Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
The Therapeutic Role of Carotenoids in Diabetic Retinopathy: A Systematic Review
Authors Fathalipour M , Fathalipour H, Safa O, Nowrouzi-Sohrabi P , Mirkhani H, Hassanipour S
Received 27 March 2020
Accepted for publication 5 June 2020
Published 3 July 2020 Volume 2020:13 Pages 2347—2358
DOI https://doi.org/10.2147/DMSO.S255783
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ming-Hui Zou
Mohammad Fathalipour,1 Hadis Fathalipour,2 Omid Safa,3 Peyman Nowrouzi-Sohrabi,4 Hossein Mirkhani,5 Soheil Hassanipour6
1Department of Pharmacology and Toxicology, Faculty of Pharmacy, Hormozgan University of Medical Sciences, Bandar Abbas, Iran; 2The Student Research Committee, Faculty of Pharmacy, Kerman University of Medical Sciences, Kerman, Iran; 3Department of Clinical Pharmacy, Faculty of Pharmacy, Hormozgan University of Medical Sciences, Bandar Abbas, Iran; 4Department of Biochemistry, Shiraz University of Medical Sciences, Shiraz, Iran; 5Department of Pharmacology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran; 6Gastrointestinal and Liver Diseases Research Center, Guilan University of Medical Sciences, Rasht, Iran
Correspondence: Soheil Hassanipour
Gastrointestinal and Liver Diseases Research Center, Guilan University of Medical Sciences, Razi Hospital, Sardar-Jangle Ave., Rasht 41448-95655, Iran
Tel +98(13)33535116
Fax +98(13)33534951
Email [email protected]
Background: Carotenoids are a large group of natural pigments that occur in many foods, fruits, and vegetables. Several studies have shown a number of biological properties of carotenoids, particularly beneficial impacts on cancer, metabolic, neurodegenerative, and cardiovascular diseases. However, recent evidence has shown that these compounds could prevent, delay, and ameliorate diabetic retinopathy (DR). The aim of current study was to review the therapeutic effects of carotenoids in the treatment of DR and discuss the molecular mechanisms that are behind these pharmacological activities.
Methods: Six online databases (Medline/PubMed, Scopus, Web of Knowledge, Embase, ScienceDirect, and ProQuest) were searched until September 2019. The systematic review was carried out using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist.
Results: A total of 25 studies were included after the final retrieval. A relationship was observed between carotenoids and management of DR. Findings also demonstrated that the underlying mechanism of beneficial effects of these compounds was antioxidant, anti-inflammatory, anti-angiogenic, and neuroprotective properties.
Conclusion: Carotenoids potentially delay the initiation and prevent the progression of DR; however, ample preclinical studies are required to confirm their effect, and adequate clinical trials are needed to really understand how well these compounds influence DR among humans.
Keywords: diabetic retinopathy, carotenoids, oxidative stress, inflammation, neuroprotection
Introduction
Diabetic retinopathy (DR) is the main leading causes of morbidity in patients with diabetes and the primary cause of vision loss around the world.1 The total prevalence of DR is about 34.6% (92.6 million adults) among diabetic patients, of which 28.4 million adults with vision impairment.2,3 The severity of DR depends on several factors, including the type of diabetes (in type 1 is more severe than type 2), duration of diabetes, glycemic control status, and the presence of some pathological conditions such as hypertension, smoking, and dyslipidemia.4
Although the underlying pathophysiology of DR is not precisely known, it is primarily caused by the metabolic impacts of chronic hyperglycemia.5 Several lines of studies have shown various biochemical mechanisms about how potentially hyperglycemia causes DR, including increased polyol pathway flux, activation of protein kinase C (PKC) pathway, increased hexosamine pathway flux, and accelerated advanced glycation end-product (AGE) formation.6 These pathogenic mechanisms involved in retinal oxidative stress and inflammation, overexpression of growth factors (particularly vascular endothelial growth factor, VEGF), retinal hemodynamic changes, and impairment in neurotrophic factor receptors and their signaling pathway, which damage to retinal vessels, neurons, and glial cells.7
The most essential strategies to delay the onset and progression of DR are strict glycemic control, educational program and treatment of high blood pressure and probably dyslipidemia.7,8 Laser photocoagulation and vitrectomy are now the only approved clinical treatments of DR.9 The introduction and development of various pharmacotherapies over recent decades have significantly improved the management of vision loss and visual acuity impairment.10 Advanced intravitreal treatment with anti-angiogenic agents (anti-VEGF therapy) or anti-inflammatory agents (glucocorticoid therapy) is the most commonly used pharmacotherapy for both prevention and treatment of established DR.11
Additionally, other pharmacological agents such as aldose reductase inhibitors, PKC inhibitors, hexosamine biosynthesis inhibitors, AGE formation inhibitors, peroxisome proliferator-activated receptor (PPAR)-gamma receptor agonists, angiotensin-converting enzyme (ACE) inhibitors, antioxidants, and anti-inflammatory agents are investigating and developing to manage DR. However, these agents are still on preclinical or clinical trial stages.12–14 Natural products as potentially valuable and easily available remedies for DR also get the attention of the researchers.
