Back to Journals » Infection and Drug Resistance » Volume 15
The Threat of Carbapenem-Resistant Gram-Negative Bacteria in Patients with Hematological Malignancies: Unignorable Respiratory Non-Fermentative Bacteria-Derived Bloodstream Infections
Authors Lu L, Xu C, Tang Y, Wang L, Cheng Q, Chen X, Zhang J, Li Y, Xiao H, Li X
Received 26 January 2022
Accepted for publication 12 May 2022
Published 4 June 2022 Volume 2022:15 Pages 2901—2914
DOI https://doi.org/10.2147/IDR.S359833
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Suresh Antony
Linli Lu,1 Cong Xu,1 Yishu Tang,2 Liwen Wang,1 Qian Cheng,1 Xin Chen,1 Jian Zhang,1 Ying Li,1 Han Xiao,1 Xin Li1
1Department of Hematology, The Third Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China; 2Department of Emergency, The Third Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China
Correspondence: Xin Li, Department of Hematology, The Third Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China, Tel/Fax +86-731-88618241, Email [email protected]
Background: Carbapenem-resistant Gram-negative bacteria (CRGNB) bloodstream infection (BSI) pose a significant threat to the prognosis of hematologic malignancies (HM) patients. Understanding the distribution of pathogenic bacteria, changes in carbapenem-resistant trends, risk factors for CRGNB infections, and exploring the early detection measures can help reduce mortality.
Methods: We conducted a multicenter retrospective study of Gram-negative bacteria (GNB) BSI in patients with HM in three university-affiliated hospitals in Hunan Province, China, from January 2010 to December 2020. Demographic and clinical data were collected from the hospital electronic medical records system.
Results: CRGNB caused 138 (15.3%) of 902 GNB BSI. The detection rate of CRGNB increased from 6.4% in 2010– 2012 to 35.4% in 2019– 2020. The 7-day mortality rate was significantly higher in patients with CRGNB BSI than in patients with carbapenem-susceptible Gram-negative bacteria (CSGNB) BSI [31.9% (44/138) vs 9.7% (74/764), P < 0.001], and the mortality rate in patients with carbapenem-resistant non-fermenting bacteria (CRNFB) bloodstream infections was generally higher than that of carbapenem-resistant Enterobacteriaceae (CRE). Urinary catheter (OR, 2.814; CI=1.395– 5.680; P=0.004) and prior exposure to carbapenem (OR, 4.372; CI=2.881– 6.635; P< 0.001) were independent risk factors for CRGNB BSI. Analysis of co-infections showed that 50%– 85% of patients with CRGNB BSI had pulmonary infections, sputum culture results suggested that sputum culture positivity rate was as high as 57.1%– 66.7% in patients with carbapenem-resistant Acinetobacter baumannii (CRAB) and Stenotrophomonas maltophilia BSI, and the results of antimicrobial susceptibility testing of sputum cultures were consistent with the blood cultures.
Conclusion: Carbapenem resistance has dramatically increased in HM patients with GNB BSI in recent years and is associated with a worse outcome, especially for non-fermenting bacteria. In high-risk patients, early screening of the respiratory tract specimens may help to detect CRNFB colonization and protect patients from breakthrough BSI.
Keywords: hematological malignancies, carbapenem-resistant, gram-negative bacteria, bloodstream infections, non-fermentative bacteria
Introduction
The prevalence of CRGNB infections has been increasing worldwide in recent years. Patients with HMs are at high risk for development of CRGNB infection due to the chemotherapy-induced gastrointestinal mucositis, primary immunodeficiency, and frequent exposure to broad-spectrum antimicrobial therapy.1–3 With an elevated prevalence reported, CRGNB BSI is a serious threat to patients, and particularly in developing countries.4–6 For example, the prevalence of CRE BSI in China increased from 12.5%5 in 2014 to 26.8% in 2019 (According to the China Antimicrobial Surveillance Network). Meanwhile, a previous study had reported the rate of carbapenem resistance in Acinetobacter baumannii was 18% in 2012, but sharply raised to 60% in 2019.7 Moreover, it has been demonstrated that the mortality rate of BSI caused by carbapenem-resistant bacteria in HM patients is significantly higher compared with non-resistant bacteria, up to 45%-80%.3,8,9 To couple with the rapidly increasing antimicrobial resistance, the World Health Organization (WHO) ranked CRGNB, which mainly includes CRE, CRAB, and carbapenem-resistant Pseudomonas aeruginosa (CRPA), as the most threatening bacteria in 2017.10 Generally, Enterobacteriaceae (EB) and non-fermentative bacteria (NFB) are the major pathogens that result in the GNB BSI in patients with HM.11,12 However, these patients with GNB BSI had different clinical characteristics due to the considerable heterogeneity among the two types of bacteria. Local knowledge of the epidemiology and resistance patterns of isolates could help improve the prognosis of patients.
