Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
The Use of Urea Cream for Hand Eczema and Urea Foam for Seborrheic Dermatitis and Psoriasiform Dermatoses of the Scalp
Authors Celleno L, D’amore A, Cheong WK
Received 7 June 2022
Accepted for publication 13 October 2022
Published 11 November 2022 Volume 2022:15 Pages 2445—2454
DOI https://doi.org/10.2147/CCID.S377718
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Leonardo Celleno,1 Alessandra D’amore,1 Wai Kwong Cheong2
1Department of Dermatology, Catholic University of Rome, Rome, Italy; 2Specialist Skin Clinic, Singapore, Singapore
Correspondence: Leonardo Celleno, Via Cesare Beccaria 98, Rome, 00196, Italy, Tel +390636006629, Email [email protected]
Purpose: Urea as an ingredient in topical skin applications can aid skin integrity and hydration and have keratolytic, anti-fungal, anti-bacterial, and anti-pruritic effects. Skin conditions that urea-containing formulations have been utilized to treat include hand eczema/dermatitis, seborrheic dermatitis and psoriasiform dermatoses of the scalp. Two monocentric, simple blind, observational studies were carried out in healthy participants to examine the efficacy and safety of two urea-containing products in these skin conditions.
Patients and Methods: Study 1 tested the actions of a commercially available 30% urea topical cream on hand eczema. The product was applied ≥ 2/day for 28 ± 2 days. Transepidermal water loss, skin redness, skin hydration, and participant ratings of efficacy and qualities were assessed prior to first product application and on days 14 and 29. Study 2 tested the actions of a commercially available foaming product containing 10% urea on seborrheic dermatitis and scalp psoriasiform dermatoses. The product was applied ≥ 2/day for 28 ± 2 days. Desquamation index and surface occupied by squames, analysis of extracted squames, microscopic assessment of scalp photos and participant ratings of product efficacy and qualities was carried out prior to first product application and on days 14 and 29.
Results: In Study 1 (n = 20 females), results showed a significant (p < 0.05) decrease in transepidermal water loss, with an increase in hydration level of the upper skin layers, and a decrease in skin redness. In Study 2 (n = 13 females, 7 males), product use led to significant (p < 0.05) decreases in desquamation measures and dryness. In both studies, the majority of participants “agreed” or “slightly agreed” that the product had good efficacy and was easy to apply. No adverse reactions were reported.
Conclusion: These findings point to the utility of urea in topically applied vehicles for hand eczema, seborrheic dermatitis, and psoriasiform dermatoses.
Keywords: natural moisturizing factor, anti-pruritic, transepidermal water loss, keratinocytes, topical formulations
Introduction
The Use of Urea in Dermatology
The polar molecule urea (CH4N2O) is part of the skin stratum corneum’s natural moisturizing factor (NMF) where it contributes to integrity and hydration via its hygroscopic properties.1,2 Topically applied urea has for decades been known for its keratolytic, anti-fungal, anti-bacterial, and anti-pruritic effects due to several mechanisms of action.1–4 For instance, it can reduce transepidermal water loss (TEWL), promote water retention, and replace water, thus increasing stratum corneum moisturization and fluidity,1,5 and can aid penetration of drugs, including hormones, anti-fungals and corticosteroids.2
Due to urea transporters being present in keratinocytes, uptake of urea can lead to the expression of genes involved in keratinocyte differentiation, including for the proteins transglutaminase 1, filaggrin and loricrin.6 This action can help in antimicrobial defense and skin barrier function. At high concentrations, urea can be keratolytic due to its actions on protein structure leading to conformational changes or bond breakages.2,7
Depending on urea concentration, its properties have been utilized in a number of topical formulations aimed at, in lower concentrations, basic skin maintenance and alleviation/prevention of xerosis, in medium concentrations, the treatment of atopic dermatitis, psoriasis and ichthyosis and, at higher concentrations, targeting areas of hyperkeratosis such as corns, keratoderma, and some nail disorders. Formulation vehicle of choice is dependent on the target for urea’s actions and can include creams, nail lacquers, foams, gels, ointments, or shampoos.2 Tolerance-wise, topical application of urea may lead to mild skin irritation at higher concentrations but this is usually transient.2