Carotenoids are a large group of organic and lipophilic pigments, which are produced by plants, algae, and several bacteria and fungi.15 More than 600 ubiquitous carotenoids have been known in nature, but only about 40 carotenoids are present in human diets, including foods, fruits, and vegetables, and fewer carotenoids have been identified in human serum and organs.16
The chemical composition of carotenoids contains a central carbon chain with alternating single and double bonds and various groups on this backbone.17 These compounds are classified into two groups; carotenes (which purely contain carbons and hydrogens) and xanthophylls (which additionally contain oxygen).18 The most important, naturally occurring carotenoids were summarized in Table 1.
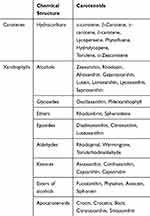 |
Table 1 The Most Important Naturally Occurring Carotenoids |
Several lines of studies have conducted on the beneficial effects of carotenoids in the prevention and management of a large number of diseases, including cancer,19 cardiovascular diseases,20 diabetes,21 osteoporosis,22 eye diseases,23,24 Alzheimer’s disease,25 and also infectious diseases.16 Moreover, it has been shown that the serum levels of some carotenoids are inversely associated with the progression of DR in humans.26–28 Recent studies on the biological impacts of different carotenoids have shown these compounds could prevent, delay, and ameliorate retinopathy in diabetes. A number of observed biological properties of these compounds including quenching of reactive oxygen/nitrogen species (ROS/RNS), scavenging of free radicals, immune enhancement, antimutagenesis activity, augmentation of self-defense systems, and photo-protection could justify the beneficial effect of these compounds on DR. Several studies have been conducted on the potential preventive and therapeutic effects of different carotenoids on DR. In the present review, the therapeutic effects of carotenoids in the treatment of DR screened and the molecular mechanisms that are behind these pharmacological activities completely discussed.
Method
The Search Strategy of Systematic Reviews
A literature review was carried out using six online databases (Medline/PubMed, Scopus, Web of Knowledge, Embase, ScienceDirect, and ProQuest) without any time limitation. The following MeSH terms were used for the primary search: (retinopathy or synonyms: retinal neovascularization, retinal vasculopathy, retinal neuropathy, retinal neurodegeneration, retinal histopathology, and eye tissue damage) AND (diabetes or diabetic) AND (the name of carotenoids, mentioned in Table 1). All obtained studies were imported into an EndNote X5 (Thomson Reuters, Carlsbad, USA) library. The duplicate studies were then removed. Reference lists of all sightly studies were manually checked to find additional studies.
Inclusion and Exclusion Criteria
All English-language studies, which contained results of the impacts of carotenoids on DR in preclinical and clinical studies, were included. Reviews, abstracts, posters, letters, comments, and editorial papers were excluded from the review.
Results
Description of Literature Search
The six online databases search yielded 204 citations. After the removal of duplicate papers, 99 unique studies retuned to evaluate in more detail. Duplicates (n=105), irrelevant (n=67), abstracts and reviews (n=13), and non-English language (n=2) studies were excluded. Twenty-five papers were included. No additional papers identified through manual references checking of included studies. The flowchart of the studies process through the review is shown in Figure 1.
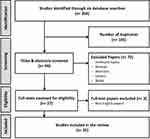 |
Figure 1 PRISMA flowchart of study selection and retrieval process.Notes: Adapted from Moher D, Shamseer L, Clarke Met al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.29 |
Studies Evaluating the Effects of Carotenoids on DR in Humans
Clinical trials and observational studies investigated the effects of carotenoids on diabetic patients with DR were reviewed. The results of these studies are summarized in Table 2.
 |
Table 2 Studies Evaluating the Effects of Carotenoids on Diabetic Retinopathy in Humans |
Moschos et al investigated the effects of carotenoid supplementation on retinal histological changes and macular function of diabetic patients in a two-year prospective cohort study. The results of the present study revealed that lutein (10 mg/day)-, zeaxanthin (2 mg/day)-, and meso-zeaxanthin (10 mg/day)-containing supplement improved retinal histological change and visual function.30
Sahli et al revealed that consumption of high lutein and zeaxanthin food was not associated with a decrease in DR incidence in a six-year prospective cohort study.31
Zhang et al study the impacts of lutein on diabetic patients with DR in a placebo-controlled clinical trial. They demonstrated intervention with lutein (10 mg/day) for 36 weeks caused a non-significant improvement of visual acuity and glare sensitivity. However, contrast sensitivity increased at special low frequencies.32
Tanaka et al reported that consumption of the carotene-containing fruits was able to decline the risk of DR among diabetic patients based on the results of an eight-week prospective cohort study.33
Hu et al investigated the effects of carotenoid-containing supplement on DR in a randomized clinical trial. They showed that lutein (6 mg/day) and zeaxanthin (0.5 mg/day) improved visual acuity, increased contrast sensitivity, and decreased macular edema after three months of intervention.34
Studies Evaluating the Effects of Carotenoids on Animal Models of DR
In this part, all experimental studies that evaluated the effects of different carotenoids on animal models of DR were reviewed. A summary of the results of these studies is presented in Table 3.
