Back to Journals » Vascular Health and Risk Management » Volume 17
The Usefulness of the Combination of D-Dimer and Soluble Fibrin Monomer Complex for Diagnosis of Venous Thromboembolism in Psychiatric Practice: A Prospective Study
Authors Takeshima M , Ishikawa H, Ogasawara M, Komatsu M, Fujiwara D, Itoh Y , Wada Y, Omori Y , Ohta H, Mishima K
Received 21 February 2021
Accepted for publication 23 April 2021
Published 21 May 2021 Volume 2021:17 Pages 239—246
DOI https://doi.org/10.2147/VHRM.S307689
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Harry Struijker-Boudier
Masahiro Takeshima,1 Hiroyasu Ishikawa,1 Masaya Ogasawara,1 Munehiro Komatsu,2 Dai Fujiwara,1 Yu Itoh,2 Yuki Wada,3 Yuki Omori,4 Hidenobu Ohta,1 Kazuo Mishima1
1Department of Neuropsychiatry, Akita University Graduate School of Medicine, Akita, Japan; 2Department of Neuropsychiatry, Akita City Hospital, Akita, Japan; 3Department of Radiology, Akita University Graduate School of Medicine, Akita, Japan; 4Department of Neuropsychiatry, Tokyo Metropolitan Geriatric Hospital, Tokyo, Japan
Correspondence: Masahiro Takeshima
Department of Neuropsychiatry, Akita University Graduate School of Medicine, 1-1-1, Hondo, Akita City, Akita, 010-8543, Japan
Tel +81-18-884-6122
Fax +81-18-884-6445
Email [email protected]
Purpose: D-dimer has the advantage of excluding venous thromboembolism (VTE) due to its high sensitivity but is disadvantageous for diagnosing VTE due to its low specificity. A method to increase the usefulness of D-dimer in the diagnosis of VTE is warranted. This study aimed to investigate the usefulness of the combination of D-dimer and soluble fibrin monomer complex (SFMC), which has been suggested as a new candidate marker for VTE, in VTE diagnosis.
Patients and Methods: This prospective study in 109 subjects was performed at a psychiatric department between August 1, 2017 and December 31, 2019. Subjects’ levels of D-dimer and SFMC were measured simultaneously. Plasma levels of D-dimer and SFMC were measured using NANOPIA® D-dimer and NANOPIA® SF. Subjects with positive D-dimer (≥ 1.0 μg/mL) results underwent contrast computed tomography for confirmation of VTE within 12 hours of D-dimer measurement. A receiver operating characteristic curve analysis was performed to examine the usefulness of SFMC for the diagnosis of VTE.
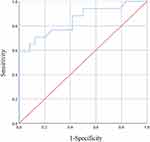
Results: Only 109 of the 783 subjects without symptoms suggestive of VTE participated in the study. Out of 41 subjects with positive D-dimer results, 17 subjects were diagnosed with VTE. A receiver operating characteristic curve analysis was performed to determine cutoff values. The area under the curves was 0.848 for SFMC (p< 0.001, 95% CI 0.722 to 0.974), and the optimal cutoff value was 10.0 μg/mL (sensitivity 58.8%, specificity 100%, positive predictive value 100%, negative predictive value 77.4%).
Conclusion: SFMC was useful for diagnosing VTE in the psychiatric patients with positive D-dimer results.
Keywords: computed tomography, D-dimer, psychiatric patients, soluble fibrin monomer complex, venous thromboembolism
Introduction
Venous thromboembolism (VTE) is a major cause of death among patients with psychiatric disorders.1 Schizophrenia, bipolar disorder, depression, antipsychotics, and antidepressants increase the risk of VTE.2–8 A recent population-based cohort study reported that the relative risk of deep vein thrombosis (DVT) was 3-fold higher, and that of pulmonary embolism (PE) was 2.6-fold higher, in patients with concurrent depressive, bipolar, and schizophrenic disorders than that in the general population.5 Previous cross sectional studies reported relatively high prevalence of VTE in patients hospitalized with psychiatric disorders, as 8.5% of patients with depression had VTE,9 and 11.6% of physically restrained patients10 and 25.3% of patients with catatonia had DVT.11 Further, previous studies reported that 62.5–76.9% of asymptomatic VTE cases were PE.9,12 Therefore, identifying psychiatric patients with a high VTE risk and conducting early VTE screening in such high-risk groups is crucial.
