Back to Journals » Psoriasis: Targets and Therapy » Volume 11
The Use of Metrics in Daily Practice and the Perception of Psoriasis-Associated Comorbidities: Discrepancies Between Research and Real-World
Received 29 September 2021
Accepted for publication 30 November 2021
Published 21 December 2021 Volume 2021:11 Pages 169—175
DOI https://doi.org/10.2147/PTT.S341215
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Uwe Wollina
Tom Hillary,1 Jo Lambert2
1Department of Dermatology, University Hospitals Leuven, Leuven, 3000, Belgium; 2Department of Dermatology, Ghent University Hospital, Gent, 9000, Belgium
Correspondence: Tom Hillary
Department of Dermatology, University Hospitals Leuven, Herestraat 49, Leuven, 3000, Belgium
Email [email protected]
Objective: To assess the feasibility of the future implementation of a recently published Belgian treat-to-target scoring in daily practice, we investigated to what extent Belgian dermatologists use metrics and take comorbidities into account in the follow-up of psoriasis patients.
Methods: Belgian dermatologists were addressed to fill out an online questionnaire in April 2020.
Results: A total of 149 dermatologists completed the survey. About 55% (n = 78) indicated to do a full-body examination during every visit. Psoriasis Area Severity Index (PASI) was the most frequently used clinical score: 25% (n = 35) and 61% (n = 87) indicated to use it every visit or sometimes (> 1/year), respectively. The most frequently used patient-reported outcome scoring system was the Dermatology Life Quality Index: 35% use it sometimes. Overall, there is awareness for the association with metabolic syndrome.
Conclusion: Among tools for follow-up on moderate-to-severe psoriasis patients, Belgian dermatologists most frequently apply full-body examination and PASI score. Patient-reported outcome scoring systems are used infrequently. Psoriasis is perceived as a disease with comorbidities beyond the skin, especially obesity and hypertension. These real-world data on the use of clinical scores and PROs indicate a discrepancy from the academic setting in which new drugs are developed and evaluated. Furthermore, these data are imperative to estimate the feasibility of implementing a treat-to-target strategy published earlier by a Belgian expert group.
Keywords: psoriasis, comorbidities, patient care, treat to target, metrics
Introduction
Psoriasis is a frequent skin disease with prevalence of up to 3% in the Western population. It is an inflammatory disease with genetic predisposition. The bulk of genes involved seems to be encoding for components of inflammatory pathways (eg PSORS1).1 Improved pathophysiological insights have led to the development of biologics targeting tumor necrosis factor (TNF)-alfa, the shared p40 subunit of interleukin (IL)-12 and IL-23, IL-17, and most recently the p19 subunit of IL-23. In Belgium, biologics are reimbursed for patients with Psoriasis Area Severity Index (PASI) and/or Body Surface Area (BSA) >10 who previously failed on or have contra-indication for ultraviolet light therapy, methotrexate and cyclosporine. Patient-reported outcome scoring systems (PROs) are currently not integrated into the reimbursement criteria for biologics in Belgium.2
In a Belgian treat-to-target (T2T) composite score, we aimed to stratify the follow-up of psoriasis patients in four target areas: disease control, patient well-being, comorbidities and burden of treatment3 (Figure 1). First, the following factors were incorporated for follow-up on disease control: PASI, BSA, visual analogue scale (VAS) for itch, and the presence or absence of body locations disturbing the patient. (Supplementary Material: Appendix 1) A suggested time frame of 12 weeks to evaluate response to initiated therapy was incorporated. Secondly, scores to follow up on patient well-being were Dermatology Life Quality Index (DLQI) and VAS for daily functioning. (Supplementary Material: Appendix 2) Third, for the assessment of comorbidities, the dermatologist was supposed to check for the presence or absence of associated symptoms and refer adequately. Obviously, thorough knowledge of associated comorbidities is a prerequisite here. Finally, the tolerability of treatment and the presence of adverse events should be monitored at all times.