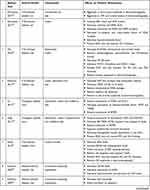 |  |  |
Table 3 Studies Evaluating the Effects of Carotenoids on Retinal Tissue of Diabetic Animal Models |
McClinton et al studied the beneficial effects of carotenoid-containing diet consumption on the DR in STZ-induced diabetic rats. The results demonstrated that the retinal function of treated animals aggravated after nine weeks of intervention.35
Sharavana et al showed treatment with lutein (0.1 mg/kg) for eight weeks suppressed retinal oxidative stress. It downregulated the retinal expression of VEGF, VEGF receptor (VEGFR), VEGF co-receptor, and VEGFR transcriptional factor in streptozotocin (STZ)-induced diabetic rats. Additionally, this compound prevented retinal morphological changes, including retinal ganglion cell (RGC) loss and different retinal layers thinning.36
Yeh et al indicated that astaxanthin (3 mg/kg) and lutein (0.5 mg/kg) in a treatment period of eight weeks reduced retinal oxidative stress and inflammatory mediators in STZ-induced diabetic rats. Moreover, these carotenoids improved retinal histological and function changes.37
Kowluru et al reported the consumption of lutein- and zeaxanthin-containing diet for 11 months decreased expression of retinal oxidative stress, inflammation, and angiogenesis process in STZ-induced diabetic rats. Retinal capillary cells apoptosis, as well as retinal function, were improved in this study.38
Yu et al investigated the effects of wolfberry (1% of total calories) in a treatment period of eight weeks on transgenic diabetic mice. Wolfberry attenuated diabetes-induced mitochondrial dysfunction, hypoxia condition, and oxidative stress in retinal tissue. The results of the study demonstrated the active compounds of wolfberry diet, zeaxanthin and lutein, declined expression of VEGF, and retinal neovascularization.39
In another study, Tang et al studied DR in transgenic diabetic mice after eight weeks of intervention with wolfberry (1% of total calories). They showed that zeaxanthin, lutein, and also cryptoxanthin as active compounds of wolfberry diet lowered retinal oxidative stress and cell apoptosis. Furthermore, these compounds restored retinal morphological changes in diabetes.40
Sasaki et al reported a lutein-rich diet (0.1 w/w) decreased retinal oxidative stress in STZ-induced diabetic mice after four months of intervention. Lutein prevented retinal neurodegeneration and apoptosis through inhibition of ERK overactivation, preservation of synaptophysin, and improvement of retinal brain-derived neurotrophic factor (BDNF) levels. This carotenoid had a protective role in retinal histology and function.41
Kowluru et al evaluated the effects of the β-carotene-containing supplement on alloxan-induced diabetic rats in two studies. The results of these studies indicated that an eight-week treatment with β-carotene (45 mg/kg) as one of the active compounds of the supplement ameliorates retinal oxidative stress and inflammation. In this study, retinal cell apoptosis was significantly decreased in the presence of the supplement.42,43
Arnal et al showed lutein (0.5 mg/kg) decreased intracellular oxidative stress in STZ-induced diabetic rats after 12 weeks of treatment. Retinal cell apoptosis also reduced by lutein administration. More importantly, this carotenoid improved the function of diabetic retinal tissue.44
Kowluru et al demonstrated that consumption of carotenoid-rich diet (0.02% w/w) for eight weeks decreased retinal oxidative stress and oxidative-induced damages in STZ-induced diabetic rats. Carotenoids inhibited hyperglycemia-induced retinal neovascularization.45
In other studies, Kowluru et al evaluated the effects of a carotenoid-rich diet (0.02% w/w) on STZ-induced diabetic rats in different studies. These compounds attenuated the retinal oxidative stress, mitochondrial overproduction of ROS, and oxidative damages of retinal microcapillary.45 Additionally, β-carotene as an active compound of this diet decreased retinal glutamate and neurotoxicity.46
Muriach et al studied the effects of two-week administration of lutein (0.2 mg/kg) on DR in alloxan-induced diabetic mice. The results of the current study revealed that lutein suppressed oxidative stress, ameliorated inflammatory responses, and improved functions of retina.47
Dene et al reported that β-carotene (10 mg/kg) had no antioxidant impacts on retinal tissue in STZ-induced diabetic rats after two weeks of treatment.48
Studies Evaluating the Effects of Carotenoids on in vitro Models of DR
Experimental studies evaluated the effects of different carotenoids on in vitro models of DR were reviewed. A summary of these studies is presented in Table 4.