Hand Eczema
Hand eczema/dermatitis is an inflammatory skin disorder than can include acute lesions – displaying erythema, papules, oedema, vesicles, oozing, and crusting – and chronic lesions –that can be lichenified, hyperkeratotic, fissured, and scaley. Skin may be described by the patient in a number of ways, such as pruritic, painful, burning, or stinging. Histologically, the epidermis may display acanthosis, parakeratosis, and intercellular oedema and spongiosis, with perivascular infiltration of lymphocytes in the upper dermis.8 Hand eczema can be classed as atopic eczema, irritant eczema, allergic contact dermatitis, or protein contact dermatitis.9 While not a necessary pre-requisite, people with atopic dermatitis have a large increased risk of developing hand eczema.8,10
A review of lifetime prevalence of hand eczema found that this is around 17.6% in women and 11.0% in men. This is most often cited as being due to women having a higher likelihood of environmental domestic and occupational exposure to precipitating factors as opposed to skin susceptibility differences.11 Hand eczema is not only bothersome, due to pain and pruritus, it can greatly impact function and quality-of-life factors such as self-esteem, ability to work, sleep, social life, family life, and hobbies.8,9,12 Psychological distress caused by hand eczema has also been reported to affect women more than men with one study showing this in 84.9% of women and 74.1% of men.12
Hand eczema is a particularly prevalent form of occupational skin condition and is most often seen in industries or lifestyles where a person’s hand is exposed to water, friction, irritants (such as chemicals and proteins), and contact allergens. The presence of hand eczema in occupational situations can lead to a person being unfit for work for short or chronic periods, sometimes leading them having to leave their job and change their profession.8
While hand eczema can most easily be prevented/alleviated by minimizing or stopping exposure to the cause (including the use of non-irritating/allergenic gloves), this is not always possible so treatment is needed. Pre-exposure, protective barrier creams can help keep the skin intact, to reduce penetration of disposing factors, and may include ingredients such as zinc oxide, aluminum chlorohydrate, and bentonite. Intact skin can also be encouraged with the use of emollients, moisturizers, and humectants, such as glycerol, sorbitol, and (at low concentrations) urea. These can also help reduce pruritus and hand eczema severity.8,9,13
In more severe and/or chronic cases of hand eczema, helpful active ingredients include topical keratolytics (eg, urea, salicylic acid), corticosteroids, calcineurin inhibitors (eg, tacrolimus), retinoids (eg, bexarotene), and tar-based products. Also available is ultraviolet phototherapy and systemic treatments including azathioprine, cyclosporine, retinoids, corticosteroids, and methotrexate. Of note though, while some ingredients, such as corticosteroids and retinoids, may help control inflammation associated with hand eczema, they may not help, or even worsen, barrier recovery, so need to be used sparingly.8,9,13
Through its actions in reducing TEWL and improving skin hydration, topical urea formulations have been shown to be useful in general for treating and preventing atopic dermatitis.14 Combined with hydrocortisone or betamethasone-17-valerate, urea’s keratolytic actions along with drug penetration enhancement properties have also been shown useful.7,15 However, few studies have directly assessed the use of urea-containing formulations for hand eczema. One, comparing two commercially available creams with 10% urea, also containing phospholipids and multisterols or betaine and lactic acid, respectively, found that both formulations alleviated hand eczema.16 Another study, utilizing a 5% urea moisturising and humectant cream compared to no cream, reported that while in the non-cream group relapse of eczema occurred on average after 2 days, this average time was 20 days in the urea-containing cream group.17
Seborrheic Dermatitis and Psoriasiform Dermatoses of the Scalp
