarity to the practice. In the 2015 Medical Standards report, the Eye Bank Association of America (EBAA) recognized that bacteriologic contamination of donor eyes does not typically lead to infection, and that culturing of eye-bank donor eyes may be performed.18 The EBAA has member eye banks operating in Canada, Europe, the Middle East, and Asia. The European Eye Bank Association (EEBA) currently states that “microbiological testing of medium and/or remaining scleral rim postoperatively is highly recommended”.19 Neither association says testing is mandatory, and the EBAA even acknowledges its questionable prognostic value. In 2012, the Global Alliance of Eye Bank Associations assigned a memorandum of understanding for formal cooperation in the eye-banking profession.20 This was signed by the EBAA, EEBA, Association of Eye Banks of Asia, Eye Bank Association of Australia and New Zealand, Pan American Association of Eye Banks, and the Eye Bank Association of India.20 Since the accompanying associations do not post public eye-banking standards, it can be assumed that the EBAA and EEBA reports are referenced. In addition to the unclear guidelines on corneoscleral rim cultures, empiric broad-spectrum antibiotics are used regardless, making the clinical utility of these tests highly questionable.
This issue also raises financial concerns, especially during a time where there is a strong focus on reducing unnecessary health-care costs. The cost of keratoplasty has increased over time. Between 2005 and 2011, the average estimated charges for a corneal transplant increased by 28%.21,22 The cost of corneal tissue processing has risen, due to increases in regulatory requirements and advancement in procedures that are required.
Inconsistent results in the literature highlight the need for an updated systematic review. If microbial evaluation of donor corneas can speed treatment and improve patient-vision outcomes, then it is of great value. If there is not a consistent ability to predict infection postoperatively, then its continued practice should be questioned, given the costs involved. A definite result as to whether this is a worthwhile procedure will allow health-care professionals and policy makers to make evidence-based decisions on resource allocations in both publicly and privately funded environments. The result of the proposed project is important, as it may help to eliminate steps in the corneal transplant procedure, potentially resulting in savings of time, labor, and funding needed to perform keratoplasty. The purpose of this systematic review is to determine the effectiveness of donor corneoscleral rim cultures in successfully predicting infections in relation to corneal transplantation, and the cost-effectiveness of this procedure.
Materials and methods
Search strategy
The following search methodology was used to assist in locating both published and unpublished studies. Research databases and conference meeting abstracts were searched for articles published from January 2000 to November 2016, and included Medline (Ovid), Embase (Ovid), Cochrane Library (Wiley), BioSis (Thomson Reuters), Web of Science (Thomson Reuters), and CINAHL (EBSCO). Grey literature was explored by searching BioSis Previews, Grey Matters, OpenGrey, Grey Literature Report, ClinicalTrials.gov, International Clinical Trials Registry, UK Clinical Trials Gateway, UK Clinical Research Network Study Portfolio, Centers for Disease Control and Prevention, Health Canada, World Health Organization, Canadian Health Research Collective, Electronic Thesis Online Services, NDLTD, Theses Canada, and Western Libraries theses and dissertations. Conference proceedings were indexed within a Web of Science Core Collection database search. The following ophthalmology-specific databases were explored: Agency for Healthcare Research and Quality, Canadian Agency for Drugs and Technology in Health, Association for Research in Vision and Ophthalmology conference abstracts, Investigative Ophthalmology and Visual Science conference abstracts, American Academy of Ophthalmology meeting archives, Canadian Society of Ophthalmology, European Ophthalmology Society, Academy Health, and the Society for Medical Decision Making. The search strategies employed database-specific subject headings and keywords for culture techniques, keratoplasty, eye infection, and their synonyms. Each strategy was structured to accommodate for database- and platform-specific terminology and syntax. Tables S1–S7 contain the complete search strategies used for the various databases. Alerts were set up for each database to receive publication notifications for new related articles until May 2017.