 |
Table 4 Studies Evaluating the Effects of Carotenoids on in vitro Models of Diabetic Retinopathy |
Hwang et al investigated the effect of various concentrations of lutein on human adult retinal pigment epithelial cells (ARPE-19) cultured in high concentrations of glucose. Their results demonstrated lutein inhibited hyperglycemia-induced oxidative stress and premature senescence in cells. They also showed these beneficial effects are related to the upregulation of silent information regulator 2 (SIRT1) mRNA and protein levels.49
Yang et al reported the result of their study on the retinal microglial cell lines, BV-2 and N9, which cultured in high-glucose and free fatty acid conditions. Crocin prevented the oxidative stress and inflammatory response of hyperglycemic and hyperlipidemic stress. Moreover, they showed these neuroprotective effects of crocin are related to activation of PI3K/Akt signaling pathway.50
Baccouche et al studied the effect of astaxanthin on primary retinal cells isolated from Psammomys obesus cultured in high-glucose condition. The results revealed that astaxanthin decreased cell apoptosis, improved mitochondrial function, and enhanced the neurons and glial cells viability.51
Umigai et al evaluated the effects of crocetin on human retinal microvascular endothelial cells (HRMEC) and human umbilical vein endothelial cells (HUVEC), which cultured in the presence of VEGF and high concentration of glucose. Although crocetin had no beneficial effect on cell viability, VEGF-induced formation of capillary-like structures in HUVEC culture, and migration of HRMEC suppressed. Furthermore, activation of p38 MAPK and expression of adhesion and tight junction proteins decreased.52
Tang et al investigated lutein and zeaxanthin impacts on the ARPE-19, which cultured in high concentrations of glucose. The study demonstrated that both zeaxanthin and lutein increased the activation of AMPK, resulting in attenuation of oxidative stress, and normalization of endoplasmic reticulum stress.40
Sun et al investigated the effects of lutein and astaxanthin on ARPE-19 in another study. These carotenoids exhibited suppression of oxidative stress and inhibition of carboxymethyllysine formation, an AGE indicator. Additionally, the deleterious effects of high exogenous AGE on ARPE-19 attenuated in the presence of these compounds.53
Kowluru et al found that β-carotene attenuated intracellular oxidative stress and also prevented apoptosis in HRMEC, which cultured in high concentrations of glucose.42
Discussion
DR is the leading cause of vision loss around the world with a high socioeconomic burden.1 Despite the advances in the pharmacotherapy of diabetes, the global prevalence of DR has increased over the last years.3 Hyperglycemia is the most important known cause of retinopathy.6 The polyol pathway flux increases under hyperglycemic conditions. Aldose reductase reduces intracellular glucose to sorbitol and aldehydes to inactive alcohols with depleting of NADPH and reduced glutathione (GSH). Subsequently, sorbitol dehydrogenase oxidizes sorbitol to fructose with the consumption of NAD+.54 Moreover, the intracellular production of advanced glycation end-products increases. Non-enzymatic modifications of intracellular and matrix proteins alter various cellular functions and cause abnormal interactions between several matrix proteins and integrins. Changes of plasma proteins produce ligands which bind to advanced glycation end-products receptors and causes the generation of intracellular reactive oxygen species, and changes in gene expression.55 Intracellular hyperglycemia also increases diacylglycerol content and activates protein kinase C, which has numerous pathogenic effects, especially activation of nuclear factor-κB, and NAD(P)H oxidases.56 Furthermore, the hexosamine pathway activation in hyperglycemic condition results in several alternations in both gene expression and protein function.57 Oxidative stress as a common link of the four pathogenic mechanisms results in hyperglycemia-induced damages. A body of evidence has demonstrated hyperglycemia-induced oxidative stress is responsible for retinal microvasculopathy as well as early neuropathy in the pathogenesis of DR.1,58,59
Carotenoids are a large group of organic and lipophilic pigments produced by plants, algae, and several bacteria and fungi.15 Several studies have conducted on the beneficial effects of these compounds in the prevention and management of a large number of diseases, particularly diabetes and diabetic complications. It has also been shown that the levels of serum carotenoids are associated with the prediction and severity of DR.26–28 Although the therapeutic effects of carotenoids in DR have been evaluated in several different types of studies, the exact effects of these individual compounds on DR are not completely established.
In the present review, all studies evaluating the effects of a number of carotenoids on DR were systematically reviewed. The underlying mechanism of carotenoids on the retinal oxidative stress, inflammation, neovascularization, neurodegeneration, histology, and function in different types of diabetes models and diabetic patients were screened.