Currently, there are a series of studies focusing on the clinical characteristics and prognosis of CRE BSI in HM patients, and most results show that the incidence of CRE BSI exhibits an increased tendency.4,6,11 Actively surveillance and infection control measures dramatically decreased CRE colonization thereby reducing mortality.13–15 Importantly, BSI caused by CRNFB should be a matter of great concern, since limited data suggested that NFB displayed a greater carbapenem resistance rate as well as a higher mortality rate than CRE,16,17 30-day mortality rate up to 45%–100%.18–20 Our previous data also demonstrated a higher mortality rate of CRNFB BSI in HM patients compared to CRE (44.7% vs 36.0%, respectively).11 However, the studies concerning the early detection and constrain the spread of CRNFB among haematological patients are scarce. Limited researches have shown that respiratory tract infections were significantly associated with CRNFB bacteremia,19,21,22 while other studies supported that catheter-related infection was the most common primary source of bacteremia.23
Given the substantial mortality related to CRGNB BSI, elucidating the clinical characteristics of patients will enhance our understanding of how the carbapenem-resistant (CR) strains impact the outcome of HMs patients. Therefore, to address this knowledge gap, our present study aims to summarize the epidemiological characteristics of HMs patients with CRE and CRNFB BSI, and further identify the association between subtypes of CR bacteria and mortality of patients. Moreover, exploring the early detection measures will help optimize antimicrobial therapy, rapid diagnostic tests may lower mortality, hospitalization, and costs.
Methods
Patients and Study Design
A retrospective study of GNB BSI in patients with HMs who were hospitalized between January 2010 to December 2020 was performed in three university-affiliated hospitals in Hunan Province, China. Only patients ≥ 16 years of age were included in this study. For patients who had more than one positive culture with the same speciation and sensitivity, only the first one was counted. Patients with non-malignant diseases, bacteremia without GNB or polymicrobial bacteria were excluded. We collected the following clinical data on each individual case: demographics, malignancy diagnosis and status, comorbidities, prior healthcare exposures within 30 days of BSI onset, co-infections, microbiological, and imaging findings. Considering that differences in treatment protocols can also have an impact on treatment outcomes, all patients received empiric anti-infective therapy immediately after blood culture samples were collected and the use of antimicrobials was according to the related clinical guidelines.24,25 After obtaining blood culture results, antibiotic treatment regimen can be selected according to the identified bacteria and drug sensitivity results. If the detected bacteria belong to carbapenem resistant bacteria, targeted antibacterial drugs will be administrated according to the pathogen and its MIC. The primary outcome was all-cause mortality within 7 days after the onset of BSI. Since HM patients with BSI have more serious symptoms, usually concomitant with infections of other systems or organs, we also summarized the multi-organ co-infections in patients before and during each episode of bloodstream infection. Carbapenem resistance was defined as resistant to any carbapenem antimicrobial (ie, minimum inhibitory concentrations of ≥4 mcg/mL for meropenem, imipenem, or doripenem, or ≥2 mcg/mL for ertapenem) or produce carbapenemase. For bacteria that have intrinsic imipenem nonsusceptibility, resistance to carbapenems other than imipenem is required.26
Ethics
This retrospective study was approved by the Ethics Committee of the Central South University with exemption from a formal review as no personally identifiable information would be collected. The requirement for informed consent from patients was also waived. This study complies with the Declaration of Helsinki.
Definitions
The date of BSI onset was defined as the first time of positive blood culture collection. BSI was defined by the isolation of bacteria from any blood culture in the context of fever or other clinical signs consistent with infection.27 Disease status was assessed by the most recently available bone marrow biopsy and categorized as remission, relapsed, or uncontrolled malignancy, as previously defined.28 Neutropenia was defined as an absolute neutrophil count (ANC) of <500 cells/mm3 or an ANC that is expected to decrease to <500 cells/mm3 during the next 48 hours.29 The use of any antibiotic for >48 hours within the last 30 days before the onset of BSI was regarded as prior antimicrobial exposure.30 Nosocomial acquisition of BSI was defined as the onset of BSI > 48h after admission to hospital.25 Pulmonary infection was diagnosed when patients had an acute respiratory disorder and the new onset of lung infiltration findings on chest computed tomography.31 Perianal infection was diagnosed when patients had local pain and local infections characterized by the presence of an anal abscess.31 Skin and soft-tissue infections (SSTIs) encompass a variety of pathological conditions that involve the skin and underlying subcutaneous tissue, fascia, or muscle, ranging from simple superficial infections to severe necrotizing infections.32
Microbiologic Methods
All isolates identification and antibiotic susceptibility tests were performed on VITEK 2 Compact (bioMe´rieux SA, Marcy l’Etoile, France). Carbapenem resistance was verified by the determination of minimum inhibitory concentrations (MICs) using E-test (AB Biodisk, Solna, Sweden) strips. Carbapenem resistance was defined as an ertapenem MIC ≥2 µg/mL and meropenem and/or imipenem MIC ≥4 µg/mL.33
Statistical Analysis
SPSS (26.0) and R (4.0.2) were used for statistical analysis. Continuous variables were expressed as medians and ranges and categorical variables using numbers and percent, Categorical variables were compared using chi-square tests. Variables with P≤0.05 (two-tailed) in the bivariate analysis were taken as candidates for multivariate analysis. Pearson’s coefficient was used for checking correlations between test parameters. Logistic regression was used for multivariate analysis to identify independent risk factors for CRGNB BSI. The log-rank method was used for survival analysis and hazard ratio (HR) estimated. P values < 0.05 (two-tailed) were considered statistically significant.