Papulosquamous SD occurs most frequently in childhood, adolescence, and between the ages of 40−60, when it may be detrimental to quality-of-life due to impacts on physical functioning, emotional well-being and social functioning.19–21 SD has an overall prevalence of around 3−7%,22 rising to around 14% in middle- and old-ages and particularly affecting males and people with immune compromising or certain neurological conditions.21,23 Adult SD (ASD) usually has a remitting/relapsing pattern with both personal (eg, poor sleep, stress) and seasonal (more prevalent in winter months) aspects involved.20,21
ASD prevalence on the scalp is due to a high concentration of sebaceous glands in this area. Appearance most often includes folliculocentric, salmon-colored, white scaled papules, and plaques that may display a greasy, yellow crust and cause pruritus.20 ASD occurs following disruption in commensurable Malassezia species fungi at the epidermis, followed by an inflammatory reaction, including appearance of perivascular lymphocytes and natural killer cells, then epidermal and skin barrier disruption. Epidermal hyperproliferation may manifest as parakeratotic cells.21,24 There is also a decrease in levels of keratins, sphingolipids, and ceramides.25,26 Collectively, these lead to a stratum corneum that is structurally impaired such that moisture vapor transmission is disrupted and external factors can penetrate.24 If lesions are chronic, psoriasiform hyperplasia and parakeratosis, along with blood vessel dilation, can resemble psoriasis but without accelerated keratinocyte differentiation.21
Treatment for ASD focuses on normalizing the structure and function of the skin and ameliorating symptoms such as pruritus.21,24 This may be applied via a cream, foam or shampoo, or taken as an oral medication. Treatments include anti-fungals (eg, ketoconazole), anti-pruritics (eg, ciclopirox), anti-inflammatories (eg, hydrocortisone, betamethasone dipropionate), keratolytics (eg, urea, zinc pyrithione), anti-bacterials (eg, sulfacetamide), and immunomodulators (eg, tacrolimus).20,21,27 These can be used in rotation to limit any adverse events occurring. Long-term maintenance may also be needed, usually in the form of a shampoo containing one or more of these active ingredients.27 Oral treatment is generally limited to more severe and/or chronic ASD and may include ketoconazole, itraconazole, terbinafine, and fluconazole, though the adverse event profile of all needs to be considered.20,21,27
As with hand eczema, few studies have investigated the use of urea-containing vehicles for the treatment of ASD. For adults with mild-severe ASD, one such topical formulation, also including propylene glycol and lactic acid, was investigated when applied daily. Clinically assessed erythema and desquamation scores, along with patient ratings of dandruff, all showed statistically significant improvements over a placebo preparation after 4 weeks’ use. Overall, the proportion of people with cleared or almost healed ASD was significantly higher compared to the placebo. Adverse events potentially related to treatment included mild, generally transient smarting pain and redness but few participants discontinued treatment due to an adverse event.28
Another study combined 40% urea and 1% bifonazole into a daily-use ointment and included patients with ASD and those with psoriasis capitis. Participants also shampooed daily with a 1% bifonazole shampoo. Here, 42.1% of participants were considered fully remitted and 24.6% to have marked improvement by Week 12 with most showing a response at the Week 1 assessment that included analysis of redness, scaling, and pruritus. Treatment was well-tolerated and it was noted that there was no damage to the hair.29
Psoriasiform dermatoses (PD) covers a wide range of skin conditions including psoriasis, lichen simplex chronicus, and pityriasis rubra pilaris. These conditions include aspects of inflammatory dermic activity and epidermal changes with keratinocyte hyperproliferation. A number of immune system cells are involved, including dendritic cells, macrophages, T lymphocytes, and neutrophils. A decrease in tolerance to cutaneous microorganisms may also be involved in disease pathogenesis.30
Psoriasis is the most prevalent form of PD, affecting around 2% of the population.1 It histopathologically appears as hyperkeratotic plaques with parakeratosis or orthokeratosis, psoriasiform acanthosis, and fused rete ridges. Oedema and capillary dilation in the superficial papillary dermis can lead to thinning of this layer. Additionally present in psoriasis may be pyknotic neutrophils in the stratum corneum, perivascular lymphocyte infiltration in the superficial dermis underlying lesions, and CD11c-positive macrophages in the basement membrane.30
