Back to Journals » Clinical Ophthalmology » Volume 10
The use of dry amniotic membrane in pterygium surgery
Authors Noureddin G, Yeung S
Received 17 December 2015
Accepted for publication 20 February 2016
Published 18 April 2016 Volume 2016:10 Pages 705—712
DOI https://doi.org/10.2147/OPTH.S80102
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Gelareh S Noureddin, Sonia N Yeung
Department of Ophthalmology and Visual Sciences, University of British Columbia, Vancouver, BC, Canada
Abstract: Pterygium is a fibrovascular growth of the bulbar conjunctiva that crosses the limbus and extends over the peripheral cornea, in some cases resulting in significant visual morbidity. When treatment is indicated, surgery is necessary, and several management options exist. These include excision, conjunctival autografting, and the use of adjuvant therapies. This paper reviews the incidence and prevalence of pterygia and also describes the various techniques currently used to treat this condition. These management options are compared to the use of dry amniotic membrane grafting (AMG), specifically with regard to recurrence rates, time to recurrence, safety and tolerability, as well as patient factors including cosmesis and quality of life. AMG has been used in the treatment of ocular surface disease due to a variety of benefits, including its anti-inflammatory properties, as well as its ability to promote epithelial growth and suppress transforming growth factor-β signaling and fibroblast proliferation. However, rates of recurrence for AMG following pterygium excision still surpass other commonly used techniques, including conjunctival and limbal autografting. Nevertheless, there are circumstances in which AMG may be most beneficial to the patient, such as when preexisting conjunctival scarring is present, when the conjunctiva must be spared for future glaucoma filtering surgery, or in cases of large or double-headed pterygia. Therefore, surgeons should be prepared to offer this procedure as an option to their patients for the treatment of pterygia.
Keywords: cornea, pterygium, amniotic membrane, conjunctival autograft
Introduction to the incidence and prevalence of pterygium
Pterygium is a wing-shaped, fibrovascular growth of the bulbar conjunctiva that crosses the limbus and extends over the peripheral cornea.1 This invasion of the corneal surface can lead to significant visual morbidity caused by irritation of the ocular surface, irregular astigmatism, obstruction of the visual axis, and loss of corneal transparency.2
The incidence and prevalence of this condition vary among different populations and are influenced by a variety of factors including age, sex, and geographic location.3 To date, the majority of research evaluating the prevalence of pterygium has focused on population-based studies, with few studies providing a more global understanding of the burden of disease. In a recent meta-analysis by Liu et al,3 20 population-based studies published between 2000 and 2013 were reviewed. The pooled worldwide prevalence of pterygium was found to be 10.2%,3 with prevalence rates ranging from 2.8% in a study by Wu et al4 and 33% in a study by McCarty et al.5 The prevalence of pterygium in men was also higher than that in women, with rates of 14.5% and 13.6%, respectively.3 Pterygium was more prevalent with increasing age up to 69 years.3 Populations living in geographic latitudes ranging from 20° to 30° also had a higher prevalence of pterygium compared with any other area.3
The nasal limbus is the most common site for pterygium formation. This predilection has been attributed to the focusing of light passing through the anterior chamber at the nasal limbus, causing damage to the limbal stem cells and inducing oxidative stress.6,7 Many population-based studies have also revealed an association between pterygium formation and outdoor occupation and activities, most likely a result of exposure to ultraviolet (UV) radiation, the pathogenesis of which has been described.8,9 Conjunctival UV autofluorescence, a biomarker of ocular exposure to UV light, has been shown to be higher in individuals with pterygium than those without.10 An increase in prevalence has also been noted in rural populations when compared to urban populations, likely reflecting differences in lifestyle and lifetime exposure to UV radiation.3,11,12
Many tumor-like features, including the propensity to invade normal tissue, the high rate of recurrence, and the coexistence with premalignant lesions, challenge the idea that pterygia are benign lesions.6 In one study, including 100 cases of pterygia, concurrent ocular surface disease included five cases of ocular surface squamous neoplasia, six cases of primary acquired melanosis, and two compound nevi (one of which was suspicious for melanoma).6 It is thus recommended that all pterygia be sent for histopathology to rule out concurrent ocular surface disorders, including those with malignant potential.6
Management approaches
The treatment of pterygia is surgical; however, because of the high rate of recurrence, careful consideration of the risks and benefits for surgery is necessary before primary excision is undertaken. Indications for treatment include any one or more of the following: vision loss secondary to astigmatism or progressive encroachment on the visual axis, restriction of ocular movement, or discomfort and irritation.13 Current management options for pterygium include excision, conjunctival autografting, and the use of adjuvant therapies including mitomycin C, 5-fluorouracil, anti-vascular endothelial growth factor (anti-VEGF) agents, and β-irradiation.