Different studies have reported that carotenoids exert retinal protection during oxidative stress, which is the most important underlying mechanism involved in the pathogenesis of DR.60,61 As previous studies have reported, carotenoids or their active metabolites may act as both free radicals quenching and ROS\RNS scavenging in oxidative milieu.62 The expression and activity of retinal oxidoreductases, including SOD,36,40,45 glutathione reductase,48 and glutathione peroxidase,44,63 as well as intracellular antioxidant molecules including GSH36 and thioredoxin,40 increased in the presence of carotenoids. However, the levels of retinal GSH did not improve after intervention with carotenoids.45 Moreover, retinal gamma-glutamyl transferase, a crucial enzyme involved in glutathione metabolism, increased.48 Forkhead box protein O1 (FOXO1), a factor involved in catalase and SOD upregulation, enhanced after exposure with carotenoids.40
Carotenoids also have improved mitochondrial dysfunction as an important source of ROS/RNS in DR. These compounds enhanced the expression of mitochondrial dehydrogenase51 and electron transport complex III45 in the retinal tissue of diabetic animals. Heat shock protein 60 (HSP60) as a major mitochondrial biomarker of stress ameliorated after treatment with carotenoids.39 Oxidative stress-induced endoplasmic reticulum (ER) stress has critical effects on retinal cell apoptosis. Both activating transcription factor 6 (ATF6) and protein kinase RNA-like ER kinase (PERK), signaling pathways involved in ER stress, and ER stress sensor protein binding immunoglobulin protein (BiP) ameliorated by carotenoids.40
The production of ROS and RNS in retinal tissue38,41 or cell culture40,50,53 of diabetes models decreased in the presence of carotenoids. High glucose-induced oxidative stress intracellular products including nitrotyrosine,37,45 protein carbonyl,36 acrolein,37 oxidized deoxyguanosine,37,45 and lipid peroxides36,42–47,53 also diminished after treatment with carotenoids. Retinal expression of inducible nitric oxide synthase (iNOS)43,45,46,50 and overproduction of NO-induced damages modulated by carotenoids.
Some studies reported that carotenoids are able to decrease hyperglycemia-induced AGE. Carotenoid reduced the formation of carboxymethyllysine and prevented neuronal and vascular damages of this AGE.53
Retinal inflammation, along with oxidative stress, is an essential part of DR pathogenesis. Treatment with carotenoids augmented the activation of retinal microglia, which have critical participation in the inflammatory responses of retina.50 The expression of pro-inflammatory mediators in retinal tissue, including different cytokines,37,38,45,50 chemokines,37 adhesion molecules for leukocytes,37 caspases-12,40 and glutamate decreased in treatment with Catranides. The activation of NFκB37,38,43,47 and extracellular signal-regulated kinases (ERK) signaling pathways41 in the activated retinal microglia significantly decreased after consumption of carotenoids.
Additionally, carotenoids enhanced the expression of SOD and attenuated ER stress by upregulating the expression and activation of AMP-activated protein kinase (AMPK).39,40 Carotenoids also activated the retinal phosphoinositide-3-kinase-protein kinase/protein kinase B (PI3K/Akt) signaling pathway, which plays a crucial role in ameliorating oxidative stress, diminishing the pro-inflammatory response, and reducing neurodegeneration.50
Retinal vasculopathy also plays a fundamental role in the process of DR initiation and progression. Carotenoids can significantly improve retinal endothelial dysfunction.64 It has been shown that retinal capillary in DR reduced in the presence of carotenoids.45 Blood-retinal barrier breakdown and vascular leakages have recovered after treatment with carotenoids.52 More importantly, carotenoids had significant beneficial effects on retinal neovascularization, particularly by decrease the expression of VEGF,36,38,39,45,52 VEGF receptors,36 and VEGF transcription factors.36 Increased hypoxia-inducible factor (HIF) due to retinal vasculopathy modulated by carotenoids.36,39
Retinal neuronal and vascular cell apoptosis also involved in the pathogenesis of DR. Several studies showed carotenoids decreased apoptosis in retinal tissue and cell of diabetes models.38,41,44,51 Moreover, it has been shown that these compounds mitigated the activation of caspase-3.40,42
Recent studies have shown that retinal neuropathy plays a crucial role, especially in the early stage of DR.65,66 Carotenoids increased the expression of retinal BDNF and prevented retinal neurodegeneration.41 Furthermore, it has been demonstrated that carotenoids diminished RGC loss,36,37,40,41 thinning of the different retinal layer including total retina (TR),37 inner plexiform layer (IPL),37,41 inner nuclear layer (INL),36,37,40,41 outer retinal layers (ORL),37 retina photoreceptor layer (RPL)40 in animal models of diabetes.