Inclusion and exclusion criteria
Articles included were from any country, published in the English language, published from 2000 to present, and were research articles. The study population comprised adults undergoing cornea transplant (any type). Articles must have had a sample size of at least 20 grafts undergoing cornea transplant with corneoscleral rim-culture results. To be included, articles were required to have reported any postoperative complications and the results of the corneoscleral rim cultures. The primary outcome was the incidence of postkeratoplasty infection.
Articles published prior to 2000 were excluded. This date restriction was chosen to ensure generalizability to today’s standards, as storage media, culture techniques, and postoperative infection rates have evolved and improved with time. For example, a systematic review conducted in 2005 found that there was a change in the incidence of endophthalmitis during different periods of time: “The rate of endophthalmitis was 0.200% in the 2000–2004 period, 0.453% in the 1990s, 0.376% in the 1980s, and 0.142% during the 1970s.”23 There are several possible explanations for this downward trend in postkeratoplasty endophthalmitis. Internationally, there have been great advancements in eye-banking techniques and standards. The increased use of povidone–iodine24 to disinfect the ocular surface of the donor and recipient reduces bacteria at the time of surgery.25 The use of broad-spectrum antibiotics, such as moxifloxacin and gatifloxacin,26 for gentamicin-resistant species in the culture media when storing corneal buttons may also have contributed to a decrease in postoperative infections. In 2001, Everts et al reviewed rim cultures between January 1992 and November 1997. They found that the culture-positivity rate decreased significantly from 18.6% to 4.5% in June 1992.13 This decrease was credited to the laboratory switching from thioglycolate broth to sheep-blood agar for culturing the rims. The advancement of eye-banking techniques has played an unquestionable role in the reduction of postkeratoplasty endophthalmitis. For these reasons, we limited our search to studies published in 2000 and after in an effort to present results that are timely, unbiased, and accurate with regard to modern-day corneal transplantations and eye-banking techniques. Additionally, nonresearch articles, such as editorials, opinion pieces, commentaries, methodology papers, and review articles, were excluded.
A total of 397 articles were retrieved from relevant databases and an additional 609 identified through grey-literature search, conference proceedings, and hand searches (Web of Science and BioSis Previews). These records were imported into Covidence to remove duplicates. After removal of duplicates, 590 articles were included for the two-level screening process. Level 1 involved screening titles and abstracts simultaneously, while level 2 involved full-text reviews of each included study. Two reviewers (EK and ST) independently screened all articles. If there was disagreement in screening, the reviewers would discuss the article and come to a consensus. If a consensus was not reached, then a third reviewer (WGH) intervened to solve disagreements on article eligibility. After level 1 and 2 screening, 12 articles were included for qualitative and quantitative synthesis (Figure 1). All studies reported on the number of infections postkeratoplasty and their relation to corneoscleral rim-culture results.
  | Figure 1 PRISMA flow diagram. Note: Reproduced from Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.46 Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses. |
Quality-assessment strategy
Articles were assessed using Downs and Black’s27 checklist for measuring study quality. This checklist of 27 questions provides an appropriate assessment of randomized and nonrandomized studies, providing an overall score for study quality ranging from 0 to 32. A modified version reported by previous studies28–31 was used, where the power question is simplified into whether or not the study has sufficient power to detect a clinically important effect (P<0.05). This question was scored out of 1 rather than 5, resulting in a score range of 0–28. O’Connor et al31 graded papers with a score of 24–28 as excellent, 19–23 as good, 14–18 as fair, and <14 as poor. This method was used to evaluate the quality-assessment results. Each included article was independently appraised by two reviewers (EK and JA). The results indicated that all 12 articles were of good quality. All articles were included in the analysis (Table 1).