VTE is commonly screened using D-dimer testing. D-dimer has the advantage of a high negative predictive value of 97–100% and a high sensitivity of 93–100% when used in high-sensitivity assays for the diagnosis of VTE.13–17 However, D-dimer has a low positive predictive value and low specificity,13–17 and has therefore mainly been used for excluding VTE.16,18 If the screening is positive, VTE is definitively diagnosed by imaging, which is not always easy because psychiatric hospitals generally do not have sufficient laboratory equipment or skilled staff to diagnose VTE, and patients with severe mental disorders sometimes cannot be undergo imaging tests for VTE due to inability to maintain rest. Therefore, developing an easy and useful method for VTE diagnosis in the psychiatric field is warranted.
Soluble fibrin monomer complex (SFMC) is a serological marker for the activation of the coagulation system, and a quantitative SFMC testing method of plasma has been established. SFMC, like D-dimer, has been suggested as a marker for VTE. However, the results of previous studies examining the usefulness of SFMC for VTE diagnosis are inconsistent. In one study, setting the cutoff value of NANOPIA® SF to 5.9 μg/mL yielded a sensitivity of 98.5% and specificity of 80.1%. The authors concluded that SFMC is useful for VTE diagnosis and exclusion.19 In another study, however, setting the cutoff value of NANOPIA® SF to 7.0 μg/mL yielded a sensitivity of only 38.9% and a specificity of only 64.3%, indicating that SFMC is not useful for VTE diagnosis or exclusion.20 In orthopedic surgery, an optimal cutoff level of 13.9 µg/mL yielded a sensitivity of 67.9% and a specificity of 78.2%.21 Although previous studies have not directly compared SFMC and D-dimer for VTE, SFMC seems to have a higher specificity than D-dimer.19–21
D-dimer has the disadvantage of low specificity; however, we hypothesized that the combination of D-dimer and SFMC would increase its usefulness for diagnosing VTE. Therefore, we conducted a prospective study to investigate this in psychiatric practice, using contrast computerized tomography (CT) for confirmation of VTE.
Patients and Methods
We performed this prospective study from August 2017 to December 2019 at the psychiatric department of our institution. Participants were recruited from consecutive inpatients with mental disorders during the study period. The inclusion criteria were patients with psychiatric disorders who were admitted at the psychiatric department of our institution for treatment of psychiatric disorder during the study periods. The exclusion criteria were: (1) iodine or iodine contrast agent sensitivity; (2) severe thyroid disease; (3) bronchial asthma; (4) severe renal dysfunction; (5) severe liver dysfunction; (6) severe heart failure; (7) macroglobulinemia; (8) multiple myeloma; (9) tetany; (10) pheochromocytoma; (11) age <20 years; and (12) pregnancy or nursing.
After participants agreed to participation in this study, their DVT risk was assessed using Well’s score.22 Plasma levels of D-dimer and SFMC were measured using NANOPIA® D-dimer and NANOPIA® SF (SEKISUI MEDICAL CO., LTD., Tokyo, Japan). NANOPIA® D-dimer uses a monoclonal antibody specific for fragment DD, while NANOPIA® SF uses a monoclonal antibody J2-23, which recognizes an epitope in the C-terminal region of the fibrin Aα chain (Aα502–521).23 The sensitivity of NANOPIA® D-dimer in diagnosing VTE has been reported as 99.6% with a cutoff value of 1.0 μg/mL.24 The measuring equipment consisted of the automated coagulation analyzer COAPRESTA 2000 (Sekisui Medical Co., Ltd., Tokyo, Japan) until October 2019 and the COAPRESTA 3000 (Sekisui Medical Co., Ltd., Tokyo, Japan) from November 2019.