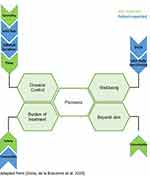 |
Figure 1 A Belgian treat to target approach consists of four target areas: disease control, patient well-being, comorbidities and burden of treatment. Metrics in blue are patient reported; metrics in green are physician reported. Adapted from Grine L, de la Brassinne M, Ghislain PD, et al. A Belgian consensus on the definition of a treat-to-target outcome set in psoriasis management. J Eur Acad Dermatol Venereol. 2020;34(4):676–684. © 2019 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.3 |
Psoriasis is associated with a number of comorbidities. As part of the psoriatic disease concept, psoriatic arthritis is reported in up to 30% of psoriatic patients.4,5 In the last decade, the focus of research has been metabolic syndrome (MetS) as comorbidity of psoriasis.6 The prevalence of MetS in psoriasis patients is in a disease severity-dependent way increased compared to the general population.7–10 Associated with these findings, it was observed that the relative risk of myocardial infarction in severe psoriasis patients is increased.11,12 Also, thrombotic events in the venous compartment have been reported to manifest more frequently in psoriasis patients than in healthy controls.13,14 Furthermore, an association between psoriasis and inflammatory bowel disease has been established.15,16 A possible link between psoriasis and lymphoma has been investigated with conflicting results: it seems that psoriasis patients have a modestly increased relative risk of lymphoma, however the absolute risk attributable to psoriasis is low, and the magnitude of association is minimal.17
At this point, we considered it useful to investigate the readiness of the average Belgian dermatologist to use metrics, as they have been suggested in our recent treat-to-target approach consensus document. Moreover, the awareness around comorbidities was surveyed as we consider this to be part of the overall approach of a psoriasis patient.
Materials and Methods
Context
In this project, Belgian dermatologists were invited to complete an online questionnaire. Six hundred and fifty-one Belgian dermatologists (both Dutch and French speaking) on the mailing list of the Royal Belgian Society of Dermatology and Venereology received an e-mail with a link to an online questionnaire. The survey was accessible for a period of two weeks in April 2020. Approval of the ethics committee of UZ Leuven to collect these data anonymously was obtained (S63770). No written informed consent was obtained in order to protect the anonymity of responders; completion of the survey was deemed informed consent.
Questionnaire
This questionnaire was designed to test the use and knowledge of parameters included in the Belgian T2T score. The language of the questionnaire was English. (Supplementary Material: Appendix 3) A first part generated general information on the participant: year of graduation and professional setting. A second part investigated the use of physician- and patient-reported outcome scoring systems in daily practice: full-body examination, PASI, Body Surface Area (BSA), Physician Global Assessment (PGA), DLQI, Hospital Anxiety and Depression Scale (HADS), VAS itch, VAS daily functioning, and blood pressure were evaluated. A third part looked into the perception of Belgian dermatologists on associated comorbidities of psoriasis: high blood sugar, inflammatory bowel disease, skin infection, associated lymphoma, pulmonary embolism, deep venous thrombosis, acute myocardial infarction, obesity, increased blood lipids and blood pressure were assessed. Finally, the actions when a comorbidity is suspected were questioned: referral to general practitioner or specialist, initiate treatment, or no action.
Results
A total of 149 dermatologists completed the survey (median year of graduation 2001–2010). 74% (n = 110) worked in a private practice, 26% (n = 39) in a hospital setting.
The Use of Patient-Reported Outcome Scoring Systems
The most frequently used PRO instrument was the Dermatology Life Quality Index (DLQI): 35% of the participating dermatologists reported to use it sometimes. Notably, over 50% (n = 77) expressed to never use this score. With only 18% (n = 26) of dermatologists expressing to use the VAS itch sometimes, this PRO is the second most important score. VAS daily functioning and Hospital Anxiety and Depression Scale (HADS) were never used by 88% (n = 125) and 91% (n = 130), respectively.
Follow-Up of Psoriasis Patients in Daily Practice
Participants could indicate whether they use a score: never; 1/year; sometimes; every visit.
Physician-Reported Outcomes
55% (n = 78) of participants indicated to do a full-body examination during every visit, whereas 43% (n = 66) responded to do this ‘sometimes’ or 1/year. Psoriasis Area Severity Index (PASI) was the most frequently used clinical score: 25% (n = 35) and 61% (n = 87) of dermatologists claimed to use it every visit or sometimes (>1/year), respectively. 7% (n = 10) reported to never use PASI. Almost 40% (n = 56) of participating dermatologists disclosed to never use BSA. Approximately the same number of dermatologists (n = 59) expressed to use BSA sometimes, whereas 14% (n = 20) uses it every visit. The PGA was never used by 80% (n = 114) of the participating dermatologists; 17% (n = 25) uses it sometimes.
Perception of Psoriasis and Its Comorbidities
Participants could indicate whether they believe the risk among psoriasis patients compared to the general population was: decreased; the same; slight increase; moderate to high increase.