Results
Epidemiology—The Detection Rate of CRGNB Showed an Increasing Trend Over the Years, Especially for CRNFB
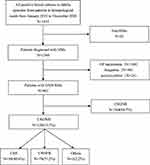
A total of 1452 strains were cultured and identified in the blood samples, including 902 GNB strains, of which 138 (15.3%) were CRGNBs (Figure 1). CRNFBs were the most common strains among all CRGNBs, accounting for 57.2% (79/138), followed by CRE with 40.6% (56/138) (Table 1). Among all the CRGNB isolates, Klebsiella pneumonia (K. pneumonia) was the most common species (n=33; 23.9%), followed by Stenotrophomonas maltophilia (S. maltophilia) (n=23; 16.7%), Pseudomonas aeruginosa (P. aeruginosa) (n=22; 15.9%), Acinetobacter baumannii (A. baumannii) (n=20; 14.5%) and Escherichia coli (E. coli) (n=13; 9.4%). As expected, the detection rate of CRNFB was higher compared with CRE.
 |
Table 1 The Distributions of the Microorganisms Causing BSI and CR Rates |
We next set out to investigate the antibiotic resistance of strains. As shown in Figure 2, our findings demonstrated that the overall rates of CR increased from 6.4% to 35.4% in the past decade, with the noted that this upward tendency existed in all identified strains. Additionally, the detected proportion of CRE and CRNFB was 0.9% and 23.3% during 2010–2012, but raised to 22.2% and 83.3% during 2019–2020, respectively. In terms of each strain, the rate of CR in A. baumannii was quite fluctuated and high during the course of the monitor (50%–80%). However, it is alarming that P. aeruginosa had a more sharply increased CR rate, from 9.1% soared to 88.9% during the same monitored period.
Outcome—Carbapenem Resistance Significantly Increases Mortality
To interrogate the roles of CR strains in the outcome of patients with HMs, we further explored the effect of CR on the prognosis of patients with different types of bacteremia. Not unexpectedly, our findings showed that CR led to increased early mortality in almost all strains of bacteria. As shown in Table 2, the 7-day mortality rate was significantly higher for patients with CRGNB BSI compared with CSGNB BSI [31.9% (44/138) vs 9.7% (74/764), P<0.001, OR=4.365 (2.837–6.715)]. Similarly, in the Enterobacteriaceae, CRE BSI also drives increased early mortality of patients with HMs compared to carbapenem-susceptible Enterobacteria (CSE) [28.6% (16/56) vs 8.5% (54/633), P<0.001, OR=4.289 (2.254–8.161)]. Notably, carbapenem-resistant Klebsiella pneumonia (CRKP) showed the most significant increase in mortality compared to carbapenem-susceptible Klebsiella pneumonia (CSKP) [39.4% (13/33) vs 7.3% (14/191), P<0.001, OR=8.218 (3.391–19.917)]. Besides, among NFBs, there were also evidently greater 7-day mortality of patients with BSI caused by CRNFB than carbapenem-susceptible non-fermentative bacteria (CSNFB) [35.4% (28/79) vs 11.8% (13/110), P<0.001, OR=4.097 (1.954–8.586)]. Even though the general high mortality caused by CRNFB BSI compared to CSNFB was identified, under subgroup analysis between different subtypes of NFB, there were no statistical differences. For example, the mortality of patients with CRPA BSI was higher than carbapenem-susceptible Pseudomonas aeruginosa (CSPA) [27.3% (6/22) vs 11.7% (11/94)], but the difference did not meet the cut-off for statistical significance (P=0.063), similar result was obtained in A. baumannii [65.0% (13/20) vs 33.3% (2/6), P=0.169].
 |
Table 2 The 7-Day Mortality Rates of BSI with Different Pathogenic Bacteria of CR or CS |
Considering the high early mortality of patients with CRGNB BSI, we next analyzed the survival time of patients (Figure 3). As anticipated, the results showed that the most majority of death cases occurred in the first week, and the median time from blood culture specimen collection to death in CRGNB BSI patients was only three days, generally shorter than in CSGNB BSI patients (6 days). Clearly, CR was a significantly unfavorable factor for the outcomes of patients with BSI (HR = 1.755, 95% CI = 1.168–2.637, p = 0.006).
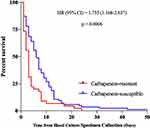 |
Figure 3 Survival in HMs patients with BSI caused by carbapenem-resistant bacteria and carbapenem-susceptible bacteria. Abbreviations: HR, hazard ratio; CI, confidence interval. |
Independent Risk Factors for CRGNB BSI—Urinary Catheter and Prior Exposure to Carbapenem
In further analysis, we attempted to identify related risk factors in CRGNB BSI. According to our results (as shown in Figure 4), the following factors were associated with CRGNB BSI by univariate analysis: central venous catheter(CVC), urinary catheter, prior antimicrobial exposures, prior exposure to carbapenem, pulmonary infection, perianal infection; In multivariable analysis, the urinary catheter (OR, 2.814; CI=1.395–5.680; P=0.004) and prior exposure to carbapenem (OR, 4.372; CI=2.881–6.635; P<0.001) were independently associated with CRGNB BSI.
Co-Infections—The Highest Incidence of Pulmonary Infection
Generally, the HM patients with BSI have more serious symptoms, usually concomitant with infections of other systems or organs. Here, we retrospectively analyzed the association between identified strains and multi-organ combined infections. Based on our findings, pulmonary involvement was the most predominant event in patients with BSI (49.9%,450/902). More importantly, there was an obviously higher incidence of patients with CRGNB BSI than CSGNB will concomitant with pulmonary infection, and the former types commonly included A. baumannii (85.0%), K. pneumoniae (78.8%), S. maltophilia (69.6%) and E. coil (69.2%). Combined intestinal infections occurred mainly in patients with CSGNB BSI (8.4% [64/764]), for CRGNB BSI was relatively low (6.5% [9/138]), whereas combined skin soft tissue or perianal infections most commonly happened in patients with BSI caused by the E. coli and K. pneumonia (N=30 and N=10, respectively) (Table 3).
 |
Table 3 | Co-Infections in Patients with BSI in HMs |
Considering the highest incidence of combined pulmonary infection in patients with BSI, we further analyzed the screening data of respiratory specimens in patients with CRGNB BSI. The results indicated that patients with CRAB BSI and S. maltophilia BSI had the most frequent detected rates of sputum culture, up to 66.7% and 57.1%, respectively. Besides, the results of antimicrobial susceptibility testing of sputum cultures were consistent with the blood cultures (Supplementary Table 1).