Subjects with positive D-dimer results underwent contrast CT within 12 hours of D-dimer measurements for the diagnosis of VTE. CT scans for detecting VTE (PE or DVT) were performed using multidetector row helical CT scanners (August 2017–January 2018: Discovery CT750HD or Discovery CT750HD-A; February 2018–December 2019: Revolution CT; GE Healthcare Japan, Tokyo, Japan) with intravenously injected low-osmolar iodinated contrast mediums (the mainly used contrast mediums were Omnipaque 300; DAIICHI SANKYO COMPANY, Limited, Tokyo, Japan and Iomeron 350; Eisai Corporation, Limited, Tokyo, Japan). Slice thickness was 1.25 mm. The total amount of contrast medium was determined according to body weight. Between August 2017 and January 2018, the total amount was calculated according to the formula “body weight (kg) × 2+30 (mL),” up to 150 mL, for Omnipaque and according to the formula “body weight (kg) × 2.5 (mL),” up to 135 mL, for Iomeron. Between February 2018 and December 2019, the total amount was calculated according to the formula “body weight (kg) × 600/iodine concentration of contrast medium (mgI/mL)”; for example, “body weight (kg) × 600/350 (mL)” for Iomeron 350 and “body weight (kg) × 600/300 (mL)” for Omnipaque 300. The contrast medium was intravenously injected at a rate of 3.5–4.0 mL/s using a power injector, and scanning was performed at 20–30 seconds (early phase) for detecting pulmonary arterial embolization (CT angiography) and at 210–240 seconds (delayed phase) for detecting DVT (CT venography). These protocols were sometimes modified according to the patients’ renal function, allergies to specific contrast media, and diameter of intravenous access. Image readings of CT scans were performed by radiological diagnostic specialists of the Japan Radiological Society or by doctors specializing in diagnosis who had exclusively worked at radiology departments for more than 10 years.
The following clinical characteristics of the participants were collected from their medical charts: age, sex, body mass index (BMI; kg/m2), diagnosis of psychiatric disorders (International Classification of Diseases 10th Revision: ICD-10), restraint, catatonia according to the Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition, daily dose of antipsychotic converted to chlorpromazine, daily dose of antidepressant converted to imipramine,25 history of VTE, anticoagulant use, D-dimer value (µg/mL), SFMC value, surgery within 4 weeks, trauma within 4 weeks, active cancer, infection, disseminated intravascular coagulation (DIC), hypertension, hyperlipidemia, diabetes mellitus, and fever (°C).
Calculation
All statistical analyses were performed with IBM SPSS Statistics version 25.0 (IBM Corp., Chicago, IL, USA). Fisher’s exact test was performed for nominal variables, and the Mann–Whitney U-test was used for continuous variables to examine the differences between the VTE and non-VTE subject groups. Using SFMC values and presence or absence of VTE in patients with positive D-dimer results, the usefulness of SFMC for the diagnosis of VTE was examined using receiver operating characteristic (ROC) curve analysis. Optimal cutoff value of SFMC was determined by the ROC analysis. The non-parametric (trapezoidal rule) area under the ROC curve was calculated to indicate overall accuracy. The McNemar chi-square test was used to estimate the statistical significance of differences in specificity when utilizing different threshold SFMC values.
Power Analysis
Power analysis was performed with R version 4.0.2. From previous studies, (9, 12) we estimated that one-third of psychiatric inpatients with a D-dimer value of 1 μg/mL or higher had VTE. Based on: (1) a one-sided significance level of 0.05; (2) power = 0.90; (3) Area Under the Curve (AUC) = 0.8; and (4) 1:3 ratio of the VTE-positive to the VTE-negative group, the minimum sample size to detect statistically significant differences was 38 subjects (10 subjects for the VTE-positive group and 28 subjects for the VTE-negative group, respectively). We therefore set a target sample size of 40 psychiatric inpatients with a D-dimer value of 1 μg/mL or higher.