The scalp is commonly affected in people with psoriasis, where it may be associated with pain, pruritus, and quality-of-life and psychosocial impacts. First-line treatment is usually with topically applied treatments such as with a corticosteroid, vitamin D analogue, keratolytic, or coal tar formulation, or a combination of two or more active ingredients in a gel, shampoo, spray, foam, or solution. Phototherapy may also be of use, including with targeted broadband ultraviolet B or via an excimer laser. Systemic agents may be needed if topical therapies fail, most often in the form of phosphodiesterase-4 inhibitors, biologics, methotrexate, acitretin, or cyclosporine.31
Keratolytics targeting PD include both urea and salicylic acid; however, there are a paucity of studies looking at the action of urea as a single active agent and none to date in scalp PD. In a study utilizing a 10% urea ointment for 2 weeks, application to limb plaques was found to reduce erythema, scaling and induration and increase epidermal hydration with no reported adverse events. Histological analysis found significantly decreased epidermal thickening and increased epidermal proliferation along with loss of parakeratosis and reappearance of the granular layer.32 Another study found use of a 10% urea cream led to a significant decrease in TEWL and hygroscopicity and increase in water content.33 At a concentration of 30% urea, a case study report illustrated complete resolution of hyperkeratosis after 2 weeks of treatment.4 Investigation with a much higher concentration of urea in the form of a 50% urea paste applied to a single plaque found it led to resolution of hyperkeratosis, proposed to be due to urea’s actions on increasing basal cell turnover and inhibiting hyperproliferation of keratocytes.34
To investigate the actions of urea on hand eczema, the authors conducted an observational study of a commercially available topical cream. To investigate urea’s action on SD and scalp PD, the authors conducted an observational study using a commercially available foaming product for scalp application.
Methods
Two studies were carried out to test urea-containing formulations. These were monocentric observational studies performed in a simple blind manner with a panel of healthy participants each acting as their own control. For both studies, participants were drawn from a volunteer database held by the study center, inclusion in which was following a detailed cosmetological questionnaire and clinical examination. The studies were carried out according to the general principles of the Declaration of Helsinki, the International Conference on Harmonization/Good Clinical Practice and cosmetic product testing guidelines.18 Testing took place at Eurofins BioPharma Product Testing Kalibios S.r.l. As these were observational studies of cosmetic products, formal submission to an ethics committee was not required; however, the protocols, informed consent forms, and preclinical information concerning the safety of the study products were submitted to and approved by the Internal Survey Committee of the study center.
Inclusion criteria for both studies included no sensitivity to any of the ingredients and that the participant had not recently received any medication or cosmetic/medical procedure that could interfere with the study (eg, use of systemic retinoids or hormonal treatment) and had not had any recent excessive/intensive sun exposure.
Study 1: A Urea-Containing Formulation for Hand Eczema
To investigate the actions of urea on participants with hand eczema, the authors conducted an observational study (human in use test under dermatological control) of U-LifeTM 30 (RELIFE Menarini Group, Florence, Italy), a commercially available topical cream containing among its ingredients 30% urea, glycerin, dimethicone, hydrogenated polydecene, and hydrogenated lecithin. This product was developed in three phases (3P™), the first of which determined the percentage of urea in the formulations, the second added specific ingredients to the formulations, and the third tested the formulation clinically in dermatological disorders.
The product, supplied in neutral packaging, was applied at least twice a day for 28 ±2 days (with reapplication after hand cleaning) in a sufficient amount to cover the hands, then massaged in. Prior to first product application (Day 0), the hands were examined under a standard “daylight” light source by the lead investigator, a dermatologist. Hands were re-examined after 14 (Day 15) and 28 ±2 days (Day 29).