Antimicrobial Susceptibility Test of CRGNB Isolates
According to the results of antimicrobial susceptibility testing for CRGNB, most CRE isolates demonstrated less sensitivity to the typical antibiotics such as cephalosporins, β-lactam/β-lactamase inhibitors and carbapenems (<50%). In K. pneumoniae, E. coli, P. aeruginosa and A. baumannii isolates, more than 85% were sensitive to polymyxin B. In Enterobacteria, 91.7% of K. pneumoniae and 100% E. coli were sensitive to tigecycline. The sensitivity of P. aeruginosa for piperacillin-tazobactam, cefperazone-sulbactam, ciprofloxacin and levofloxacin were 76.2%, 81.8%, 95.2% and 100%, respectively. Additionally, more than 90% of S. maltophilia were sensitive to ceftazidime, levofloxacin. A. baumannii was only sensitive to polymyxin B (Table 4).
 |
Table 4 | Antimicrobial Susceptibility of CRGNB Isolates (N=111) |
Discussion
This study suggests that carbapenem-resistant non-fermentative bacteria deserve attention, and specific measures are proposed for the early diagnosis and treatment of bloodstream infections with such bacteria that seriously affect the prognosis of patients. The awareness of hematologists about CRE bloodstream infection in HM patients has been gradually improved, but much less about CRNFB bloodstream infection. Our study revealed the annually increasing incidence of CRE bloodstream infections in HM patients, as well as the great threat that CRE poses to the prognosis of patients, and provided a systematical analysis for CRNFB. The results showed that the incidence of CRNFB bloodstream infections and the resulting mortality are higher than those of CRE, so clinicians also need to be more alert to CRNFB infections. Our results also showed that patients with CRNFB bloodstream infections were prone to lung co-infections, and had a higher rate of positive sputum cultures of which the sputum culture drug sensitivity results were consistent with those of blood cultures, indicating that there may be a close association between CRNFB respiratory tract infections and bloodstream infections. These results preliminarily support that early respiratory specimen screening for CRNFB in high-risk patients may help to detect pathogenic bacteria.
In broad outline, CRGNB mainly includes CRE and CRNFB, and a previous study showed that in HM patients with CRGNB BSI, CRE and CRNFB accounted for 61.2% and 35.9%, respectively,3 in this study, among the different CRGNBs strains, the most common was CRKP (44.7%). However, our study data indicated that CRNFB accounted for the main proportion (57.2%), with an overall detection rate of 31.2% for CRAB and S. maltophilia, probably due to the regular rectal screening of hospitalized patients for CRE in our study center from 2018 and the implementation of contact isolation and infection control measures for CRE carriers, which resulted in a lower incidence of CRE bloodstream infections. Recently, several studies have consistently proved an increasing trend of CR rates among these two major groups of bacteria in HMs patients with BSI.4,6,11 Similarly, in line with the results of a European study,4 our findings revealed that CR was undetectable among Enterobacteriaceae in 2010–2012, but upgraded to 14.8% in E. coli and 32% in K. pneumoniae during 2019–2020. Additionally, CR is more commonly observed in non-fermenting bacteria compare to Enterobacteriaceae, especially in A. baumannii and P. aeruginosa. Most studies reported a varied detection rate of CRAB, ranging from 60% to 80%,4,5,7 which was consistent with our data. Notably, the detection rate of CRPA displayed a worrisome trend, increasing significantly from 9.1% in 2010–2012 to 88.9% in 2019–2020, which may result from antibiotic exposure and regional differences.1,20
CRGNB BSI poses a significant threat to the prognosis of HM patients, overall mortality rates of CRGNB BSI in HM patients ranging from 45% to 80% were reported across different studies.3,6,8,9 Nizar Andria et al reported that the 14-day mortality rate was higher in patients with CRGNB BSI compared to CSGNB [45.6% (47/103) versus 15% (48/320), respectively (P < 0.001)].3 In our study, a significantly higher 7-day mortality was also observed in patients with CRGNB BSI compared to patients with CSGNB BSI (31.9% vs 9.7%, P < 0.001), but it was still lower than other studies. The possible reason for this discrepancy was that adequate supportive treatment was administered based on the prognostic high-risk factors of HM patients with BSI, like blood transfusion, albumin supplementation, and reduction of inappropriate initial antimicrobial therapy (IIAT).28,30 Moreover, we identified that CR as an unfavorable factor of which drives increased early mortality in patients with BSI. A multicenter prospective survey reported that patients with CRKP BSI had a significantly higher mortality rate than those with BSI caused by CSKP (52.2% vs 14.5%; P < 0.001).34 In our study, compared to CSKP, there was also obviously higher early mortality observed in patients with CRKP-caused BSI (39.4% vs 7.3%, P < 0.001), which was in agreement with the previously published studies.11,34,35 It is well documented that the type of pathogenic bacteria can affect the prognosis of patients with HM BSIs, of which A. baumannii BSI causes the highest mortality11 and CRAB BSI leads to a worse prognosis.11,36 Our study also reached the same conclusion. Taken together, CR significantly impacts the prognosis of patients. To make matters worse, according to our results, the median survival time of patients with CRGNB BSI was only 3 days, which means that the results of blood culture were usually not obtained. Therefore, it is urgently needed to reduce the emergence of drug resistance and conduct early screening, thereby timely performing an intervention for high-risk patients.