  | Table 1 Quality assessment Note: Down's and Black 27 item checklist for the assessment of the methodological quality both of randomized and non-randomized studies.27 The Downs and Black quality assessment scale contains 27 questions that assesses reporting, external validity, internal validity, bias, confounding, and power. An answer of “yes” is marked as 1, whereas a “no” or “unable to determine” response is given 0. |
Data-extraction strategy
Qualitative and quantitative data were obtained from each article by one reviewer (EK) using an Excel template. Information on study design, study period, study location, operation, preservation antibiotic, culture media, number of grafts, number of postoperative infections, and number of culture-positive donor rims was collected. Additionally, the number of true positives, false positives, true negatives, and true positives for each article was required to be included in the review, so that a 2×2 contingency table could be created. If any of these values was missing, authors were emailed to obtain the absent values. No authors were able to provide additional information. Cost data were obtained from the London Laboratory Services Group (LLSG) microbiology lab in London, ON, Canada. Table 2 lists the characteristics and outcomes of the extracted studies.
  | Table 2 Characteristics and outcomes of included studies |
Data analysis
Contingency tables for patients who had a clinical diagnosis of keratitis or endophthalmitis within 6 months of keratoplasty that were attributable to the donor cornea were constructed according to the corneoscleral rim-culture results. Sensitivity, specificity, positive predictive value (PPV), and negative PV (NPV) were calculated to determine the performance of the diagnostic test.
Cost analysis
The costs of positive and negative rim-culture tests were obtained from the LLSG for their process of culturing corneoscleral rim cultures for bacterial and fungal microorganisms. Additionally, the cost of conducting fungal cultures without bacterial cultures was estimated. The total cost of each diagnostic test was applied to the pooled estimate of positive and negative cultures from the 12 included studies. From these data, an incremental cost-effectiveness ratio (ICER) was calculated using the following formula:
|
where C1 = cost in intervention group, C0 = cost in control group, E1 = effect in intervention group, and E0 = effect in control group.
Results
Study characteristics
A total of 12 studies were included in the analysis. Table 2 shows the study characteristics and outcomes of each study. Studies were conducted in the US,5,13,32–37 Israel,38 Italy,39 Saudi Arabia,40 and Japan.16 All articles were published between 2001 and 2016. The cumulative number of all corneal transplants was 7,870. All studies were observational. There was minimal variation in study populations. These comprised adult patients with a corneal disorder requiring penetrating keratoplasty, anterior lamellar keratoplasty, endothelial keratoplasty, Descemet-stripping automated endothelial keratoplasty, femtosecond laser-enabled keratoplasty, or deep anterior lamellar keratoplasty. One of the main outcome measures was PPV, which describes the performance of the diagnostic test. The PPV is directly proportional to the prevalence of the disease or condition. If the test is conducted in a population with high prevalence, it is more likely that the persons who test positive truly have the disease. If we test in a low-prevalence setting, it is more likely that persons who test positive do not have the disease. Table 3 lists quantitative outcome measures (sensitivity, specificity, PPV, NPV, and positive and negative likelihood ratios).
Primary outcome – infection
Of 7,870 grafts, 954 had a positive rim culture (12.1%), with 12 of those 954 positive cultures going on to develop keratitis or endophthalmitis (1.3%). The prevalence of keratitis and endophthalmitis in this study was 0.15% (12 of 7,870). The cumulative PPV of this test was 1.5%, meaning that of those with a positive screening test, only 1.5% would truly have the disease. Of the 12 infections, nine were fungal (six keratitis, three endophthalmitis) and three bacterial (one keratitis, two endophthalmitis). A 2×2 contingency table evaluating the association between the test result and postkeratoplasty infection is shown in Table 4.
  | Table 4 Two-by-two contingency table of pooled numbers from all studies included in the systematic review |
All studies provided information regarding ocular infection postkeratoplasty and corneoscleral rim-culture results. Seven of the studies resulted in no infections postkeratoplasty, which inhibited the ability to calculate the pooled results for performance of donor-rim cultures quantitatively without bias.