TEWL (g/m2/h) was examined using an Evaporimeter Ep-1 (ServoMed Co, Egypt). Skin redness was measured using a Colorimeter CL 400 (Courage & Khazaka Electronic, GmbH, Germany). Determination of the hydration level of the upper layers of the skin on hands was evaluated using a Corneometer CM 825 (Courage & Khazaka Electronic, GmbH, Germany). All measures were performed at inclusion then after 14 and 28 consecutive days (±2 days) of product use. Measures were performed by the same technician, supervised by the investigator, throughout the study. All data were subjected to statistical treatment using Student’s t-test for repeated measures and for independent samples. Results were considered statistically significant if p < 0.05.
Participant appreciation of the product’s cosmetic efficacy and qualities were conducted via a questionnaire adapted to the investigational product on Days 15 and 29 . For efficacy, participants rated on a scale of 0 (disagree) to 3 (agree) how much the product affected aspects of the eczema and their skin.
Study 2: A Urea-Containing Formulation for Seborrheic Dermatitis and Psoriasiform Dermatoses of the Scalp
To investigate the actions of urea on SD and scalp PD, the authors conducted an observational study (human in use test under dermatological control) of U-LifeTM 10 Ecofoam (RELIFE Menarini Group, Florence, Italy), a commercially available foaming product for scalp application containing among its ingredients 10% urea, glycerin, lactic acid, dimethicone, betaine and prunus amigdalus dulcis oil (sweet almond oil). This product was also developed in three phases (3P™).
The foam, supplied in neutral packaging, was applied in sufficient amounts to cover affected areas of the scalp once a day, then gently massaged in until absorbed, for 28 ±2 days. Prior to first and subsequent examination at the study center, applicants were asked to refrain from hair washing and not use any hair styling products for 2 days or change their usual shampoo or treat/color their hair for the study duration. No sun or ultraviolet A exposure was permitted for the study duration.
At Days 0 (prior to first product application), 15 and 28 (±2 days), the scalp was examined under a standard “daylight” light source by the lead investigator. Desquamation index and surface occupied by squames was assessed using D-squame® (CuDerm Corporation, USA). Analysis of squames extracted from the scalp to an adhesive disc was performed using a charge-coupled device (CCD) camera and Monaderm’s Quantisquam® software (MonaDerm, France). Also assessed was microscopic analysis of photos of the fronto-temporal area of the scalp using a 50X Video camera Dermascope to evaluate scalp dandruff, redness, and dryness. Clinical evaluation of the scalp by the investigator provided a score of desquamation intensity on a scale from 0 (no desquamation) to 10 (severe desquamation). Participants were not identifiable from photos. All measures were performed at inclusion then after 14 and 28 consecutive days (±2 days) of product use. Measures were performed by the same technician, supervised by the investigator, throughout the study. All data were subjected to statistical treatment using Student’s t-test for repeated measures and for independent samples. Results were considered statistically significant if p < 0.05.
Participant appreciation of cosmetic efficacy and qualities were conducted via a questionnaire adapted to the investigational product on Days 15 and 29. For efficacy, participants rated on a scale of 0 (disagree) to 3 (agree) how much the product affected aspects of the hand eczema and their skin.
Results
Study 1: A Urea-Containing Formulation for Hand Eczema
The study took place between March 31st, 2017 and April 28th, 2017. Participants included 20 females with hand eczema due to external factors who had a mean age of 40 (range 20−65 years), with a phototype (Fitzpatrick) skin type of II (n = 6), III (n = 11), or IV (n = 3).
As can be seen from Table 1 and Figure 1, examination of TEWL showed a large and significant (p < 0.05) decrease on Day 15, maintained and bettered by Day 29. Also significantly decreased in a similar manner over the study period (p < 0.05) was skin redness. Significant increases in hydration level of the upper skin layers, from “very dry skin” to “dry skin”, were shown in corneometry values (p < 0.05).
 |
Table 1 Clinical Measures |
 |
Figure 1 Percentage change from baseline for hand eczema study of TEWL, colorimetry, corneometry and clinical evaluation. |
On the questionnaire (Table 2), the majority of participants agreed or slightly agreed (score of 2 or 3) that the product gave hands a healthy look, reduced skin redness and itching, and had a nourishing, moisturising, softening, smoothing, and protective effect. The majority also agreed/slightly agreed that the produce was pleasant to use and easy to apply and absorb.
 |
Table 2 Hand Eczema Study: Assessment of Cosmetic Qualities and Efficacy |
There were no reports of adverse reactions or sensations of discomfort when using the product.