Ethics
This study was approved by the Ethics Committee Akita University Graduate School of Medicine and Faculty of Medicine (No. 1821) and conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from each participant after a full explanation of the study protocol prior to study commencement.
Results
During the research period, 783 subjects were admitted to our hospital for the treatment of psychiatric disorders. Of these, 598 patients were excluded because of their refusal to participate, and 76 were excluded based on the exclusion criteria (2: iodine or iodine contrast agent sensitivity; 10: severe renal dysfunction; and 64: age < 20 years). Finally, 109 subjects without VTE symptoms participated in the study. Forty-seven subjects showed positive test results (D-dimer ≥ 1.0 µg/mL). SFMC was not measured in four subjects. Two patients were withdrawn from the study after the VTE screening: in one patient, severe renal dysfunction was revealed by a blood test conducted as part of the VTE screening and the other patient refused to undergo CT. Finally, 41 subjects underwent contrast CT. Participant selection is shown in Figure 1.
Of these 41 patients, 17 had VTE (DVT only: three, PE only: eight, both DVT and PE: six). Of all patients, DVT was observed in 8.4% (8/95) of low-risk, 0% (0/12) of medium-risk, and 50% (1/2) of high-risk patients, categorized according to their Well’s score.
The subjects’ clinical characteristics are shown in Table 1. Compared to the VTE-negative group, the VTE-positive group had higher levels of D-dimer and SFMC (p<0.001 and p<0.001) (see Figure 2). There were no differences between both groups in age, sex, BMI, operation within 4 weeks, trauma within 4 weeks, malignancy, infection, DIC, history of VTE, or use of anticoagulants.
 |
Table 1 Clinical and Demographic Characteristics |
The ROC analysis of SFMC is shown in Figure 3. The SFMC ROC curve had an AUC of 0.848 (p<0.001, 95% confidence interval [CI] 0.722 to 0.974). The Youden index, used to automatically calculate the optimal cutoff value (sensitivity + specificity −1), was 10.0 µg/mL for SFMC (sensitivity 58.8%, specificity 100%, positive predictive value 100%, negative predictive value 77.4%).
Discussion
This is the first study to explore the usefulness of the combination of D-dimer and SFMC for the diagnosis of VTE. The results of this study provide cutoff values of SFMC that are sufficient for diagnosing VTE clinically, with a sensitivity of 58.8% and specificity of 100% among patients with psychiatric disorders and positive D-dimer results. Although previous studies have shown inconsistent results on the usefulness of SFMC,19–21 the ROC analysis conducted in the current study shows that SFMC is a useful marker for the diagnosis of VTE. It is unclear why the specificity of the cutoff value for SFMC in this study was high. Possible reasons that affected the results were that (1) VTE screening by D-dimer was performed simultaneously with SFMC measurement, and that (2) the subjects were psychiatric patients with few physical factors that elevate SFMC other than VTE compared to non-psychiatric patients. Since few studies have examined the usefulness of SFMC, further studies are warranted to validate these findings.
In a previous study using Well’s score, 5% (95% CI 4 to 8%) of low-risk, 17% (95% CI 13 to 23%) of middle-risk, and 53% (95% CI 44 to 61%) of high-risk patients had DVT.22 In this study, these corresponding values were 8.4%, 0%, and 50%. Here, the prevalence of DVT was almost within range in the low-risk and high-risk groups, but it was lower in the middle-risk group than in the respective patient groups in the previous study. The reason for these differences between studies is unclear, but the small sample size may have affected the results of this study. Studies with larger sample sizes are warranted to determine whether Well’s score is also useful in the evaluation of patients with psychiatric disorders.