Patients with HMs are more susceptible to developing CRGNB-related BSI than patients with other malignancies.6 In our study, prior exposure history to carbapenem and the presence of urinary catheters were independent risk factors for CRGNB-caused BSI in patients with HM. In addition, studies from others also suggest that patients with colonization or infection history of CRGNB were also at high risk of CRGNB BSI.2,9,34,37 In HM patients, we suggest to minimize possible invasive procedures. If such events cannot be avoided due to the illness itself, reducing antibiotic exposure and early screening for resistant bacteria colonization are required. In terms of antibiotic exposure, previous studies have shown that prophylactic use of antibiotics does not improve the prognosis of bloodstream infections in patients with HM, while avoiding the overuse of antibiotics, especially carbapenems, significantly reduces the incidence of CRGNB infections.31,38 Current studies have confirmed that chemotherapy-induced mucosal destruction is a risk factor favoring CRE colonization and BSIs in patients with hematologic malignancies.2,34 Asymptomatic colonized patients may serve as potential sources of infection, but cultures of clinical samples from patients can only identify a small proportion of CRE infections. Therefore, it is indispensable to conduct active surveillance for identifying the CRE carriers in high-risk patients, perform a timely intervention, reduce the incidence of breakthrough BSI, and further decrease the risk of infection-related mortality. An Italian retrospective study confirmed that the implementation of control measures, particularly regular rectal screening of hospitalized patients for CRKP and contact precautions for CRKP carriers and new admissions, significantly reduce the transmission of CRKP, the rate of CRKP colonization infection also decreased from 10.7% to 2.1% (P=0.001).39 In this regard, various national guidance documents recommend CRE anal swab screening for colonized bacteria in high-risk patients to control infection.40,41
In HM patients with GNB BSI, several studies have confirmed that NFB is an independent risk factor related to patient prognosis, while CRNFB has a greater impact on the prognosis and account for 45%-100% mortality,18–20 our study also showed that the incidence of CRNFB bloodstream infection and mortality are significantly higher than CRE in HM patients. Although intense studies have revealed strong evidence on the role of active surveillance for the prevention and treatment of CRE BSI, more understanding about early screening and infection control measures for CRNFB is still needed. In our study, the most common CRNFB isolates were S. maltophilia (29.1%), CRPA (27.8%) and CRAB (25.3%). Coupled with several previous studies, which suggested that respiratory tract infections are the principal source of CRNFB bacteremia,21,22 our data also supported the evidence that pulmonary involvement was the most common event in patients with CRNFB BSI. The results of a study by Marya D. Zilberberg et al also showed that CRNFB were isolated in more than 90% of patients with CRGNB respiratory infections, the most common of which were CRPA, CRAB and S. maltophilia.42 In different NFBs, Pseudomonas aeruginosa is one of the leading causes of the hospital-acquired pneumonia (HAP), which has the ability to disrupt the lung barrier and leading to the P. aeruginosa bacteremia.43 Also, it has been confirmed that the genome of bloodstream isolates was highly homologous to that of respiratory isolates, suggesting that A. baumannii BSI may originate from the respiratory tract.44 At present, specimens for non-fermentative bacterial screening mainly include sputum, pharyngeal swabs, alveolar lavage fluid. Under the premise of ensuring the positive rate of bacterial screening, non-invasive methods were priority for specimen collection. Therefore, sputum specimens may be more suitable for respiratory screening. In addition, by further analysis of sputum culture results from patients with CRNFB bloodstream infection in our study. We found 8 patients had positive sputum cultures, and 4 of them (50%) developed bloodstream infection within one week after sputum culture specimen collection, the results of antimicrobial susceptibility testing of sputum cultures were consistent with the blood cultures, suggesting the importance of early respiratory screening in high-risk patients.
CRGNB BSI has become a rapidly growing global threat with limited antibiotic options and significant mortality,3,6,9 therefore, early identification of CRGNB colonization and rapid initiation of targeted therapy once concomitant with febrile neutropenia or signs of infection is essential to control CRGNB infections. Generally, the treatment of CRE BSI is based on polymyxins, tigecycline, aminoglycosides, and their combinations.2,8,13 Our data also showed that polymyxins are the essential treatment for infections caused by CRKP and carbapenem-resistant Escherichia coli (CREC). In contrast, for CRNFB, the therapy schemes should be administered according to the subtypes of bacterial species. For example, levofloxacin, cefoperazone sulbactam, and ceftazidime are recommended for patients with S. maltophilia BSI, which was in concordance with that obtained from other studies.18,45 Given that current concerns regarding the efficacy of CRAB, regimens containing polymyxins are the only feasible initial treatment for patients suspected of having CRAB BSI.19,23 However, several studies have shown that the therapeutic dose of polymyxin, polymyxin monotherapy or combination regimens can also have an impact on the prognosis of patients with CRAB infection.46–48 Fortunately, there has a relatively more choice of available antimicrobial regimens for patients with CRPA associated BSI, such as enzyme inhibitors, quinolones, and some cephalosporins.