Study 2: A Urea-Containing Formulation for Seborrheic Dermatitis and Psoriasiform Dermatoses of the Scalp
The study took place between March 31st, 2017 and April 28th, 2017. Thirteen female and seven male participants with SD or PD on the scalp had a mean age of 35 (range 20−65 years), with a phototype (Fitzpatrick) skin type of II (n = 8), III (n = 8) or IV (n = 4).
With regard to desquamation, as can be seen from Table 1 and Figure 2, a statistically significant reduction (p < 0.05) in the desquamation index and the surface occupied by squames was noted after 14 consecutive days of product use. The results improved further after 28 days’ use.
Microscopic analysis of photos found a general improvement after product use, in particular an appreciable and significant (p < 0.05) reduction in desquamation and dryness. The three most improved cases are shown in Figure 3. Clinical evaluation showed a significant (p < 0.05) visible reduction in desquamation in 75% of participants at Day 15, rising to 85% at Day 29.
 |
Figure 2 Percentage change from baseline for seborrheic dermatitis and psoriasiform dermatoses of desquamation index and surface occupied by squames. |
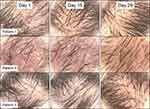 |
Figure 3 Microscopic analysis of the scalp, showing scalps from the three participants with the highest amount of change (reduction on redness, dandruff and dryness). |
On the questionnaire (Table 3), the majority of participants agreed or slightly agreed (score of 2 or 3) that the product had a good moisturising and soothing efficacy and reduced desquamation, itching and skin redness. The majority of participants also deemed the produce easy to apply and absorb and that it did not oil the skin.
 |
Table 3 Seborrheic Dermatitis and Psoriasiform Dermatoses of the Scalp: Assessment of Cosmetic Qualities and Efficacy |
There were no reports of adverse reactions or sensations of discomfort when using the cream.
Discussion
Study 1 provides some indication that the use of a urea-containing topical formulation can aid in the alleviation of hand eczema. However, it is noted that this may also be due to the combination of ingredients and further testing would need to study the urea-containing formulation against a non-urea formulation to provide more direct evidence of the efficacy of urea itself. Further studies could also include skin parameter measurement in an environmentally conditioned room; using participants as their own controls by using the emollient device on only one hand; an equal gender balance; and a wider range of Fitzpatrick skin types.
With regard to Study 2, for ASD, it is known that the use of urea-based moisturizers can reduce TEWL, increase water retention, act as a humectant, strengthen impaired skin barrier function, counteract dry skin, and reduce the prevalence of inflammatory skin diseases.2,5 These actions were confirmed in this study, highlighting a urea-containing cream’s efficacy in restoring the skin barrier and, as also shown by a decrease in corneometry measures, providing moisturising efficacy. This is of particular use for people with ASD where increased TEWL and skin barrier dysfunction are major features.14
Studies have also shown that the use of urea on non-scalp areas reduced, in ASD, desquamation, and pruritus28,29 and, in PD, plaque-associated features including scaling, while increasing epidermal hydration.32,33 This is reflected here where measures of desquamation and dryness were reduced, which may suggest an action on controlling keratinocyte proliferation.2
Limitations of this study include that there was no control condition and that other ingredients may have contributed to the results. Further studies are needed to more formally test this urea-containing formulation under full clinical trial conditions.
Conclusion
Studies, including the two presented here, indicate that urea-containing formulations could aid in the treatment of hand eczema, ASD, and scalp PD.
Acknowledgments
The authors wish to thank study site staff and participants, and Eleanor Roberts, PhD, of Beeline Science Communications, Ltd, for help with manuscript preparation. Dr Roberts received funding from the RELIFE S.r.l. (Menarini Group).