Strengths and Weaknesses of the Study
The strengths of this study are (1) its prospective design, and (2) the detailed examination for VTE (both DVT and PE) using contrast-enhanced CT. Nevertheless, this study also has some limitations: (1) Only 109 of the 783 (13.9%) patients admitted to our department participated in the study. Selection bias may have affected the results of this study. (2) VTE may have been missed because patients with D-dimer levels < 1.0 µg/mL did not undergo contrast-enhanced CT, based on the criteria for the 1st screening; and (3) the ROC curve of D-dimer or SFMC alone could not be plotted because contrast-enhanced CT was not performed in patients with negative D-dimer results; (4) some participants might exhibit confounding factors that increase D-dimer or SFMC levels; (5) although a fixed 1.0 D-dimer level was used, the age-adjusted D-dimer level, which is recommended by several guidelines,26 may be more useful in combination with SFMC. These methodological issues should be resolved through future carefully designed studies.
Conclusion
SFMC was useful for diagnosing VTE in the psychiatric patients with positive D-dimer results.
Abbreviations
AUC, area under the curve; BMI, body mass index; CI, confidence interval; CT, computerized tomography; DIC, disseminated intravascular coagulation; DVT, deep vein thrombosis; PE, pulmonary embolism; ROC, receiver operating characteristic; SFMC, soluble fibrin monomer complex; VTE, venous thromboembolism.
Data Sharing Statement
The datasets used and analyzed in the current study are available from the corresponding author on reasonable request.
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
Author Contributions
All authors made substantial contributions to the conception and design of the study and to the acquisition of data or the analysis and interpretation of data; they all took part in drafting the article or revising it critically for important intellectual content; they gave their final approval of the version to be published; and all authors agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Manu P, Kane JM, Correll CU. Sudden deaths in psychiatric patients. J Clin Psychiatry. 2011;72(7):936–941. doi:10.4088/JCP.10m06244gry
2. Barbui C, Conti V, Cipriani A. Antipsychotic drug exposure and risk of venous thromboembolism: a systematic review and meta-analysis of observational studies. Drug Saf. 2014;37(2):79–90. doi:10.1007/s40264-013-0127-6
3. Hsu WY, Lane HY, Lin CL, Kao CH. A population-based cohort study on deep vein thrombosis and pulmonary embolism among schizophrenia patients. Schizophr Res. 2015;162(1–3):248–252. doi:10.1016/j.schres.2015.01.012
4. Kunutsor SK, Seidu S, Khunti K. Depression, antidepressant use, and risk of venous thromboembolism: systematic review and meta-analysis of published observational evidence. Ann Med. 2018;50(6):529–537. doi:10.1080/07853890.2018.1500703
5. Lin CE, Chung CH, Chen LF, Chien WC. Increased risk for venous thromboembolism among patients with concurrent depressive, bipolar, and schizophrenic disorders. Gen Hosp Psychiatry. 2019;61:34–40. doi:10.1016/j.genhosppsych.2019.10.003
6. Parkin L, Balkwill A, Sweetland S, et al. Antidepressants, depression, and venous thromboembolism risk: large prospective study of UK women. J Am Heart Assoc. 2017;6(5):e005316. doi:10.1161/JAHA.116.005316
7. Wu CS, Lin CC, Chang CM, et al. Antipsychotic treatment and the occurrence of venous thromboembolism: a 10-year nationwide registry study. J Clin Psychiatry. 2013;74(9):918–924. doi:10.4088/JCP.12m08117
8. Zhang R, Dong L, Shao F, Tan X, Ying K. Antipsychotics and venous thromboembolism risk: a meta-analysis. Pharmacopsychiatry. 2011;44(5):183–188. doi:10.1055/s-0031-1280814