Strengths and Limitations
This is a large sample multicenter study. It provides preliminary evidence of a possible close association between CRNFB respiratory tract infections and bloodstream infections, and that screening of respiratory specimens may help to reduce the morbidity and mortality of CRNFB bloodstream infections in patients with HM. Our study has several limitations. First, as a retrospective study, we could not obtain bacterial samples to prove the homology of the infection pathogens in bloodstream infection and pulmonary infection. Second, our study relied on hospitalization records, and we could only analyze objective and easily measured outcomes, such as patients’ 7-day all-cause mortality. Therefore, a large-scale, multicenter, prospective evaluation would help to optimize the conclusion.
Conclusions
Collectively, in the present study, we identified that CRNFB was the predominant pathogen among HM patients with CRGNB caused BSI. Moreover, the detective rates of CRGNBs had a rising tendency in recent years, which could increase the early mortality of patients with BSI, especially in CRNFB. It was noteworthy that HM patients with BSI had a high probability of concomitant with pulmonary infection. Therefore, conducting the early screening of the respiratory tract specimens may help to detect CRNFBs colonization and protect patients from breakthrough BSI. However, the screening population, screening methods and their frequency need to be further investigated.
Abbreviations
CRGNB, carbapenem-resistant Gram-negative bacteria; BSI, bloodstream infection; HM, hematologic malignancies; GNB, Gram-negative bacteria; CSGNB, carbapenem-susceptible Gram-negative bacteria; CRNFB, carbapenem-resistant non-fermenting bacteria; CRE, carbapenem-resistant Enterobacteriaceae; CRAB, carbapenem-resistant Acinetobacter baumannii; WHO, World Health Organization; CRPA, carbapenem-resistant Pseudomonas aeruginosa; EB, Enterobacteriaceae; NFB, non-fermentative bacteria; CR, carbapenem-resistant; MICs, minimum inhibitory concentrations; HR, hazard ratio; K. pneumonia, Klebsiella pneumonia; S. maltophilia, Stenotrophomonas maltophilia; P. aeruginosa, Pseudomonas aeruginosa; A. baumannii, Acinetobacter baumannii; E. coli, Escherichia coli; CSE, carbapenem-susceptible Enterobacteria; CRKP, carbapenem-resistant Klebsiella pneumonia; CSKP, carbapenem-susceptible Klebsiella pneumonia; CSNFB, carbapenem-susceptible non-fermentative bacteria; CSPA, carbapenem-susceptible Pseudomonas aeruginosa; CVC, central venous catheter; CREC, carbapenem-resistant Escherichia coli.
Acknowledgments
We thank all those who helped us in this study, in particular, the Department of Hematology and the Department of Clinical Laboratory for making this study possible.
Author Contributions
All authors meet the ICMJE authorship criteria. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was supported by a grant from National Natural Science Foundation of China for Xin Li(No. 81870165 and No. 82170204) and Natural Science Foundation of Hunan Province for Qian Cheng(No. 2021JJ40916).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nordmann P, Poirel L. Epidemiology and diagnostics of carbapenem resistance in gram-negative bacteria. Clin Infect Dis. 2019;69(Suppl Supplement_7):S521–S528. doi:10.1093/cid/ciz824
2. Lalaoui R, Javelle E, Bakour S, Ubeda C, Rolain JM. Infections due to carbapenem-resistant bacteria in patients with hematologic malignancies. Front Microbiol. 2020;11:1422. doi:10.3389/fmicb.2020.01422
3. Andria N, Henig O, Kotler O, et al. Mortality burden related to infection with carbapenem-resistant Gram-negative bacteria among haematological cancer patients: a retrospective cohort study. J Antimicrob Chemother. 2015;70(11):3146–3153. doi:10.1093/jac/dkv218
4. Kara Ali R, Surme S, Balkan II, et al. An eleven-year cohort of bloodstream infections in 552 febrile neutropenic patients: resistance profiles of Gram-negative bacteria as a predictor of mortality. Ann Hematol. 2020;99(8):1925–1932. doi:10.1007/s00277-020-04144-wF
5. Li Y, Sun QL, Shen Y, et al. Rapid Increase in Prevalence of Carbapenem-Resistant Enterobacteriaceae (CRE) and emergence of colistin resistance Gene mcr-1 in CRE in a Hospital in Henan, China. J Clin Microbiol. 2018;56(4). doi:10.1128/jcm.01932-17
6. Chen J, Hu C, Wang R, et al. Shift in the dominant sequence type of carbapenem-resistant Klebsiella pneumoniae bloodstream infection from ST11 to ST15 at a Medical Center in Northeast China, 2015–2020. Infect Drug Resist. 2021;14:1855–1863. doi:10.2147/IDR.S311968
7. Liang C, Zhang X, Zhou L, Meng G, Zhong L, Peng P. Trends and correlation between antibacterial consumption and carbapenem resistance in gram-negative bacteria in a tertiary hospital in China from 2012 to 2019. BMC Infect Dis. 2021;21(1):444. doi:10.1186/s12879-021-06140-5
8. Caston JJ, Lacort-Peralta I, Martin-Davila P, et al. Clinical efficacy of ceftazidime/avibactam versus other active agents for the treatment of bacteremia due to carbapenemase-producing Enterobacteriaceae in hematologic patients. Int J Infect Dis. 2017;59:118–123. doi:10.1016/j.ijid.2017.03.021
9. Satlin MJ, Cohen N, Ma KC, et al. Bacteremia due to carbapenem-resistant Enterobacteriaceae in neutropenic patients with hematologic malignancies. J Infect. 2016;73(4):336–345. doi:10.1016/j.jinf.2016.07.002
10. World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics.2017. Available from: http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf.