The technique of excising a pterygium without repairing the remaining defect is called bare sclera excision. This technique is no longer recommended because of its high rate of recurrence, which ranges from 38% to 88%.14 This recurrence rate is higher than for any other treatment modality.13 Moreover, there are no advantages conferred by this technique except for its simplicity and short surgical time.13
Primary closure is a technique that involves excision of the pterygium, followed by suturing of the remaining conjunctiva on either side of the wound over the bare sclera, to close the defect. This procedure has also been reported to have an unacceptably high rate of recurrence compared to newer techniques (45%–70%).14
Given the unacceptable recurrence rates of both bare sclera and primary closure techniques, advances in pterygium excision have focused on the use of grafts and adjuvant therapies, of which conjunctival autografts are the most commonly used. In this method, the pterygium is excised and the remaining defect closed with the patient’s own grafted conjunctiva and attached using fibrin glue or sutures (Figure 1). Although the ipsilateral superior conjunctiva is typically used, both superior and inferior conjunctival autografts have been found to be reasonable options.15 Recurrence rates for this procedure have been cited between 2% and 20%.14 Alpay et al13 observed that all recurrences in patients who had undergone conjunctival autografting in their case series (3/18=16.65%) occurred in eyes that had undergone previous pterygium surgery (no primary pterygia treated with conjunctival autografting recurred). Interestingly, Syam et al16 found that 36.66% of patients developed conjunctival scarring at the site of the donor conjunctiva.
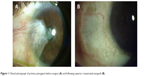  | Figure 1 Clinical photograph of primary pterygium before surgery (A) and following superior conjunctival autograft (B). |
The P.E.R.F.E.C.T. technique, which stands for “Pterygium Extended Removal Followed by Extended Conjunctival Transplantation”, was pioneered by Lawrence Hirst and differs from traditional conjunctival autografting by the extensive removal of Tenon’s layer after pterygium excision. This technique was shown by Hirst, in a prospective trial of 250 consecutive patients with primary pterygia, to have a recurrence rate of 0% and a good cosmetic outcome.17 In another study, he went on to show that the P.E.R.F.E.C.T. technique could also be used for excision of recurrent pterygia with a recurrence rate of 0% and few complications.18
Limbal conjunctival autografting involves transplantation of limbal stem cells in addition to autologous conjunctiva in order to cover the defect created from excision of the pterygium. The benefit of this method is that, in addition to decreasing recurrence rates, the limbal stem cells promote healing.14 Sutured limbal conjunctival autografts have a recurrence rate ranging from 0% to 14.29%.14 The use of fibrin glue was shown in one study to significantly decrease the rate of recurrence.19
A conjunctival flap requires undermining of the conjunctiva at the donor site without detaching the tissue from its origin. The surgeon then rotates the flap to cover the defect left by excision of the pterygium. Few complications have been reported for this procedure, apart from conjunctival cyst formation and flap retraction.13 Alpay et al13 found a recurrence rate of 33.33% with this technique and noted poor cosmesis, which improves with time.