Belgian dermatologists reported an overall concern for psoriasis patients to be at increased risk for metabolic syndrome. 68% of dermatologists indicate they believe psoriasis patients are at moderate-to-high risk for developing obesity. Moreover, most dermatologist (59% and 53%, respectively) believe the risk of high blood lipids and high blood pressure are moderate to high compared to the general population. Also, 35% of dermatologists believe the risk of high blood sugar is moderate to high.
61% of dermatologists believes the risk of myocardial infarction is slightly increased in psoriasis patients. In contrast, 63% and 64% of the participants share the opinion that, respectively, pulmonary embolism and deep venous thrombosis occur at the same rate in psoriasis patients and healthy controls.
A correlation with IBD is suspected by 84% of the responders. 56% of the Belgian dermatologists believe psoriasis patients are not at increased risk for developing lymphoma, whereas 41% believes there is a slightly increased risk.
Referral of Psoriasis Patients with a Suspected Comorbidity
Participants ranked the following options: I don’t take comorbidities into account; I start a treatment for a comorbidity; I refer to the general practitioner; I refer to a specialist. A general score was calculated.
With an average of 3.53/4, referral to the patients’ general practitioner when a comorbidity is suspected, is the most popular strategy among Belgian dermatologists. Secondly, with a score of 2.9/4, dermatologists indicate feeling comfortable referring to a specialist. The least favorite strategy is to initiate treatment when a comorbidity is suspected (eg start antihypertensive).
Discussion
Psoriasis is a frequent skin disease with associated comorbidities. Treatment options have expanded in recent years, and follow-up of psoriasis patients can be challenging. In April 2020, we sent out an online questionnaire to investigate the common practice in the use of metrics in the follow-up of psoriasis patients in Belgium and the awareness of associated diseases. We found that the average Belgian dermatologist follows up on his psoriasis patient mostly through a full body examination (55% every visit), PASI score (25% every visit and 61% sometimes (>1/year)), and DLQI (35% use it sometimes). From a different perspective, this means that a significant number of dermatologists are not or rarely using standardized metrics to follow up on their psoriasis patients. Furthermore, the use of PROs as VAS for itch or daily functioning is almost never used.
Next, we evaluated the awareness of dermatologists on comorbidities of psoriasis: Belgian dermatologists are conscious about the association of metabolic syndrome, cardiovascular disease and inflammatory bowel disease, which is in line with published data (Figure 2). Most dermatologists do not believe psoriasis and lymphoma, VTE or PE are related. However, some literature for the latter two is available, warranting more attention for this. Dermatologists feel most comfortable referring to the general practitioners to diagnose and/or treat a suspected comorbidity.
It is of interest to point out the discrepancy between the Belgian dermatologists reported use of metrics in daily practice, and the use of metrics in guidelines, clinical trials, and also our Belgian treat-to-target approach. Only recently, the importance of using multiple outcome measures was pointed out by Svoboda et al, suggesting that the use of both physician- and patient-reported data can assist clinicians in evaluating the disease burden, ultimately enhancing quality of care.18
It is therefore surprising that very little literature is available on the actual use of metrics for the follow-up of psoriasis in real-world clinical practice. Knuckles et al investigated how dermatologists define and treat moderate psoriasis: they contacted 174 dermatologists, of whom 150 dermatologists completed the survey.19 They reported BSA to be the most frequently used tool to assess disease severity. Nazeer et al assessed the variation in management practices of psoriasis among dermatologists in Kerala, South India.20 They found that most dermatologists rely on BSA, rather than PASI or PGA, due to time constraints. Furthermore, DLQI was not assessed by the majority of dermatologists. In the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey, van de Kerkhof et al reported that dermatologists most commonly consider the location or size of skin lesions to be the most important factor contributing to disease severity.21 However, they do not report in detail, which metrics are used to assess and follow-up on psoriasis patients.
Drawbacks of our work are the fact that 149 dermatologists completed the questionnaire, correlating with a response rate of approximately 25%. However, this number and the partition of the profile of the responders can be considered representative for the Belgian dermatologists as a group. It should be kept in mind that in the survey period a number of practices were closed because of the COVID-19 pandemic. Furthermore, the questionnaire was in English, which may have been less comfortable for native Dutch or French-speaking dermatologists.
Conclusion
To conclude, this study reports on the current psoriasis-related real-world practices and opinions among Belgian dermatologists: there is a preference for the use of PASI and BSA scores in the follow-up of patients and in general patient-reported outcome-scores are not used. Furthermore, the association of psoriasis with systemic comorbidities is well known. Specifically, awareness of the association with metabolic syndrome is high. We conclude that daily practice differs significantly from the approach we suggested earlier in a previously published treat-to-target recommendation. Next, we plan to investigate whether this treat-to-target approach leads to different clinical decisions, compared to common practice. We intend to do this in a pilot group of patients. If future research confirms the benefits of this T2T approach, hurdles in daily practice to implement this strategy should be investigated and overcome.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author, [TH], upon reasonable request.