11. Tang Y, Xu C, Xiao H, Wang L, Cheng Q, Li X. Gram-negative bacteria bloodstream infections in patients with hematological malignancies – the impact of pathogen type and patterns of antibiotic resistance: a Retrospective Cohort Study. Infect Drug Resist. 2021;14:3115–3124. doi:10.2147/IDR.S322812
12. Scheich S, Weber S, Reinheimer C, et al. Bloodstream infections with gram-negative organisms and the impact of multidrug resistance in patients with hematological malignancies. Ann Hematol. 2018;97(11):2225–2234. doi:10.1007/s00277-018-3423-5
13. Yang -T-T, Luo X-P, Yang Q, et al. Different screening frequencies of carbapenem-resistant Enterobacteriaceae in patients undergoing hematopoietic stem cell transplantation: which one is better? Antimicrob Resist Infect Control. 2020;9(1):49. doi:10.1186/s13756-020-0706-0
14. Richter SS, Marchaim D. Screening for carbapenem-resistant Enterobacteriaceae: who, when, and how? Virulence. 2017;8(4):417–426. doi:10.1080/21505594.2016.1255381
15. Garpvall K, Duong V, Linnros S, et al. Admission screening and cohort care decrease carbapenem resistant Enterobacteriaceae in Vietnamese pediatric ICU’s. Antimicrob Resist Infect Control. 2021;10(1):128. doi:10.1186/s13756-021-00994-9
16. Lima EM, Cid PA, Beck DS, et al. Predictive factors for sepsis by carbapenem resistant Gram-negative bacilli in adult critical patients in Rio de Janeiro: a case-case-control design in a prospective cohort study. Antimicrob Resist Infect Control. 2020;9(1):132. doi:10.1186/s13756-020-00791-w
17. Routsi C, Pratikaki M, Platsouka E, et al. Risk factors for carbapenem-resistant Gram-negative bacteremia in intensive care unit patients. Intensive Care Med. 2013;39(7):1253–1261. doi:10.1007/s00134-013-2914-z
18. Bao H, Qiao Y, Liu D, et al. The clinical impact of Stenotrophomonas maltophilia bacteremia on the 30-day mortality rate in patients with hematologic disorders: a single-institution experience. Infection. 2020;48(2):205–212. doi:10.1007/s15010-019-01369-4
19. Shargian-Alon L, Gafter-Gvili A, Ben-Zvi H, et al. Risk factors for mortality due to Acinetobacter baumannii bacteremia in patients with hematological malignancies – a retrospective study. Leuk Lymphoma. 2019;60(11):2787–2792. doi:10.1080/10428194.2019.1599113
20. Aviv T, Lazarovitch T, Katz D, et al. The epidemiological impact and significance of carbapenem resistance in Pseudomonas aeruginosa bloodstream infections: a matched case-case-control analysis. Infect Control Hosp Epidemiol. 2018;39(10):1262–1265. doi:10.1017/ice.2018.181
21. Amat T, Gutierrez-Pizarraya A, Machuca I, et al. The combined use of tigecycline with high-dose colistin might not be associated with higher survival in critically ill patients with bacteraemia due to carbapenem-resistant Acinetobacter baumannii. Clin Microbiol Infect. 2018;24(6):630–634. doi:10.1016/j.cmi.2017.09.016
22. Britt NS, Ritchie DJ, Kollef MH, et al. Importance of site of infection and antibiotic selection in the treatment of carbapenem-resistant pseudomonas aeruginosa sepsis. Antimicrob Agents Chemother. 2018;62(4). doi:10.1128/aac.02400-17
23. Freire MP, de Oliveira Garcia D, Garcia CP, et al. Bloodstream infection caused by extensively drug-resistant Acinetobacter baumannii in cancer patients: high mortality associated with delayed treatment rather than with the degree of neutropenia. Clin Microbiol Infect. 2016;22(4):352–358. doi:10.1016/j.cmi.2015.12.010
24. Chinese Society of Hematology CMA, Chinese Medical Doctor Association HB. [Chinese guidelines for the clinical application of antibacterial drugs for agranulocytosis with fever (2020)]. Zhonghua Xue Ye Xue Za Zhi. 2020;41(12):969–978. Chinese. doi:10.3760/cma.j.issn.0253-2727.2020.12.001
25. Averbuch D, Orasch C, Cordonnier C, et al. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4th European Conference on Infections in Leukemia. Haematologica. 2013;98(12):1826–1835. doi:10.3324/haematol.2013.091025
26. Centers for disease control and prevention. Facility guidance for control of carbapenem- resistant Enterobacteriaceae (CRE) 2015 update [S/OL]; 2015. Available from: https://www.cdc.gov/.