A forest plot of the sensitivity and specificity of the included articles was constructed using RevMan 5.3 (Figure 2).41 A summary receiver-operating characteristic (ROC) curve (Figure 3) was also constructed to reveal pooled sensitivity and specificity (for the five studies where sensitivity and specificity were both estimable). One can see that this is a biased graph, as the ROC line indicates the rim-culture tests are “good” at predicting infection. We know this is not true, since the ROC curve included only five of the twelve studies, and the PPV of the test was 1.5%. For this same reason, the diagnostic odds ratio was unable to be calculated for risk of biasing the results.
  | Figure 2 Predictive values for corneoscleral rim cultures. |
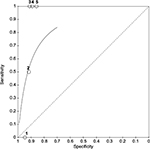  | Figure 3 Summary Receiving Operating Characteristic Curve (SROC) plot for the five studies with post-keratoplasty infections, 1, Everts et al;13 2 Garg et al;36 3 Rauen et al;35 4 Tsui et al;37 and 5 Rehany et al.38 |
Cost-effectiveness analysis: corneoscleral culture results from LLSG
The cost of positive and negative rim-culture tests was obtained from the LLSG for their process of culturing corneoscleral rim cultures for bacterial and fungal infections (Figure 4). One author (EK) contacted the lab supervisor, and was given an estimate of the equipment used and time spent during each step of the rim-culturing process. First, the corneoscleral rim is put in a thioglycolate broth. If the culture is clear after the allotted time, then it is negative for bacteria. The test would then end here, totaling CAD$4.54. If the rim is cloudy, then it is put through an aerobic and anaerobic test to determine if it is positive for microorganisms. The plates are observed for 8–10 days for growth. Where a culture is positive for microorganisms, it is assessed by matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry, which identifies which microorganisms have contaminated the corneoscleral rim. It is important to note that not all fungi can be identified by MALDI-TOF;42 these can be limited to Candida spp. and other yeastlike fungi. That being said, the specific algorithm for fungal identification may change depending on geographic location. In Canada, Candida spp. are common, making MALDI-TOF an acceptable means of fungal identification. Finally, antimicrobial susceptibility tests are used to determine to which specific antibiotics a bacteria or fungus is sensitive. As a result, there are five possible costs to culturing a corneoscleral rim, depending on the outcome:
- negative thioglycolate-broth culture = $4.54 (negative)
- negative anaerobic test with negative aerobic test = $11.88 + $11.88 = $23.76 (negative)
- positive anaerobic test with negative aerobic test = $28.55 + $11.88 = $40.43 (positive)
- negative anaerobic test with positive aerobic test = $11.88 + 28.55 = $40.43 (positive)
- positive anaerobic test with positive aerobic test = $28.55 + $28.55 = $57.10 (positive).
  | Figure 4 Cost estimates of bacterial and fungal corneoscleral rim culturing (in CAD). Abbreviation: MALDI-TOF, matrix-assisted laser desorption/ionization time of flight. |
Given that there are two possible costs for a negative test and three possible costs for a positive test, we calculated the averages to determine the cost to be applied to our 2×2 table. A negative test cost $14.15 ([4.54+23.76]÷2) and a positive test cost $45.99 ([40.43+40.43+57.10]÷3). If we apply these estimates to the number of pooled positive and negative tests, the result is $43,874.46 (45.99×954) and $97,861.40 (14.15×6,916), totaling $141,735.86 to have 7,870 corneoscleral rims cultured. The ICER for the cost of potential cases averted is:
|
The ICER to run all 7,870 tests in this systematic review is estimated to be $40,215.70. The ICER is the cost that on average needs to be sustained to obtain an additional success. It costs $40,215.70 to obtain every additional true-positive result (positive corneoscleral rim culture with subsequent infection).
Cost-effectiveness analysis – fungal only
Figures and tables herein present an estimation of the process and cost to limit the corneoscleral rim-culture procedure to fungal cultures only. Evaluation of the association between the fungal test result and postkeratoplasty infection is shown in Table 5.