9. Takeshima M, Ishikawa H, Umeta Y, et al. Prevalence of asymptomatic venous thromboembolism in depressive inpatients. Neuropsychiatr Dis Treat. 2020;16:579–587. doi:10.2147/NDT.S243308
10. Ishida T, Katagiri T, Uchida H, et al. Incidence of deep vein thrombosis in restrained psychiatric patients. Psychosomatics. 2014;55(1):69–75. doi:10.1016/j.psym.2013.04.001
11. Ishida T, Sakurai H, Watanabe K, Iwashita S, Mimura M, Uchida H. Incidence of deep vein thrombosis in catatonic patients: a chart review. Psychiatry Res. 2016;241:61–65. doi:10.1016/j.psychres.2016.04.105
12. Takeshima M, Ishikawa H, Shimizu K, Kanbayashi T, Shimizu T. Incidence of venous thromboembolism in psychiatric inpatients: a chart review. Neuropsychiatr Dis Treat. 2018;14:1363–1370. doi:10.2147/NDT.S162760
13. Di Nisio M, Squizzato A, Rutjes AW, Buller HR, Zwinderman AH, Bossuyt PM. Diagnostic accuracy of D-dimer test for exclusion of venous thromboembolism: a systematic review. J Thromb Haemost. 2007;5(2):296–304. doi:10.1111/j.1538-7836.2007.02328.x
14. Fronas SG, Wik HS, Dahm AEA, et al. Safety of D-dimer testing as a stand-alone test for the exclusion of deep vein thrombosis as compared with other strategies. J Thromb Haemost. 2018;16(12):2471–2481. doi:10.1111/jth.14314
15. Ghanima W, Abdelnoor M, Mowinckel MC, Sandset PM. The performance of STA-Liatest D-dimer assay in out-patients with suspected pulmonary embolism. Br J Haematol. 2006;132(2):210–215. doi:10.1111/j.1365-2141.2005.05859.x
16. Vermeer HJ, Ypma P, Van Strijen MJL, et al. Exclusion of venous thromboembolism: evaluation of D-Dimer PLUS for the quantitative determination of D-dimer. Thromb Res. 2005;115(5):381–386. doi:10.1016/j.thromres.2004.09.005
17. Waser G, Kathriner S, Wuillemin WA. Performance of the automated and rapid STA Liatest D-dimer on the STA-R analyzer. Thromb Res. 2005;116(2):165–170. doi:10.1016/j.thromres.2004.12.003
18. Stein PD, Hull RD, Patel KC, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med. 2004;140(8):589–602. doi:10.7326/0003-4819-140-8-200404200-00005
19. Tsuji A, Wada H, Matsumoto T, et al. Elevated levels of soluble fibrin in patients with venous thromboembolism. Int J Hematol. 2008;88(4):448–453. doi:10.1007/s12185-008-0173-5
20. Yano S, Yoshida Y, Notsu Y, et al. Significance of D-dimer and soluble fibrin testing in screening of incident venous thromboembolism. Vasc Fail. 2019;3(1):26–30. doi:10.30548/vascfail.3.1_26
21. Hasegawa M, Wada H, Wakabayashi H, et al. The relationships among hemostatic markers, the withdrawal of fondaparinux due to a reduction in hemoglobin and deep vein thrombosis in Japanese patients undergoing major orthopedic surgery. Clin Chim Acta. 2013;425:109–113. doi:10.1016/j.cca.2013.07.009
22. Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA. 2006;295(2):199–207. doi:10.1001/jama.295.2.199
23. Suzuki A, Ebinuma H, Matsuo M, Miyazaki O, Yago H. The monoclonal antibody that recognizes an epitope in the C-terminal region of the fibrinogen α-chain reacts with soluble fibrin and fibrin monomer generated by thrombin but not with those formed as plasmin degradation products. Thromb Res. 2007;121(3):377–385. doi:10.1016/j.thromres.2007.05.008
24. Yamaki T, Nozaki M, Sakurai H, et al. Combined use of pretest clinical probability score and latex agglutination D-dimer testing for excluding acute deep vein thrombosis. J Vasc Surg. 2009;50(5):1099–1105. doi:10.1016/j.jvs.2009.06.059
25. Inada T, Inagaki A. Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci. 2015;69(8):440–447. doi:10.1111/pcn.12275
26. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J. 2019;54(3):1901647. doi:10.1183/13993003.01647-2019
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.