An amniotic membrane (AM) graft can also be used to cover bare sclera following pterygium excision (Figure 2). These grafts are thought to promote healing and reduce rates of recurrence because of their anti-inflammatory properties, their promotion of epithelial growth, and their suppression of transforming growth factor β (TGF-β) signaling and fibroblast proliferation.14 Recurrence rates of pterygia following amniotic membrane grafting (AMG) are cited between 14.5% and 27.3%.14 The use of postoperative steroid injections following AMG also reduces the rate of recurrence.14 When compared to conjunctival and limbal autografting, recurrence rates are higher for AMG.20 AMG shows particular promise over the other grafting procedures in certain circumstances, such as when preexisting conjunctival scarring precludes the harvesting of donor conjunctiva for an autograft. AMG is also helpful when the superior conjunctiva must be spared for future glaucoma filtering surgery, as well as in cases of large or double-headed pterygia.21
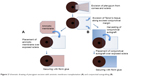  | Figure 2 Schematic drawing of pterygium excision with amniotic membrane transplantation (A) and conjunctival autografting (B). |
While grafts have greatly improved recurrence rates following pterygium surgery, they are not without their complications. Reported complications include wound dehiscence, Tenon’s granuloma, conjunctival cysts, necrotizing scleritis, and subconjunctival fibrosis from the donor site.9
Adjuvant therapies, including the use of mitomycin C, have been used both independently and in conjunction with grafting techniques to reduce the rate of recurrence following pterygium surgery. Mitomycin C is an antibiotic from the bacteria Streptomyces caespitosus that inhibits DNA, RNA, and protein synthesis.14 Its use has been shown to lower the rate of recurrence when used in conjunction with bare sclera, limbal autograft, conjunctival autograft, and AMG techniques. However, many serious complications have been noted with its use, including scleral melt, punctate keratitis, infectious scleritis, corneal perforation, secondary glaucoma, cataract, iritis, and a possible effect on corneal endothelial cells.9,14 A safe, minimal, and effective dose has not been established.13 For this reason, caution must be taken when using mitomycin C to ensure that the risk of treatment does not outweigh the benefit of a reduced recurrence rate.
β-Irradiation has also been found to be a relatively well-tolerated procedure, with recurrence rates similar to chemotherapeutic agents and conjunctival autografting. Rare but significant complications of this procedure include scleral thinning, ulceration, infection, and radiation-induced cataract.22 Other medical therapies are also currently being investigated, including the chemotherapeutic agent 5-fluorouracil, and anti-VEGF agents such as ranibizumab and bevacizumab.23 These treatments are beyond the scope of this review.
Introduction to the use of AM
Amniotic membrane transplantation (AMT) was first described by Davis24 for use as a surgical material in skin transplantation.24,25 In the 1940s, its use in the treatment of ocular surface conditions was described.25 Since 1995, it has been increasingly used to treat a variety of ocular surface conditions,26 including persistent corneal epithelial defects, acute chemical burns, and cicatrizing conditions such as Stevens–Johnson syndrome and ocular cicatricial pemphigoid.26 AMT has been used in the reconstruction of fornices, as a covering following excision of conjunctival lesions, and in limbal stem cell deficiency with concomitant limbal stem cell grafting.26
Several characteristics of AM make it useful for the treatment of ocular surface conditions. It has been shown to promote epithelialization, it contains important growth factors including epithelial growth factor and keratocyte growth factor (both of which promote wound healing), it inhibits scarring by interfering with the TGF-β signaling cascade in corneal and conjunctival fibroblasts, and it inhibits inflammation by releasing anti-inflammatory cytokines from its epithelium and stroma, such as interleukin-10 and interleukin-1 receptor antagonists.26 Furthermore, because AM does not express HLA-A, B, or DR antigens, tissue rejection seldom occurs.25
AM can be prepared fresh or preserved using either freeze-drying of the membrane (dry AM) or cryopreservation. Fresh AM is more commonly used in the developing world, where preservation techniques are not easily performed.27 Unfortunately, the use of fresh AM is less advantageous, not only because it must be used in a limited time and does not exploit the size of the membrane for multiple tissue transplantations, but also poses a greater risk of transmitting infection.27 This is because the donor, who may or may not be screened when the membrane is retrieved, is not rescreened following a period of 6 months for communicable infectious diseases, which may only manifest after this period of time has elapsed.27