  | Table 5 Two-by-two contingency table of fungal culture results from the included studies in the systematic review |
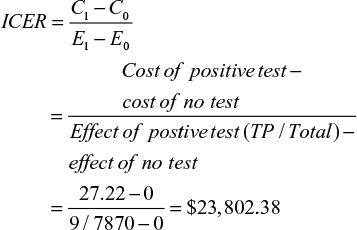
The cost of a positive and negative fungal rim-culture test was obtained from the LLSG. A flowchart was created to display the process and cost of a fungal rim culture using data from the LLSG (Figure 5). A negative test cost $4.54 and a positive test $27.22. If we apply these estimates to the number of pooled positive and negative tests, the result is $2,286.48 (27.22×84) and $35,348.44 (4.54×7,786), totaling $37,634.84 to have 7,870 corneoscleral rims cultured for fungus. The ICER for the cost of potential cases averted is:
|
  | Figure 5 Cost estimates of fungal rim culture testing (in CAD). Abbreviation: MALDI-TOF, matrix-assisted laser desorption/ionization time of flight. |
The ICER to run all 7,870 tests was estimated to be $23,802.38. The ICER is the cost that on average needs to be sustained to obtain an additional success. In this situation, it costs $23,802.38 to obtain every additional true-positive result (positive corneoscleral rim culture with subsequent infection).
Conclusion
This is a systematic review of all research studies since 2000 that examined corneoscleral rim cultures and their ability to predict postkeratoplasty infections accurately in recipients. Positive cultures of donor corneal rims were found in 12.1% of the 7,870 donor rims, with a range of 5%–21%. This is in concordance with previous results: Wilhelmus and Hassan14 found that positive rims were present in 14% of donor corneas. The range may reflect different culturing techniques, preservation mediums, and equipment. From 1985 to 2000, six studies4,7,9,12,43,44 that investigated corneoscleral rim cultures reported contamination rates that ranged from 11% to 39%. The decrease in the rate of positive cultures may be due to the advancement of eye-banking techniques and standards.
The PPV of this test was found to be 1.5%. However, the results of the pooled estimates for diagnostic accuracy have shown corneoscleral rim cultures to be highly specific. The NPV was very high. However, the sensitivity was unable to be determined, as only five of the 12 studies had infectious outcomes. On the other hand, this study has demonstrated the inability for rim cultures to predict postkeratoplasty endophthalmitis or keratitis accurately, especially in bacterial cases. In bacterial cases, no rim culture predicted a clinical infection. The poor PPV results in a loss of time and resources. Furthermore, test results are not available to the surgeon until 24–72 hours after the procedure. Given empiric antibiotic therapy is usually used if a patient has an infection, it would also be extremely unlikely that this low PPV would change management if a patient developed a bacterial infection that matched the rim culture.
The PPV was not as poor for fungal infections. As can be seen from Table 5, the PPV of fungal cultures was 10.7% (nine of 84). Further, the ICER for fungal infections alone was a little under $24,000, which may be a reasonable amount to pay to alter management for a fungal infection. To illustrate the difference between bacterial and fungal cultures, there were 12 infections in total found in our study: nine fungal and three bacterial. All three bacterial infections had negative cultures. However, 100% of the fungal infections had positive cultures with microorganisms that matched the infection. Since empiric therapy postkeratoplasty is antibacterial treatment, is it possible that management may change with a positive fungal culture, as antifungal therapy is seldom part of empiric therapy. Table 6 lists the 12 infections in the review, and if any management change occurred after the results of the rim cultures were available. One study32 did not report if postoperative management was changed due to the donor-culture result (four cases of infection). Three cases of infections had negative bacterial culture results; therefore, empiric therapy would not have changed. The five remaining infections were positive for fungal microorganisms, yet in each case the surgeon elected to observe the patient rather than begin antifungal therapy. When a fungal infection was detected in the recipient’s eye (9–41 days later), antifungal therapy was administered, but in no case did it alleviate the infection, and further surgery was required. Nevertheless, it is possible that using the results of the fungal rim culture earlier may have changed the outcome in these cases. Therefore, there was a much higher association between fungal donor-rim contamination and postoperative infection than there was in bacterial cases, with the potential for management changes that may have affected the outcome.