27. Genga KR, Russell JA. Update of sepsis in the intensive care unit. J Innate Immun. 2017;9(5):441–455. doi:10.1159/000477419
28. Tang Y, Wu X, Cheng Q, Li X. Inappropriate initial antimicrobial therapy for hematological malignancies patients with Gram-negative bloodstream infections. Infection. 2020;48(1):109–116. doi:10.1007/s15010-019-01370-x
29. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2011;52(4):e56–93. doi:10.1093/cid/cir073
30. Tang Y, Cheng Q, Yang Q, et al. Prognostic factors and scoring model of hematological malignancies patients with bloodstream infections. Infection. 2018;46(4):513–521. doi:10.1007/s15010-018-1151-3
31. Zhao Y, Lin Q, Liu L, et al. Risk factors and outcomes of antibiotic-resistant pseudomonas aeruginosa bloodstream infection in adult patients with acute leukemia. Clin Infect Dis. 2020;71(Suppl 4):S386–S393. doi:10.1093/cid/ciaa1522
32. Sartelli M, Guirao X, Hardcastle TC, et al. 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World J Emerg Surg. 2018;13:58. doi:10.1186/s13017-018-0219-9
33. Tamma PD, Goodman KE, Harris AD, et al. Comparing the outcomes of patients with carbapenemase-producing and non-carbapenemase-producing carbapenem-resistant Enterobacteriaceae bacteremia. Clin Infect Dis. 2017;64(3):257–264. doi:10.1093/cid/ciw741
34. Trecarichi EM, Pagano L, Martino B, et al. Bloodstream infections caused by Klebsiella pneumoniae in onco-hematological patients: clinical impact of carbapenem resistance in a multicentre prospective survey. Am J Hematol. 2016;91(11):1076–1081. doi:10.1002/ajh.24489
35. Tofas P, Skiada A, Angelopoulou M, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections in neutropenic patients with haematological malignancies or aplastic anaemia: analysis of 50 cases. Int J Antimicrob Agents. 2016;47(4):335–339. doi:10.1016/j.ijantimicag.2016.01.011
36. Wang X, Zhang L, Sun A, et al. Acinetobacter baumannii bacteraemia in patients with haematological malignancy: a multicentre retrospective study from the infection working party of Jiangsu society of hematology. Eur J Clin Microbiol Infect Dis. 2017;36(7):1073–1081. doi:10.1007/s10096-016-2895-2
37. Palacios-Baena ZR, Giannella M, Manissero D, et al. Risk factors for carbapenem-resistant Gram-negative bacterial infections: a systematic review. Clin Microbiol Infect. 2021;27(2):228–235. doi:10.1016/j.cmi.2020.10.016
38. Wanla W, Katip W, Supakul S, Apiwatnakorn P, Khamsarn S. Effects of an antimicrobial restriction system on appropriate carbapenem use in a hospital without infectious diseases consultation. Int J Gen Med. 2017;10:443–449. doi:10.2147/IJGM.S145133
39. Micozzi A, Gentile G, Minotti C, et al. Carbapenem-resistant Klebsiella pneumoniae in high-risk haematological patients: factors favouring spread, risk factors and outcome of carbapenem-resistant Klebsiella pneumoniae bacteremias. BMC Infect Dis. 2017;17(1):203. doi:10.1186/s12879-017-2297-9
40. Chinese Society of Hematology CMA, Chinese Hematology Association CMDA. [Management of Carbapenem-resistant Enterobacteriaceae (CRE) infection in patients with hematological malignancies: Chinese consensus (2020)]. Zhonghua Xue Ye Xue Za Zhi. 2020;41(11):881–889. Chinese. doi:10.3760/cma.j.issn.0253-2727.2020.11.001
41. Girmenia C, Viscoli C, Piciocchi A, et al. Management of carbapenem resistant Klebsiella pneumoniae infections in stem cell transplant recipients: an Italian multidisciplinary consensus statement. Haematologica. 2015;100(9):e373–6. doi:10.3324/haematol.2015.125484
42. Zilberberg MD, Nathanson BH, Sulham K, Fan W, Shorr AF, Novel A. Algorithm to analyze epidemiology and outcomes of carbapenem resistance among patients with hospital-acquired and ventilator-associated pneumonia: a retrospective cohort study. Chest. 2019;155(6):1119–1130. doi:10.1016/j.chest.2018.12.024
43. Berube BJ, Rangel SM, Hauser AR. Pseudomonas aeruginosa: breaking down barriers. Curr Genet. 2016;62(1):109–113. doi:10.1007/s00294-015-0522-x
44. Li H, Zhang J, Wang Z, et al. Evolution of Acinetobacter baumannii in clinical bacteremia patients. Infect Drug Resist. 2021;14:3553–3562. doi:10.2147/IDR.S320645
45. Cai B, Tillotson G, Benjumea D, Callahan P, Echols R. The burden of bloodstream infections due to stenotrophomonas maltophilia in the United States: a large, retrospective database study. Open Forum Infect Dis. 2020;7(5):ofaa141. doi:10.1093/ofid/ofaa141
46. Katip W, Uitrakul S, Oberdorfer P. Clinical efficacy and nephrotoxicity of the loading dose colistin for the treatment of carbapenem-resistant Acinetobacter baumannii in critically ill patients. Pharmaceutics. 2021;14(1). doi:10.3390/pharmaceutics14010031
47. Katip W, Oberdorfer P. Clinical efficacy and nephrotoxicity of colistin alone versus colistin plus vancomycin in critically ill patients infected with carbapenem-resistant Acinetobacter baumannii: a propensity score-matched analysis. Pharmaceutics. 2021;13(2). doi:10.3390/pharmaceutics13020162
48. Katip W, Uitrakul S, Oberdorfer P. A comparison of colistin versus colistin plus meropenem for the treatment of carbapenem-resistant Acinetobacter baumannii in critically ill patients: a propensity score-matched analysis. Antibiotics. 2020;9(10). doi:10.3390/antibiotics9100647
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.