Back to Journals » Clinical Ophthalmology » Volume 9
The use of endoillumination probe-assisted Descemet membrane endothelial keratoplasty for bullous keratopathy secondary to argon laser iridotomy
Authors Kobayashi A, Yokogawa H , Yamazaki N, Masaki T, Sugiyama K
Received 26 September 2014
Accepted for publication 1 November 2014
Published 8 January 2015 Volume 2015:9 Pages 91—93
DOI https://doi.org/10.2147/OPTH.S74981
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Akira Kobayashi, Hideaki Yokogawa, Natsuko Yamazaki, Toshinori Masaki, Kazuhisa Sugiyama
Department of Ophthalmology, Kanazawa University Graduate School of Medical Science, Kanazawa, Japan
Purpose: To report the first case of Descemet membrane endothelial keratoplasty (DMEK) for bullous keratopathy (BK) secondary to argon laser iridotomy (ALI).
Patient: A 71-year-old woman presented with decreased visual acuity in her right eye due to BK secondary to ALI that was performed 10 years prior.
Results: Phacosurgery was performed first, followed by successful DMEK 4 months later. A DMEK shooter was used for donor insertion, which allowed for a stable anterior chamber during donor insertion, even when the anterior chamber was quite shallow. Also, removal of edematous epithelial cells and endoillumination probe-assisted DMEK was quite useful to visualize DMEK graft on the background of the dark brown iris seen in Asian eyes. The patient’s best corrected visual acuity rapidly increased from 20/200 to 25/20 after 1 month, with complete resolution of corneal edema.
Conclusion: We reported the first successful DMEK case for BK secondary to ALI. The use of a DMEK shooter for donor insertion and endoillumination assistance to visualize the DMEK graft was a useful technique for BK secondary to ALI.
Keywords: argon laser iridotomy, bullous keratopathy, endoillumination, DMEK
A Letter to the Editor has been received and published for this article
Introduction
To date, Descemet-stripping automated endothelial keratoplasty (DSAEK) has been the most popularized endothelial keratoplasty technique to treat bullous keratopathy (BK).1,2 We have previously reported for the first time the usefulness of DSAEK for BK secondary to argon laser iridotomy (ALI); these patients usually have small eyes with a shallow anterior chamber, making DSAEK quite challenging.3–6 Recently, to achieve better and more rapid visual acuity when compared to DSAEK, selective transplantation of only donor Descemet membrane (DM) and endothelium was established, and the procedure has been called DM endothelial keratoplasty (DMEK).7–9 Herein, we report the first successful DMEK case for BK secondary to ALI. A surgical technique using endoillumination probe-assisted DMEK was presented.
Patient
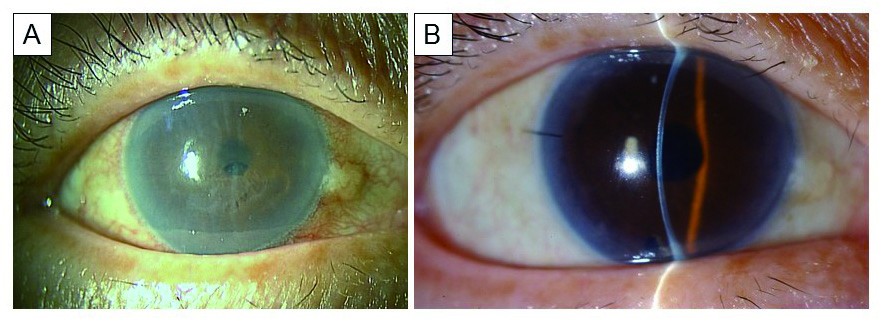
This study was approved by the ethics committee of the Kanazawa University Graduate School of Medical Science (Kanazawa, Japan) and followed the tenets of the Declaration of Helsinki. A 71-year-old woman presented with decreased visual acuity (20/200) in her right eye due to BK secondary to ALI that was performed 10 years prior (Figure 1A). The patient had hypertension and rheumatoid arthritis.
Results
Surgery
The surgery was performed at the Department of Ophthalmology, Kanazawa University Graduate School of Medical Science. The patient read and signed an informed consent form prior to surgery.
Cataract surgery (phacoemulsification and intraocular lens implantation) was performed in the 4 months prior to DMEK. Both cataract and DMEK surgery were performed under peribulbar anesthesia. DMEK was performed according to methods previously reported.7,8 In brief, after removal of the edematous host epithelial cells for better visualization of the anterior chamber, the host DM was removed (approximately 9.0 mm in diameter) under viscoelastic materials, which filled the anterior chamber (Figure 2A). An inferior iridectomy at the 6 o’clock position was created using a 25-gauge vitreous cutter. Then, the donor graft was prepared using the submerged cornea with a backgrounds away technique (Figure 2B). The harvested and stained DM roll stained by trypan blue (8.0 mm in diameter) was then inserted into the anterior chamber via a 2.4 mm temporal clear corneal incision using a DMEK shooter (G-38630; GEUDER AG, Heidelberg, Germany) (Figures 2C and D). After securing the wound with a 10-0 nylon suture, the DMEK roll was correctly oriented with the endothelium side facing down. A small air bubble was then injected over the DM graft and used to unfold the DM graft (Figures 2E–G). To obtain further visualization, oblique light via an endoillumination probe held by an assistant surgeon was used (Figures 2E–G). The endoillumination probe was not inserted into the anterior chamber, but attached at the peripheral cornea. This technique improved the contrast between the blue-stained DM roll and the background of the dark brown iris. Finally, the anterior chamber was filled with air to attach the DM graft completely to the posterior stromal surface (Figure 2H). No corneal fenestrations were made to drain the interface fluid. No recipient peripheral stromal scraping was performed to improve donor recipient adhesion. The anterior chamber was left full of air, and the patient was instructed to lie on her back for 2–3 hours.