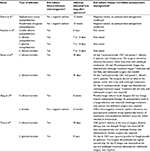  | Table 6 Management change after culture results Abbreviation: CRC, corneoscleral rim culture. |
Finally, routine rim cultures have a substantial cost. To screen 7,870 rims in this study, our estimated cost was $141,736, with an ICER of $40,215 (bacterial and fungal cultures). However, the ICER for fungal infections was substantially less. Reed et al45 estimated that in the US, submitting donor rims for microbiological testing costs US$2.5 million per year. Similarly, Wiffen et al4 found that the cost of routine donor rim cultures cost US$137 per cornea transplanted in 1994, and that if this number was applied to the 43,743 corneal transplants performed that year in the US, it would be equivalent to $6 million. The cost that was estimated for our study is much less. This could potentially be attributed to the decreasing costs of laboratory equipment and more efficient culturing methods. Additionally, laboratory overheads, eg, equipment amortization, utilities, and rent, were not included in our estimate.
In summary, the PPV of bacterial cornea rim cultures was poor. Bacterial infections in patients correlated poorly with rim-culture results. Management would rarely, if ever, be changed, even if rim culture and patient infection matched. The ICER was over $40,000, indicating that this inaccurate procedure is also very expensive.
For fungal cultures, the PV was better (10.7%) as was the potential for management change. The ICER was significantly lower than it was for full bacterial and fungal culturing ($24,000). Based on both accuracy and health economic principles, we could not find any evidence to support bacterial rim cultures. However, in regions where fungal infections are high, fungal rim cultures may meet the threshold of accuracy and willingness to pay, and could be considered based on these same principles.
It would be beneficial for the eye-bank associations to reevaluate their recommendations on this practice. Without the ability to predict infection postkeratoplasty, corneoscleral rim cultures do not appear to be valuable in the prevention of infection or vision loss.
Limitations
One limitation of this study is that diagnostic studies are typically less homogeneous than treatment studies. Different surgeons performed the operation at each clinic, and there may have been different procedures or anesthetic treatments that may influenced the outcomes. Slight variations in laboratory techniques (such as antibiotic supplementation, preservation medium, and culture medium) also produce less homogeneity. Next, the applicability of the cost data will differ depending on the region. Finally, a pooled sensitivity analysis was unable to be performed, due to the nature of the data.
Disclosure
The authors report no conflicts of interest in this work.
References
Eye Bank Association of America. Statistical report. Available from: http://restoresight.org/what-we-do/publications/statistical-report. Accessed July 20, 2004. | ||
Eye Bank Association of America. 2015 Eye Banking Statistical Report. Washington: EBAA; 2016. | ||
Government of Canada. Addressing the challenges and opportunities of ageing in Canada. 2007. Available from: http://www.un.org/esa/socdev/ageing/documents/review_map/Canada.pdf. Accessed July 5, 2017. | ||
Wiffen SJ, Weston BC, Maguire LJ, Bourne WM. The value of routine donor corneal rim cultures in penetrating keratoplasty. Arch Ophthalmol. 1997;115(6):719–724. | ||
Ritterband DC, Shah MK, Meskin SW, et al. Efficacy and safety of moxifloxacin as an additive in Optisol-GS a preservation medium for corneal donor tissue. Cornea. 2006;25(9):1084–1089. | ||
Leveille AS, McMullan FD, Cavanagh HD. Endophthalmitis following penetrating keratoplasty. Ophthalmology. 1983;90(1):38–39. | ||