Cryopreservation of AM is achieved by freezing fresh AM in either phosphate-buffered saline in dimethylsulfoxide or in Eagle’s Minimum Essential Medium (MEM) with glycerol, both at −80°C.27 This precludes its use outside of large medical centers capable of maintaining this temperature and results in greater cost expenditure.28 Once frozen, many of the beneficial soluble factors are depleted from the tissue, and this may cause a decrease in its efficacy.28 Despite this drawback, this type of preservation is most commonly in use.28
Dry AM is made by freeze-drying fresh AM and rehydrating it before use.27 Conventionally, this would require the membrane to be frozen before being dried, which is thought to cause damage to beneficial factors, such as epidermal growth factor and TGF-β1, in the membrane.28 Because AM is usually <100 μm thick, the membrane can be dried in a freeze-dryer vacuum without prefreezing, thus resulting in improved factor retention.28 The membrane can then be maintained at room temperature.28
Allen et al28 compared dried and cryopreserved AM as an ocular surface dressing and found that dried AM was superior to cryopreserved AM because of the effect of the preservation process on the tissue. They found that preservation affected the biochemical and structural composition of the AM. Using electron microscopy, they showed that dried AM was more similar to fresh AM than to cryopreserved AM. This was even more evident when the dried AM was pretreated with trehalose, a lyoprotectant, which inhibits destruction of intracellular organelles.28 Their study also showed that the biochemical composition, including the amount of factors such as epidermal growth factor and TGF-β1, was much more similar to fresh AM than to cryopreserved AM. Dried AM, as a preservation method, was found to decrease devitalization of epithelial cells and decrease cellular damage. In addition, the optimization of dried AM with the lyoprotectants trehalose and raffinose was found to maintain the integrity of the tissue even more than drying alone.28
Animal models using rabbits have also shown that dried AM is at least as efficacious as cryopreserved AM when used as a substrate for ocular surface reconstruction.29
Role of AM in management of postoperative pain
In addition to the aforementioned effects on the host tissue, AMT has also been observed to decrease postoperative pain. Pires et al30 studied patients with symptomatic bullous keratopathy with poor visual potential and observed that 90% of patients with intractable pain secondary to their disease preoperatively were pain free following AMT. Pirouzian et al31 treated three pediatric patients with AMG who had previously undergone deep lamellar excision of grade 1 limbal dermoid lesions. They found that postoperative pain was eliminated with the use of AM and attributed this finding to the covering of the corneal epithelial defect (and by extension, the exposed corneal nerves), as well as to the inhibition of inflammation provided by the membrane.31 In another study by Hamza et al,32 83.3% of patients treated for various ocular surface conditions reported no pain 1 month following AMT, whereas 90% of patients in the same study had reported pain on the same questionnaire before transplantation.32 In a study by Uhlig et al,33 72.1% and 78.3% of patients suffering from corneal ulcers treated with AMT (with overlay or sandwich techniques, respectively) reported either no pain or an improvement in their comfort.
Comparative efficacy of using AM in pterygium surgery
The efficacy of dry AMG in pterygium surgery has been compared to the other standard techniques in many previous studies. Significant parameters for comparison include recurrence rate, time to recurrence, complications, and cosmesis.
A meta-analysis by Li et al34 showed that the recurrence rate of pterygium after primary excision was significantly lower with conjunctival autografting than with AMG. However, the recurrence rates were equal when these techniques were used for treatment of recurrent pterygia.34 In another study, it was concluded that recurrence rates of pterygia were significantly lower after limbal conjunctival autograft transplantation when compared to AMT.35 Yet another comparative study showed that while limbal conjunctival autograft and conjunctival autograft techniques were not significantly different from one another in terms of recurrence, both were significantly better in reducing recurrence rates than AMG.20 The technique of using a limbal conjunctival flap was also shown to be superior to AMT in terms of recurrence rate in one study by Kurna et al.36 Prabhasawat et al37 showed significantly higher recurrence rates for primary, recurrent, and all pterygia treated with AMG compared to conjunctival autografting, and the time to recurrence was delayed in conjunctival autograft transplantation when compared to AMT.