Several lines of evidence demonstrated retinal electrophysiological function in preclinical and clinical models of DR has improved after treatment with carotenoids. The amplitude of a-wave,38 b-wave,37,38,44,47 and oscillatory potentials41 as well as the implicit time of b-wave44 in electroretinography of diabetic animals restored with carotenoids. However, the administration of carotenoids aggravated a-, b-waves amplitude and a-, b-wave, and oscillatory potentials latency in electroretinography.35
Although there is not much evidence for the effects of carotenoids on DR in clinical trials, some beneficial effects of these compounds have investigated in diabetic patients. These compounds improved central foveal thickness,30 retinal response density,30 visual acuity,34 contrast sensitivity,32,34 and macular edema.34 However, in some studies, carotenoids had no advantageous effects on visual acuity and glare sensitivity,32 and the absence of these effects might be related to the short period of intervention. It has been shown in an eight-year prospective cohort, consumption of carotenoid-rich diet declines the DR incidence among diabetic patients.31 On the other hand, the intake of food rich in lutein and zeaxanthin was not associated with a decrease in DR incidence.31
Carotenoids showed a higher success rate in the improvement of DR in experimental models compared to diabetic patients. This discrepancy might be related to low doses of carotenoids and short duration of intervention in clinical studies. Moreover, a carotenoids-rich supplement or diet was used in most clinical studies. These types of studies have evaluated the effects of several compounds instead of a specific carotenoid and might be reported confounding results.
It will be advantageous to evaluate the impacts of carotenoids on DR in good quality, placebo-controlled, and large prospective clinical trials in a more prolonged period of time. Furthermore, these trials must also evaluate the safety and tolerability of different carotenoids.
Conclusion
In summary, carotenoids potentially delay the initiation and prevent the progression of DR; however, ample preclinical studies are required to confirm their effect, and adequate clinical trials are needed to prove their effectiveness among humans.
Disclosure
The authors claim that there is no conflict of interest.
References
1. Hammes HP. Diabetic retinopathy: hyperglycaemia, oxidative stress and beyond. Diabetologia. 2017;61:29–38.
2. Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi:10.2337/dc11-1909
3. Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Experiment Ophthalmol. 2016;44(4):260–277. doi:10.1111/ceo.12696
4. Olivares AM, Althoff K, Chen GF, et al. Animal models of diabetic retinopathy. Curr Diab Rep. 2017;17(10):93. doi:10.1007/s11892-017-0913-0
5. Whitmire W, Al-Gayyar MM, Abdelsaid M, Yousufzai BK, El-Remessy AB. Alteration of growth factors and neuronal death in diabetic retinopathy: what we have learned so far. Mol Vis. 2011;17:300–308.
6. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi:10.1038/414813a
7. Chistiakov DA. Diabetic retinopathy: pathogenic mechanisms and current treatments. Diabetes Metab Syndr. 2011;5(3):165–172. doi:10.1016/j.dsx.2012.02.025
8. Khalaf FR, Fahmy HM, Ibrahim AK, et al. Does a diabetic retinopathy educational program raise awareness among elderly diabetic patients? Diabetes Metab Syndr Obes. 2019;12:1867–1875. doi:10.2147/DMSO.S208072
9. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298(8):902–916. doi:10.1001/jama.298.8.902
10. Duh EJ, Sun JK, Stitt AW. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight. 2017;2(14):e93751. doi:10.1172/jci.insight.93751
11. Jonas JB. Intravitreal triamcinolone acetonide for diabetic retinopathy. Dev Ophthalmol. 2007;39:96–110.
12. Coucha M, Elshaer SL, Eldahshan WS, Mysona BA, El-Remessy AB. Molecular mechanisms of diabetic retinopathy: potential therapeutic targets. Middle East Afr J Ophthalmol. 2015;22(2):135–144. doi:10.4103/0974-9233.154386
13. Simo R, Hernandez C. Advances in the medical treatment of diabetic retinopathy. Diabetes Care. 2009;32(8):1556–1562. doi:10.2337/dc09-0565
14. Khan ZA, Chakrabarti S. Cellular signaling and potential new treatment targets in diabetic retinopathy. Exp Diabetes Res. 2007;2007:31867. doi:10.1155/2007/31867