Farrell PL, Fan JT, Smith RE, Trousdale MD. Donor cornea bacterial contamination. Cornea. 1991;10(5):381–386. | ||
Poole TG, Insler MS. Contamination of donor cornea by gentamicin-resistant organisms. Am J Ophthalmol. 1984;97(5):560–564. | ||
Mathers WD, Lemp MA. Corneal rim cultures. Cornea. 1987;6(3):231–233. | ||
Liesegang TJ, Robinson N, Jones DB. Modified tissue culture medium for corneal storage – II: investigation of the effect of the corneoscleral rim on bacterial contamination. Arch Ophthalmol. 1984;102(4):625–627. | ||
Fong LP, Gladstone D, Casey TA. Corneoscleral rim cultures: donor contamination a case of fungal endophthalmitis transmitted by K-Sol stored cornea. Eye. 1988;2(6):670–676. doi:10.1038/eye.1988.123. | ||
Antonios SR, Cameron JA, Badr IA, Habash NR, Cotter JB. Contamination of donor cornea: postpenetrating keratoplasty endophthalmitis. Cornea. 1991;10(3):217–220. | ||
Everts RJ, Fowler WC, Chang DH, Reller LB. Corneoscleral rim cultures: lack of utility and implications for clinical decision-making and infection prevention in the care of patients undergoing corneal transplantation. Cornea. 2001;20(6):586–589. | ||
Wilhelmus KR, Hassan SS. The prognostic role of donor corneoscleral rim cultures in corneal transplantation. Ophthalmology. 2007;114(3):440–445. | ||
Borowsky CM, Wallau AD, Reetz A, Kwitko S, Rymer S, Locatelli CI. [Positive corneoscleral rim culture in penetrating keratoplasty at the Porto Alegre Clinical Hospital]. Arq Bras Oftalmol. 2008;71(1):13–17. Portuguese. | ||
Matsumoto M, Suzuma K, Miyamura N, Imamura N, Kitaoka T. Conjunctival swabs and corneoscleral rim cultures from corneal transplantation donors as possible early indicators for posttransplant endopthalmitis [sic]. Jpn J Ophthalmol. 2011;55(4):321–326. | ||
Cornish KS, Ramamurthi S, Butcher I, Ramaesh K. Is microbiological analysis of donor cornea transport culture media necessary? Eur J Ophthalmol. 2009;19(1):137–138. | ||
Eye Bank Association of America. Medical Standards. Washington: EBAA; 2015. | ||
European Eye Bank Association. Technical guidelines for ocular tissue. 2017. Available from: https://www.eeba.eu/files/Technical_Guidelines_Rev8_final.pdf. Accessed July 6, 2017. | ||
Machin H. Development of the Global Alliance of Eye Bank Associations. Int J Eye Bank. 2014;2(1):1. | ||
Bentley TS, Hanson SG, Hauboldt RH. 2011 U.S. organ and tissue transplant cost estimates and discussion. 2011. Available from: http://www.milliman.com/uploadedFiles/insight/research/health-rr/2011-us-organ-tissue.pdf. Accessed July 6, 2017. | ||
Ortner NJ, Cosway RG. 2005 US organ and tissue transplant cost estimates and discussion. 2005. Available from: http://www.econ.wayne.edu/agoodman/7550/week8/Previous/2005_Milliman_Report.pdf. Accessed February 21, 2017. | ||
Taban M, Behrens A, Newcomb RL, Nobe MY, McDonnell PJ. Incidence of acute endophthalmitis following penetrating keratoplasty: a systematic review. Arch Ophthalmol. 2005;123(5):605–609. | ||
Ciulla TA, Starr MB, Masket S. Bacterial endophthalmitis prophylaxis for cataract surgery: an evidence-based update. Ophthalmology. 2002;109(1):13–24. | ||
de Kaspar HM, Chang RT, Singh K, Egbert PR, Blumenkranz MS, Ta CN. Prospective randomized comparison of 2 different methods of 5% povidone-iodine applications for anterior segment intraocular surgery. Arch Ophthalmol. 2005;123(2):161–165. | ||
Bertino JS. Impact of antibiotic resistance in the management of ocular infections: the role of current and future antibiotics. Clin Ophthalmol. 2009;3:507–521. | ||
Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. | ||