Despite a body of evidence suggesting that the recurrence rate after the use of AMG is higher than the aforementioned techniques, there is also evidence that suggests that the use of AM is at least equivalent. For example, in a study by Ma et al,38 the use of AM was compared retrospectively to the use of both conjunctival autograft and topical mitomycin C. The study showed the recurrence rate of AMT to be 3.8%, compared to 5.4% and 3.7% in the conjunctival autograft and topical mitomycin C groups, respectively. The time to recurrence was found to be 12.3 months in the AM group, compared to 3 and 5.5 months in the conjunctival autograft and topical mitomycin C groups, respectively. The study showed no significant difference in either the recurrence rate or the time to recurrence in any of the three groups.38 In another study, AMT was retrospectively compared to conjunctival autograft transplantation. This study found a recurrence rate of 25% in the conjunctival autograft group and a 35% recurrence rate in the AMT group, though statistically, this was not a significant difference.39 In the same study, the mean time to recurrence was significantly shorter for the conjunctival autograft group than for the AM group, at 2.3±0.9 and 3.2±1.0 months, respectively.39
Adjuvant therapies combined with the use of AMG have also been studied. Soloman et al21 found that the rates of recurrence following pterygium surgery were improved when an intraoperative depot corticosteroid injection was used to control postoperative inflammation following AMT, with recurrence rates of 3.0%–9.5%. The use of fibrin glue instead of sutures to attach the graft has also been shown to reduce the recurrence rate in AMT, with recurrence rates of 9.4% in the fibrin glue group and 10.5% in the vicryl suture group.40 The use of topical mitomycin C in combination with AMT has also been shown to lower the rate of pterygium recurrence.16 However, in another study by Ma et al,41 the same conclusion was not confirmed to be true.
A significant weakness in the data presented here is that, in many of the references cited, the inclusion criteria included patients who were followed for <1 year before conclusions were made about recurrence rates.16,20,21,37,39–42 In several studies, the differing arms of the study had significantly different follow-up times, likely underestimating recurrence rates in those groups followed for shorter periods. In addition, the definition of pterygium recurrence varies from the standard definition of any new fibrovascular growth across the limbus in some studies. These consider pterygium recurrence to occur only once the fibrovascular growth has extended >2 mm across the limbus, again underestimating the rate of recurrence. This invalidates the results and makes comparisons between studies and techniques unreliable.
Safety and tolerability
AMT following pterygium excision has been found to be a very safe and well-tolerated procedure, without any major complications reported in the literature.
Because AM lacks the expression of HLA-A, -B, and -DR antigens, the risk of immunologic graft rejection is minimal.25 The AM is obtained from potential donors undergoing elective cesarean section.42 These donors are screened for communicable infectious diseases including HIV, hepatitis B and C, and syphilis.27,42 Serological screening of donors is again recommended at 6 months following procurement of the membrane, as some infectious diseases may not be detectable within this transmission window period.42 When both of these serological screens are negative, the AM is released for use. The membrane is prepared under sterile conditions and washed with antibiotics including penicillin, streptomycin, neomycin, and amphotericin B.42 The amnion and chorion are separated by blunt dissection.42 Microbial infection rates after AMT have been reported as low as 1.6% and occur primarily with Gram-positive organisms.43
Clinical complications of AMT are relatively minor. In one study by Ma,38 no major complications were noted in 80 eyes of 71 patients who were treated with AMG. Of these, one case (1.25%) developed a pyogenic granuloma, and one case (1.25%) developed an iatrogenic microhyphema, which was caused by an inadvertently deep limbal suture. In one of the comparative arms of the same study, where patients were treated with conjunctival autografting, two cases developed a pyogenic granuloma (3.6%) and four cases (7.3%) developed a conjunctival inclusion cyst. In the third arm of the study, where patients were treated with topical mitomycin C, one case developed a pyogenic granuloma (1.8%) and one case (1.8%) developed scleral ischemia.38 In another study, AMT resulted in pyogenic granuloma (4.3%), epithelial defects (4.3%), and dellen (4.3%) and showed no significant difference when compared to complications from conjunctival autograft.39 All complications resolved spontaneously.39 Other complications that have been reported include increased foreign body sensation, eyelid edema, conjunctival hyperemia, and symblepharon.16