Clinical outcomes
Intra- and postoperative complications, including graft dislocation, pupillary air block, and rejection, were not noted. The best corrected visual acuity in the patient’s right eye rapidly increased to 25/20 after 1 month, with complete resolution of corneal edema (Figure 1B). Postoperative endothelial cell count after 1 month was 2,212 cells/mm2, a 17.5% reduction when compared with donor counts (2,682 cells/mm2).
Discussion
BKs secondary to ALI are quite common in Japan.3–6 However, patients usually have small eyes with a shallow anterior chamber; this makes endothelial keratoplasty quite challenging.3 In particular, a donor insertion step using a conventional donor taco-folding push-in technique caused severe complications such as iris prolapse, iris bleeding and, in severe cases, vitreous prolapse with serious endothelial cell damage.6 To circumvent these difficulties, we developed the double-glide pull-through technique using a Busin glide together with an intraocular lens sheet glide (also known as Kobayashi’s double-glide technique).6
Herein, we reported the first successful DMEK case for BK secondary to ALI. DMEK for ALI was technically difficult in two ways. First, to insert the DM roll into a shallow anterior chamber using an implantable collamer lens inserter is difficult. Therefore, we found it quite useful to use a DMEK shooter for these eyes, which allowed for a stable anterior chamber during DM roll insertion. In general, the anterior chamber is quite shallow in patients after they have undergone ALI, even when the patient is pseudophakic. Second, the background of a dark brown iris, which is usually seen in Asian eyes, makes the visibility of the blue-stained DMEK donor graft difficult. To circumvent this difficulty, we used oblique light via an endoillumination probe held by an assistant surgeon, which significantly improved the visibility of the DMEK graft in the anterior chamber.
Conclusion
In conclusion, we reported the first successful DMEK case for BK secondary to ALI. The use of the DMEK shooter for donor insertion and endoillumination assistance to visualize the DMEK graft was a useful technique for BK secondary to ALI. Further clinical studies using a large number of cases are required to confirm the usefulness of these techniques.
Acknowledgments
This study was supported by a grant from the Japanese Ministry of Health, Labour and Welfare and by a Grant-in-Aid for Scientific Research (number 22591934). No sponsor or funding organization had any role in the design or conduct of this research.
Author contributions
Contributions by authors: Design (AK, HY, NY, TM, KS), interpretation of the data (AK, HY, NY, TM, KS), and review and approval of the manuscript (AK, HY, NY, TM, KS).
Disclosure
The authors report no conflicts of interest in this work.
References
Melles GR. Posterior lamellar keratoplasty: DLEK to DSEK to DMEK. Cornea. 2006;25(8):879–881. | ||
Gorovoy MS. Descemet-stripping automated endothelial keratoplasty. Cornea. 2006;25(8):886–889. | ||
Shimazaki J, Amano S, Uno T, Maeda N, Yokoi N; Japan Bullous Keratopathy Study Group. National survey on bullous keratopathy in Japan. Cornea. 2007;26(3):274–278. | ||
Ang LP, Higashihara H, Sotozono C, et al. Argon laser iridotomy-induced bullous keratopathy a growing problem in Japan. Br J Ophthalmol. 2007;91(12):1613–1615. | ||
Kobayashi A, Yokogawa H, Sugiyama K. Non-Descemet stripping automated endothelial keratoplasty for endothelial dysfunction secondary to argon laser iridotomy. Am J Ophthalmol. 2008;146(4):543–549. | ||
Kobayashi A, Yokogawa H, Sugiyama K. Descemet stripping with automated endothelial keratoplasty for bullous keratopathies secondary to argon laser iridotomy – preliminary results and usefulness of double-glide donor insertion technique. Cornea. 2008;27 Suppl 1:S62–S69. | ||
Dapena I, Moutsouris K, Droutsas K, Ham L, van Dijk K, Melles GR. Standardized “no-touch” technique for descemet membrane endothelial keratoplasty. Arch Ophthalmol. 2011;129(1):88–94. | ||
Kobayashi A, Yokogawa H, Yamazaki N, Masaki T, Sugiyama K. In vivo laser confocal microscopy after Descemet’s membrane endothelial keratoplasty. Ophthalmology. 2013;120(5):923–930. | ||
Monnereau C, Quilendrino R, Dapena I, et al. Multicenter study of descemet membrane endothelial keratoplasty: first case series of 18 surgeons. JAMA Ophthalmol. 2014;132(10):1192–1198. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.