Richmond SA, Fukuchi RK, Ezzat A, Schneider K, Schneider G, Emery CA. Are joint injury, sport activity, physical activity, obesity, or occupational activities predictors for osteoarthritis? A systematic review. J Orthop Sports Phys Ther. 2013;43(8):515–B19. | ||
Simic M, Hinman RS, Wrigley TV, Bennell KL, Hunt MA. Gait modification strategies for altering medial knee joint load: a systematic review. Arthritis Care Res. 2011;63(3):405–426. | ||
Morton S, Barton CJ, Rice S, Morrissey D. Risk factors and successful interventions for cricket-related low back pain: a systematic review. Br J Sports Med. 2014;48(8):685–691. | ||
O’Connor SR, Tully MA, Ryan B, Bradley JM, Baxter GD, McDonough SM. Failure of a numerical quality assessment scale to identify potential risk of bias in a systematic review: a comparison study. BMC Res Notes. 2015;8:224. | ||
Keyhani K, Seedor JA, Shah MK, Terraciano AJ, Ritterband DC. The incidence of fungal keratitis and endophthalmitis following penetrating keratoplasty. Cornea. 2005;24(3):288–291. | ||
Ritterband DC, Shah MK, Meskin SW, et al. Efficacy and safety of voriconazole as an additive in Optisol GS: a preservation medium for corneal donor tissue. Cornea. 2007;26(3):343–347. | ||
Hassan SS, Wilhelmus KR. Quality assessment and microbiologic screening of donor corneas. Cornea. 2007;26(8):953–955. | ||
Rauen MP, Goins KM, Sutphin JE, Kitzmann AS, Schmidt GA, Wagoner MD. Impact of eye bank lamellar tissue cutting for endothelial keratoplasty on bacterial and fungal corneoscleral donor rim cultures after corneal transplantation. Cornea. 2012;31(4):376–379. | ||
Garg S, Said B, Farid M, Steinert RF. Prevalence of positive microbiology results from donor cornea tissue in different methods of corneal transplantation. Cornea. 2012;32(2):137–140. | ||
Tsui E, Fogel E, Hansen K, et al. Candida interface infections after Descemet stripping automated endothelial keratoplasty. Cornea. 2016;35(4):456–464. | ||
Rehany U, Balut G, Lefler E, Rumelt S. The prevalence and risk factors for donor corneal button. Cornea. 2004;23(7):649–654. | ||
Fontana L, Errani PG, Zerbinati A, Musacchi Y, Di Pede B, Tassinari G. Frequency of positive donor rim cultures after penetrating keratoplasty using hypothermic and organ-cultured donor corneas. Cornea. 2007;26(5):552–556. | ||
Wagoner MD, Gonnah ES, Al-Towerki AE. Outcome of primary adult optical penetrating keratoplasty with imported donor corneas. Int Ophthalmol. 2010;30(2):127–136. | ||
Cochrane Community. RevMan 5 [software]. 2014. | ||
Chalupová J, Raus M, Sedlářová M, Sebela M. Identification of fungal microorganisms by MALDI-TOF mass spectrometry. Biotechnol Adv. 2014;32(1):230–241. | ||
Armitage WJ, Easty DL. Factors influencing the suitability of organ-cultured corneas for transplantation. Invest Ophthalmol Vis Sci. 1997;38(1):16–24. | ||
Borderie VM, Laroche L. Microbiologic study of organ-cultured donor corneas. Transplantation. 1998;66(1):120–123. | ||
Reed JW, Bealer LA, Sloop CM, Davis RM. Questionable benefit of cultures at the time of penetrating keratoplasty. Cornea. 1994;13(1):101. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. |
Supplementary material
Database-specific search strategies
  | Table S1 Medline (Ovid), October 20, 2015, updated November 25, 2016 |
  | Table S2 Embase (Ovid), October 20, 2015, updated November 25, 2016 |
  | Table S3 CINAHL, October 20, 2015, updated November 25, 2016 |
  | Table S4 Web of Science, October 20, 2015, updated November 25, 2016 |
  | Table S5 Cochrane, October 21, 2015, updated November 25, 2016 |
  | Table S6 BioSis, October 21, 2015, updated November 25, 2016 (grey literature) |
  | Table S7 Grey-literature search Note: Websites and databases searched using the search terms “corneoscleral rim culture”, “culture”, “keratoplasty”, and “postoperative infections”. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.