Patient-focused perspectives such as quality of life, patient satisfaction/acceptability
Cosmetic outcomes and patient satisfaction are also important considerations when assessing the effectiveness of AMT in the treatment of pterygia, in addition to minimizing recurrence rates and surgical complications. Conjunctival autografting following pterygium excision has been shown to be significantly superior to AMG in final cosmetic appearance.37,44 In one study, cosmetic appearance was graded from 1 to 4, where grade 3 was an unacceptable cosmetic outcome and grade 4 was true recurrence. Of the patients who underwent conjunctival autografting, 10% had an unacceptable cosmetic outcome, compared to 21.1% of patients who had undergone AMT; this result was statistically significant.44
In a study by Hirst,45 cosmetic results of the P.E.R.F.E.C.T. for pterygium technique were compared to the same results from the study by Prabhasawat et al,37 which examined conjunctival autografting, AMG, and primary closure. A cosmetic result graded 3 or 4 (which represent poor cosmetic result and frank recurrence, respectively) occurred in 5.7% of cases undergoing the P.E.R.F.E.C.T. technique, 9% of cases with conjunctival autografting, 32.6% of cases with AMG, and 85% of cases with primary closure.45
In another study, Kucukerdonmez et al40 compared the cosmetic outcome of using AMT following pterygium excision with either fibrin glue or vicryl sutures and found that while there was a decrease in surgical time and postoperative symptoms, there was no difference in the cosmetic outcome between the two groups. Of note, patient tolerability was much improved in the fibrin glue group compared with the vicryl suture group in all subjective symptoms studied, including epiphora, foreign body sensation, and irritation.40
AM has also been used to cover conjunctival defects following the removal of ocular surface neoplasms. In one study, which followed eight patients having undergone tumor excision of ocular surface neoplasms, all eight patients were reported to have satisfactory cosmetic results following AMT.46
The role of AMT in the management of postoperative pain has been detailed earlier.
Conclusion, place in therapy
AMG in the treatment of pterygium has been shown to be less effective than, or at best, equivalent to other grafting procedures including conjunctival and limbal autografting.20,34–39 In particular, AMT has been shown in many studies to have a higher rate of recurrence compared to both conjunctival and limbal autografting.20,34,35,37 Despite these findings, there is still a role for this technique in the treatment of pterygia.
AMG can be useful in the covering of wide ocular surface defects, such as in the case of large or double-headed pterygia.21 In this case, the amount of autologous conjunctival tissue that can be harvested is limited, whereas AM can cover any sized ocular surface defect.42 AM also provides an advantage in cases where the conjunctiva is scarred and cannot be harvested16 or when the bulbar conjunctiva must be spared to allow for future glaucoma filtering surgery.
Dry AM, in particular, confers advantages over both fresh and cryopreserved AM. The preservation process allows for the tissue to be quarantined during a window period of 6 months, allowing for screening of infectious diseases, including HIV, hepatitis B and C, and syphilis, from donors who may not screen positive at the time that the membrane is harvested.27
Dry AM has also been shown to be a more gentle preservation method than cryopreservation, as it allows for greater maintenance of structural and biochemical integrity, and improves the retention of beneficial factors, which give the membrane its useful properties.28 Dry AM is also less expensive to maintain than cryopreserved membrane because it can be maintained at room temperature, as opposed to cryopreservation which requires temperatures of −80°C.28 This also makes its use more versatile outside of large hospital settings, as it can be used in the developing world and in military environments.28
Therefore, while the use of AM in the treatment of pterygium may not be the ideal choice in all circumstances, there are certainly situations where its use may be most beneficial to the patient, and surgeons should keep this technique in their armamentarium of treatment options.
Disclosure
The authors report no conflicts of interest in this work.