Disclosure
AD’a was the principal investigator in this study and was funded by RELIFE S.r.l. (Menarini Group). LC and WKC are consultants for RELIFE S.r.l. (Menarini Group). WKC has served as an advisory board member for RELIFE S.r.l. (Menarini Group). The authors report no other conflicts of interest in this work.
References
1. Celleno L. Topical urea in skincare: a review. Dermatol Ther. 2018;31(6):e12690. doi:10.1111/dth.12690
2. Piquero-Casals J, Morgado-Carrasco D, Granger C, et al. Urea in dermatology: a review of its emollient, moisturizing, keratolytic, skin barrier enhancing and antimicrobial properties. Dermatol Ther. 2021;11(6):1905–1915. doi:10.1007/s13555-021-00611-y
3. Ashton H, Frenk E, Stevenson CJ. Therapeutics 13. Urea as a topical agent. Br J Dermatol. 1971;84(2):194–196.
4. Dall’Oglio F, Tedeschi A, Verzì AE, Lacarrubba F, Micali G. Clinical evidences of urea at medium concentration. Int J Clin Pract. 2020;74(187):e13815. doi:10.1111/ijcp.13815
5. Dirschka T. Mode of action of urea. Int J Clin Pract. 2020;74(187):e13569. doi:10.1111/ijcp.13569
6. Grether-Beck S, Felsner I, Brenden H, et al. Urea uptake enhances barrier function and antimicrobial defense in humans by regulating epidermal gene expression. J Invest Dermatol. 2012;132(6):1561–1572. doi:10.1038/jid.2012.42
7. Pan M, Heinecke G, Bernardo S, Tsui C, Levitt J. Urea: a comprehensive review of the clinical literature. Dermatol Online J. 2013;19(11):20392. doi:10.5070/D31911020392
8. Thyssen JP, Schuttelaar MLA, Alfonso JH, et al. Guidelines for diagnosis, prevention, and treatment of hand eczema. Contact Dermatitis. 2022;86(5):357–378. doi:10.1111/cod.14035
9. Lakshmi C, Srinivas CR. Hand eczema: an update. Indian J Dermatol Venereol Leprol. 2012;78:569–582. doi:10.4103/0378-6323.100547
10. Ruff SMD, Engebretsen KA, Zachariae C, et al. The association between atopic dermatitis and hand eczema: a systematic review and meta-analysis. Br J Dermatol. 2018;178(4):879–888. doi:10.1111/bjd.16516
11. Thyssen JP, Johansen JD, Linneberg A, Menné T. The epidemiology of hand eczema in the general population--prevalence and main findings. Contact Dermatitis. 2010;62(2):75–87. doi:10.1111/j.1600-0536.2009.01669.x
12. Meding B, Swanbeck G. Consequences of having hand eczema. Contact Dermatitis. 1990;23(1):6–14. doi:10.1111/j.1600-0536.1990.tb00076.x
13. Elsner P, Agner T. Hand eczema: treatment. J Eur Acad Dermatol Venereol. 2020;34(Suppl 1):13–21. doi:10.1111/jdv.16062
14. Wollenberg A, Christen-Zäch S, Taieb A, et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34(12):2717–2744. doi:10.1111/jdv.16892
15. Lodén M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol. 2003;4(11):771–788.
16. Fredriksson T, Gip L. Urea creams in the treatment of dry skin and hand dermatitis. Int J Dermatol. 1975;14(6):442–444. doi:10.1111/j.1365-4362.1975.tb00137.x
17. Lodén M, Wirén K, Smerud K, et al. Treatment with a barrier-strengthening moisturizer prevents relapse of hand-eczema. An open, randomized, prospective, parallel group study. Acta Derm Venereol. 2010;90(6):602–606. doi:10.2340/00015555-0964
18. Cosmetics Europe. Product test guidelines for the assessment of human skin compatibility; 1997. Available from: https://www.cosmeticseurope.eu/files/6014/6407/8875/Product_Test_Guidelines_for_the_Assessment_of_Human_Skin_Compatibility_-_1997.pdf.