15. Xavier AA, Perez-Galvez A. Carotenoids as a source of antioxidants in the diet. Subcell Biochem. 2016;79:359–375.
16. Milani A, Basirnejad M, Shahbazi S, Bolhassani A. Carotenoids: biochemistry, pharmacology and treatment. Br J Pharmacol. 2017;174(11):1290–1324. doi:10.1111/bph.13625
17. Stahl W, Sies H. Bioactivity and protective effects of natural carotenoids. Biochim Biophys Acta. 2005;1740(2):101–107. doi:10.1016/j.bbadis.2004.12.006
18. Alvarez R, Vaz B, Gronemeyer H, de Lera AR. Functions, therapeutic applications, and synthesis of retinoids and carotenoids. Chem Rev. 2014;114(1):1–125. doi:10.1021/cr400126u
19. Bolhassani A. Cancer chemoprevention by natural carotenoids as an efficient strategy. Anticancer Agents Med Chem. 2015;15(8):1026–1031. doi:10.2174/1871520615666150302125707
20. Gammone MA, Pluchinotta FR, Bergante S, Tettamanti G, D’Orazio N. Prevention of cardiovascular diseases with carotenoids. Front Biosci. 2017;9:165–171. doi:10.2741/s480
21. Roohbakhsh A, Karimi G, Iranshahi M. Carotenoids in the treatment of diabetes mellitus and its complications: a mechanistic review. Biomed Pharmacother. 2017;91:31–42. doi:10.1016/j.biopha.2017.04.057
22. Sugiura M, Nakamura M, Ogawa K, Ikoma Y, Yano M. High serum carotenoids associated with lower risk for bone loss and osteoporosis in post-menopausal Japanese female subjects: prospective cohort study. PLoS One. 2012;7(12):e52643. doi:10.1371/journal.pone.0052643
23. Wise J. Diet rich in carotenoids is linked to reduced risk of advanced age related macular degeneration. BMJ. 2015;351:h5384. doi:10.1136/bmj.h5384
24. Moeller SM, Voland R, Tinker L, et al. Associations between age-related nuclear cataract and lutein and zeaxanthin in the diet and serum in the Carotenoids in the Age-Related Eye Disease Study, an ancillary study of the women’s health initiative. Arch Ophthalmol. 2008;126(3):354–364. doi:10.1001/archopht.126.3.354
25. Mohammadzadeh Honarvar N, Saedisomeolia A, Abdolahi M, et al. Molecular anti-inflammatory mechanisms of retinoids and carotenoids in Alzheimer’s disease: a review of current evidence. J Mol Neurosci. 2017;61(3):289–304. doi:10.1007/s12031-016-0857-x
26. Murillo AG, Fernandez ML. Potential of dietary non-provitamin A carotenoids in the prevention and treatment of diabetic microvascular complications. Adv Nutr. 2016;7(1):14–24. doi:10.3945/an.115.009803
27. Brazionis L, Rowley K, Itsiopoulos C, O’Dea K. Plasma carotenoids and diabetic retinopathy. Br J Nutr. 2008;101(2):270. doi:10.1017/S0007114508006545
28. She C, Shang F, Zhou K, Liu N. Serum carotenoids and risks of diabetes and diabetic retinopathy in a Chinese population sample. Curr Mol Med. 2017;17(4). doi:10.2174/1566524017666171106112131
29. Moher D, Shamseer L, Clarke Met al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi:10.1186/2046-4053-4-1
30. Moschos MM, Dettoraki M, Tsatsos M, Kitsos G, Kalogeropoulos C. Effect of carotenoids dietary supplementation on macular function in diabetic patients. Eye Vision. 2017;4(1):23. doi:10.1186/s40662-017-0088-4
31. Sahli MW, Mares JA, Meyers KJ, et al. Dietary intake of lutein and diabetic retinopathy in the Atherosclerosis Risk in Communities Study (ARIC). Ophthalmic Epidemiol. 2016;23(2):99–108. doi:10.3109/09286586.2015.1129426
32. Zhang P-C, Wu C-R, Wang Z-L, et al. Effect of lutein supplementation on visual function in nonproliferative diabetic retinopathy. Asia Pac J Clin Nutr. 2013;26(3):406–411.
33. Tanaka S, Yoshimura Y, Kawasaki R, et al. Fruit intake and incident diabetic retinopathy with type 2 diabetes. Epidemiology. 2013;24(2):204–211. doi:10.1097/EDE.0b013e318281725e
34. Hu BJ, Hu YN, Lin S, Ma WJ, Li XR. Application of lutein and zeaxanthin in nonproliferative diabetic retinopathy. Int J Ophthalmol. 2011;4(3):303–306. doi:10.3980/j.issn.2222-3959.2011.03.19
35. McClinton KJ, Aliani M, Kuny S, Sauve Y, Suh M. Differential effect of a carotenoid-rich diet on retina function in non-diabetic and diabetic rats. Nutr Neurosci. 2019;1–11. doi:10.1080/1028415X.2018.1563664
36. Sharavana G, Baskaran V. Lutein downregulates retinal vascular endothelial growth factor possibly via hypoxia inducible factor 1 alpha and X-box binding protein 1 expression in streptozotocin induced diabetic rats. J Funct Foods. 2017;31:97–103. doi:10.1016/j.jff.2017.01.023
37. Yeh PT, Huang HW, Yang CM, Yang WS, Yang CH. Astaxanthin inhibits expression of retinal oxidative stress and inflammatory mediators in streptozotocin-induced diabetic rats. PLoS One. 2016;11(1):e0146438. doi:10.1371/journal.pone.0146438