References
Duane TD, Jaeger EA, Tasman W. Duane’s Ophthalmology. In: Ophthalmology. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. | ||
Rong SS, Peng Y, Liang YB, Cao D, Jhanji V. Does cigarette smoking alter the risk of pterygium? A systematic review and meta-analysis. Invest Ophthalmol Vis Sci. 2014;55(10):6235–6243. | ||
Liu L, Wu J, Geng J, Yuan Z, Huang D. Geographical prevalence and risk factors for pterygium: a systematic review and meta-analysis. BMJ Open. 2013;3(11):e003787. | ||
Wu K, He M, Xu J, Li S. Pterygium in aged population in Doumen County, China. Yan Ke Xue Bao. 2002;18(3):181–184. Chinese. | ||
McCarty CA, Fu CL, Taylor HR. Epidemiology of pterygium in Victoria, Australia. Br J Ophthalmol. 2000;84(3):289–292. | ||
Chui J, Coroneo MT, Tat LT, Crouch R, Wakefield D, Girolamo N. Ophthalmic pterygium: a stem cell disorder with premalignant features. Am J Pathol. 2011;178(2):817–827. | ||
Alqahtani JM. The prevalence of pterygium in Alkhobar: a hospital-based study. J Family Community Med. 2013;20(3):159–161. | ||
Di Girolamo N, Chui J, Coroneo MT, Wakefield D. Pathogenesis of pterygia: role of cytokines, growth factors, and matrix metalloproteinases. Prog Retin Eye Res. 2004;23(2):195–228. | ||
Chui J, Di Girolamo N, Wakefield D, Coroneo MT. The pathogenesis of pterygium: current concepts and their therapeutic implications. Ocul Surf. 2008;6(1):24–43. | ||
McKnight CM, Sherwin JC, Yazar S, et al. Pterygium and conjunctival ultraviolet autofluorescence in young Australian adults: the Raine study. Clin Experiment Ophthalmol. 2015;43(4):300–307. | ||
Cajucom-Uy H, Tong L, Wong TY, Tay WT, Saw SM. The prevalence of and risk factors for pterygium in an urban Malay population: the Singapore Malay Eye Study (SiMES). Br J Ophthalmol. 2010;94(8):977–981. | ||
Nangia V, Jonas JB, Nair D, Saini N, Nangia P, Panda-Jonas S. Prevalence and associated factors for pterygium in rural agrarian central India. The central India eye medical study. PLoS One. 2013;8(12):e82439. | ||
Alpay A, Ugurbas S, Erdogan B. Comparing techniques for pterygium surgery. Clin Ophthalmol. 2009;3:69–74. | ||
Janson BJ, Sikder S. Surgical management of pterygium. Ocul surf. 2014;12(2):112–119. | ||
Yeung SN, Lichtinger A, Kim P, et al. Superior versus inferior conjunctival autografts combined with fibrin glue in the management of primary pterygia. Cornea. 2013;32(12):1582–1586. | ||
Syam PP, Eleftheriadis H, Liu CSC. Inferior conjunctival autograft for primary pterygia. Ophthalmology. 2003;110:806–810. | ||
Hirst LW. Prospective study of primary pterygium surgery using pterygium extended removal followed by extended conjunctival transplantation. Ophthalmology. 2008;115(10):1663–1672. | ||
Hirst LW. Recurrent pterygium surgery using pterygium extended removal followed by extended conjunctival transplant: recurrence rate and cosmesis. Ophthalmology. 2009;116(7):1278–1286. | ||
Yüksel B, Unsal SK, Onat S. Comparison of fibrin glue and suture technique in pterygium surgery performed with limbal autograft. Int J Ophthalmol. 2010;3(4):316–320. | ||
Kaufman SC, Jacobs DS, Lee WB, Deng SX, Rosenblatt MI, Shtein RM. Options and adjuvants in surgery for pterygium: a report by the American Academy of Ophthalmology. Ophthalmology. 2013;120(1):201–208. | ||
Solomon A, Pires RT, Tseng SC. Amniotic membrane transplantation after extensive removal of primary and recurrent pterygia. Ophthalmology. 2001;108(3):449–460. | ||
Ali AM, Thariat J, Bensadoun RJ, et al. The role of radiotherapy in the treatment of pterygium: a review of the literature including more than 6,000 treated lesions. Cancer Radiother. 2011;15(2):140–147. | ||
Detorakis ET, Spandidos DA. Pathogenetic mechanisms and treatment options for ophthalmic pterygium: trends and perspectives. Int J Mol Med. 2009;23(4):439–447. | ||
Davis JW. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Med J. 1910;15:307. | ||
Dua HS, Azuara-Blanco A. Amniotic membrane transplantation. Br J Ophthalmol. 1999;83:748–752. | ||