Acknowledgment
The authors would like to thank all participating dermatologists for filling out the survey.
Funding
No funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Alshobaili HA, Shahzad M, Al-Marshood A, Khalil A, Settin A, Barrimah I. Genetic background of psoriasis. Int J Health Sci (Qassim). 2010;4(1):23–29.
2. Segaert S, Ghislain PD, Boone C. An observational study of the real-life management of psoriasis patients treated with etanercept according to the new reimbursement criteria (in Belgium). J Dermatolog Treat. 2016;27(2):103–109. doi:10.3109/09546634.2015.1055228
3. Grine L, de la Brassinne M, Ghislain PD, et al. A Belgian consensus on the definition of a treat-to-target outcome set in psoriasis management. J Eur Acad Dermatol Venereol. 2020;34(4):676–684. doi:10.1111/jdv.16104
4. Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl 2):ii14–17. doi:10.1136/ard.2004.032482
5. Gelfand JM, Gladman DD, Mease PJ, et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol. 2005;53(4):573. doi:10.1016/j.jaad.2005.03.046
6. Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and metabolic syndrome: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol. 2013;68(4):654–662. doi:10.1016/j.jaad.2012.08.015
7. Ma C, Harskamp CT, Armstrong EJ, Armstrong AW. The association between psoriasis and dyslipidaemia: a systematic review. Br J Dermatol. 2013;168(3):486–495. doi:10.1111/bjd.12101
8. Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149(1):84–91. doi:10.1001/2013.jamadermatol.406
9. Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2(12):e54–e54. doi:10.1038/nutd.2012.26
10. Miller IM, Ellervik C, Yazdanyar S, Jemec GB. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. 2013;69(6):1014–1024. doi:10.1016/j.jaad.2013.06.053
11. Samarasekera EJ, Neilson JM, Warren RB, Parnham J, Smith CH. Incidence of cardiovascular disease in individuals with psoriasis: a systematic review and meta-analysis. J Investig Dermatol. 2013;133(10):2340–2346. doi:10.1038/jid.2013.149
12. Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–1741. doi:10.1001/jama.296.14.1735
13. Ahlehoff O, Gislason GH, Lindhardsen J, et al. Psoriasis carries an increased risk of venous thromboembolism: a Danish nationwide cohort study. PLoS One. 2011;6(3):e18125. doi:10.1371/journal.pone.0018125
14. Ogdie A, Kay McGill N, Shin DB, et al. Risk of venous thromboembolism in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a general population-based cohort study. Eur Heart J. 2018;39(39):3608–3614. doi:10.1093/eurheartj/ehx145
15. Fu Y, Lee C-H, Chi -C-C. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154(12):1417–1423. doi:10.1001/jamadermatol.2018.3631
16. Cohen A, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23(5):561–565. doi:10.1111/j.1468-3083.2008.03031.x
17. Gelfand JM, Shin DB, Neimann AL, Wang X, Margolis DJ, Troxel AB. The risk of lymphoma in patients with psoriasis. J Invest Dermatol. 2006;126(10):2194–2201. doi:10.1038/sj.jid.5700410
18. Svoboda SA, Ghamrawi RI, Owusu DA, Feldman SR. Treatment goals in psoriasis: which outcomes matter most? Am J Clin Dermatol. 2020;21(4):505–511. doi:10.1007/s40257-020-00521-3
19. Knuckles MLF, Levi E, Soung J. Defining and treating moderate plaque psoriasis: a dermatologist survey. J Dermatolog Treat. 2018;29(7):658–663. doi:10.1080/09546634.2018.1443200
20. Nazeer M, Ravindran S, Gangadharan G, Criton S. A survey of treatment practices in management of psoriasis patients among dermatologists of Kerala. Indian Dermatol Online J. 2019;10(4):437–440. doi:10.4103/idoj.IDOJ_306_18
21. van de Kerkhof PC, Reich K, Kavanaugh A, et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population-based multinational assessment of psoriasis and psoriatic arthritis survey. J Eur Acad Dermatol Venereol. 2015;29(10):2002–2010. doi:10.1111/jdv.13150
22. Langley RG, Ellis CN. Evaluating psoriasis with psoriasis area and severity index, psoriasis global assessment, and lattice system physician’s global assessment. J Am Acad Dermatol. 2004;51(4):563–569.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