19. Pärna E, Aluoja A, Kingo K. Quality of life and emotional state in chronic skin disease. Acta Derm Venereol. 2015;95(3):312–316. doi:10.2340/00015555-1920
20. Tucker D, Masood S. Seborrheic dermatitis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551707/.
21. Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a Comprehensive review. J Clin Investig Dermatol. 2015;3(2). doi:10.13188/2373-1044.1000019
22. Palamaras I, Kyriakis KP, Stavrianeas NG. Seborrheic dermatitis: lifetime detection rates. J Eur Acad Dermatol Venereol. 2012;26(4):524–526. doi:10.1111/j.1468-3083.2011.04079.x
23. Sanders MGH, Pardo LM, Franco OH, Ginger RS, Nijsten T. Prevalence and determinants of seborrhoeic dermatitis in a middle-aged and elderly population: the Rotterdam Study. Br J Dermatol. 2018;178(1):148–153. doi:10.1111/bjd.15908
24. Schwartz JR, Messenger AG, Tosti A, et al. A comprehensive pathophysiology of dandruff and seborrheic dermatitis - towards a more precise definition of scalp health. Acta Derm Venereol. 2013;93(2):131–137. doi:10.2340/00015555-1382
25. Kerr K, Darcy T, Henry J, et al. Epidermal changes associated with symptomatic resolution of dandruff: biomarkers of scalp health. Int J Dermatol. 2011;50(1):102–113. doi:10.1111/j.1365-4632.2010.04629.x
26. Kerr K, Schwartz JR, Filloon T, et al. Scalp stratum corneum histamine levels: novel sampling method reveals association with itch resolution in dandruff/seborrhoeic dermatitis treatment. Acta Derm Venereol. 2011;91(4):404–408.
27. Cheong WK, Yeung CK, Torsekar RG, et al. Treatment of seborrhoeic dermatitis in Asia: a consensus guide. Skin Appendage Disord. 2016;1(4):187–196.
28. Emtestam L, Svensson Å, Rensfeldt K. Treatment of seborrhoeic dermatitis of the scalp with a topical solution of urea, lactic acid, and propylene glycol (K301): results of two double-blind, randomised, placebo-controlled studies. Mycoses. 2012;55(5):393–403. doi:10.1111/j.1439-0507.2011.02126.x
29. Shemer A, Nathansohn N, Kaplan B, Weiss G, Newman N, Trau H. Treatment of scalp seborrheic dermatitis and psoriasis with an ointment of 40% urea and 1% bifonazole. Int J Dermatol. 2000;39(7):532–534. doi:10.1046/j.1365-4362.2000.00986-3.x
30. Balan R, Grigoraş A, Popovici D, Amălinei C. The histopathological landscape of the major psoriasiform dermatoses. Arch Clin Cases. 2019;6(3):59–68. doi:10.22551/2019.24.0603.10155
31. Mosca M, Hong J, Hadeler E, Brownstone N, Bhutani T, Liao W. Scalp psoriasis: a literature review of effective therapies and updated recommendations for practical management. Dermatol Ther. 2021;11(3):769–797. doi:10.1007/s13555-021-00521-z
32. Hagemann I, Proksch E. Topical treatment by urea reduces epidermal hyperproliferation and induces differentiation in psoriasis. Acta Derm Venereol. 1996;76:353–356.
33. Sasaki Y, Tadaki T, Tagami H. The effects of a topical application of urea cream on the function of pathological stratum corneum. Acta Dermatol Kyoto. 1989;84:581.
34. Micali G, Verzi AE, Musumeci ML, Luca M, Lacarrubba F. Ultrasound assessment of the keratolytic effect of a 50% urea anhydrous paste on psoriasis plaques: a prospective study. G Ital Dermatol Venereol. 2019;154(5):509–512. doi:10.23736/S0392-0488.19.06190-X
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