Meller D, Pauklin M, Thomasen H, Westekemper H, Steuhl K-P. Amniotic membrane transplantation in the human eye. Dtsch Arztebl Int. 2011;108(14):243–248. | ||
Rahman I, Said DG, Maharajan VS, Dua HS. Amniotic membrane in ophthalmology: indications and limitations. Eye (Lond). 2009;23(10):1954–1961. | ||
Allen CL, Clare G, Stewart EA, et al. Augmented dried versus cryopreserved amniotic membrane as an ocular surface dressing. PLoS One. 2013;8(10):e78441. | ||
Libera RD, Melo GB, Lima Ade S, Haapalainen EF, Cristovam P, Gomes JA. Assessment of the use of cryopreserved x freeze-dried amniotic membrane (AM) for reconstruction of ocular surface in rabbit model. Arg Bras Oftalmol. 2008;71(5):669–673. | ||
Pires RT, Tseng SC, Prabhasawat P, et al. Amniotic membrane transplantation for symptomatic bullous keratopathy. Arch Ophthalmol. 1999;117(10):1291–1297. | ||
Pirouzian A, Holz H, Merrill K, Sudesh R, Karlen K. Surgical management of pediatric limbal dermoids with sutureless amniotic membrane transplantation and augmentation. J Pediatr Ophthalmol Strabismus. 2012;49(2):114–119. | ||
Hamza MS, Ullah MR, Hashmi AH, Sahaf IA. Amniotic membrane transplantation in ocular surface disorders. Pak J Ophthalmol. 2011;27(3):138–141. | ||
Uhlig CE, Frings C, Rohloff N, et al. Long-term efficacy of glycerine-processed amniotic membrane transplantation in patients with corneal ulcer. Acta Ophthalmol. 2015;93(6):e481–e487. | ||
Li M, Zhu M, Yu Y, Gong L, Zhao N, Robitaille MJ. Comparison of conjunctival autograft transplantation and amniotic membrane transplantation for pterygium: a meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2012;250(3):375–381. | ||
Ozer A, Yildirim N, Erol N, Yurdakul S. Long-term results of bare sclera, limbal-conjunctival autograft and amniotic membrane graft techniques in primary pterygium excisions. Ophthalmologica. 2009;223(4):269–273. | ||
Kurna SA, Altun A, Aksu B, Kurna R, Sengor T. Comparing treatment options of pterygium: limbal sliding flap transplantation, primary closing, and amniotic membrane grafting. Eur J Ophthalmol. 2013;23(4):480–487. | ||
Prabhasawat P, Barton K, Burkett G, Tseng SC. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. 1997;104(6):974–985. | ||
Ma DH, See LC, Liau SB, Tsai RJ. Amniotic membrane graft for primary pterygium: comparison with conjunctival autograft and topical mitomycin C treatment. Br J Ophthalmol. 2000;84(9):973–978. | ||
Memarzadeh F, Fahd AK, Shamie N, Chuck RS. Comparison of de-epithelialized amniotic membrane transplantation and conjunctival autograft after primary pterygium excision. Eye (Lond). 2008;22(1):107–112. | ||
Kucukerdonmez C, Karalezli A, Akova YA, Borazan M. Amniotic membrane transplantation using fibrin glue in pterygium surgery: a comparative randomized clinical trial. Eye (Lond). 2010;24(4):558–566. | ||
Ma DH, See LC, Hwang YS, Wang SF. Comparison of amniotic membrane graft alone or combined with intraoperative mitomycin C to prevent recurrence after excision of recurrent pterygia. Cornea. 2005;24(2):141–150. | ||
Malhotra C, Jain AK. Human amniotic membrane transplantation: different modalities of its use in ophthalmology. World J Transplant. 2014;4(2):111–121. | ||
Marangon FB, Alfonso EC, Miller D, Remonda NM, Muallem MS, Tseng SC. Incidence of microbial infection after amniotic membrane transplantation. Cornea. 2004;23(3):264–269. | ||
Küçükerdönmez C, Akova YA, Altinörs DD. Comparison of conjunctival autograft with amniotic membrane transplantation for pterygium surgery: surgical and cosmetic outcome. Cornea. 2007;26(4):407–413. | ||
Hirst LW. Cosmesis after pterygium extended removal followed by extended conjunctival transplant as assessed by a new, web-based grading system. Ophthalmology. 2011;118(9):1739–1746. | ||
Ozcan A, Esen E, Ciloglu E. Sutureless amniotic membrane transplantation following excision of ocular surface neoplasia. Int J Ophthalmol. 2015;8(3):637–640. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