38. Kowluru RA, Zhong Q, Santos JM, Thandampallayam M, Putt D, Gierhart DL. Beneficial effects of the nutritional supplements on the development of diabetic retinopathy. Nutr Metab. 2014;11(1):8. doi:10.1186/1743-7075-11-8
39. Yu HF, Wark L, Ji H, et al. Dietary wolfberry upregulates carotenoid metabolic genes and enhances mitochondrial biogenesis in the retina of db/db diabetic mice. Mol Nutr Food Res. 2013;57(7):1158–1169. doi:10.1002/mnfr.201200642
40. Tang L, Zhang Y, Jiang Y, et al. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Exp Biol Med. 2011;236(9):1051–1063. doi:10.1258/ebm.2011.010400
41. Sasaki M, Ozawa Y, Kurihara T, et al. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia. 2010;53(5):971–979. doi:10.1007/s00125-009-1655-6
42. Kowluru RA, Koppolu P. Diabetes-induced activation of caspase-3 in retina: effect of antioxidant therapy. Free Radic Res. 2002;36(9):993–999. doi:10.1080/1071576021000006572
43. Kowluru RA, Koppolu P, Chakrabarti S, Chen S. Diabetes-induced activation of nuclear transcriptional factor in the retina, and its inhibition by antioxidants. Free Radic Res. 2003;37(11):1169–1180. doi:10.1080/10715760310001604189
44. Arnal E, Miranda M, Johnsen-Soriano S, et al. Beneficial effect of docosahexanoic acid and lutein on retinal structural, metabolic, and functional abnormalities in diabetic rats. Curr Eye Res. 2009;34(11):928–938. doi:10.3109/02713680903205238
45. Kowluru RA, Menon B, Gierhart DL. Beneficial effect of zeaxanthin on retinal metabolic abnormalities in diabetic rats. Invest Ophthalmol Vis Sci. 2008;49(4):1645–1651. doi:10.1167/iovs.07-0764
46. Kowluru RA, Engerman RL, Case GL, Kern TS. Retinal glutamate in diabetes and effect of antioxidants. Neurochem Int. 2001;38(5):385–390. doi:10.1016/S0197-0186(00)00112-1
47. Muriach M, Miranda M, Arnal E, Bosch-Morel F, Romero FJ. Effect of lutein in the retina of diabetic mice. Eur J Ophthalmol. 2007;17(3):477–478.
48. Dene BA, Maritim AC, Sanders RA, Watkins JB. Effects of antioxidant treatment on normal and diabetic rat retinal enzyme activities. J Ocul Pharmacol Th. 2005;21(1):28–35. doi:10.1089/jop.2005.21.28
49. Hwang JS, Han SG, Lee CH, Seo HG. Lutein suppresses hyperglycemia‐induced premature senescence of retinal pigment epithelial cells by upregulating SIRT1. J Food Biochem. 2018;42(3):e12495. doi:10.1111/jfbc.12495
50. Yang X, Huo F, Liu B, et al. Crocin inhibits oxidative stress and pro-inflammatory response of microglial cells associated with diabetic retinopathy through the activation of PI3K/Akt signaling pathway. J Mol Neurosci. 2017;61(4):581–589. doi:10.1007/s12031-017-0899-8
51. Baccouche B, Mbarek S, Dellaa A, et al. Protective effect of astaxanthin on primary retinal cells of the gerbil Psammomys obesus cultured in diabetic milieu. J Food Biochem. 2017;41(1):e1745. doi:10.1111/jfbc.12274
52. Umigai N, Tanaka J, Tsuruma K, Shimazawa M, Hara H. Crocetin, a carotenoid derivative, inhibits VEGF-induced angiogenesis via suppression of p38 phosphorylation. Curr Neurovasc Res. 2012;9(2):102–109. doi:10.2174/156720212800410830
53. Sun Z, Liu J, Zeng X, et al. Protective actions of microalgae against endogenous and exogenous advanced glycation endproducts (AGEs) in human retinal pigment epithelial cells. Food Funct. 2011;2(5):251–258. doi:10.1039/c1fo10021a
54. Lorenzi M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp Diabetes Res. 2007;2007(3):61038. doi:10.1155/2007/61038
55. Chen M, Curtis TM, Stitt AW. Advanced glycation end products and diabetic retinopathy. Curr Med Chem. 2013;20(26):3234–3240. doi:10.2174/09298673113209990025
56. Galvez MI. Protein kinase C inhibitors in the treatment of diabetic retinopathy. Review. Curr Pharm Biotechnol. 2011;12(3):386–391. doi:10.2174/138920111794480606
57. Semba RD, Huang H, Lutty GA, Van Eyk JE, Hart GW. The role of O-GlcNAc signaling in the pathogenesis of diabetic retinopathy. Proteomics Clin Appl. 2014;8(3–4):218–231. doi:10.1002/prca.201300076
58. Kowluru RA, Mishra M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim Biophys Acta. 2015;1852(11):2474–2483. doi:10.1016/j.bbadis.2015.08.001
59. Behl T, Kaur I, Kotwani A. Implication of oxidative stress in progression of diabetic retinopathy. Surv Ophthalmol. 2016;61(2):187–196. doi:10.1016/j.survophthal.2015.06.001
60. Wu MY, Yiang GT, Lai TT, Li CJ. The oxidative stress and mitochondrial dysfunction during the pathogenesis of diabetic retinopathy. Oxid Med Cell Longev. 2018;2018:3420187. doi:10.1155/2018/3420187
61. Sahajpal NS, Goel RK, Chaubey A, Aurora R, Jain SK. Pathological perturbations in diabetic retinopathy: hyperglycemia, AGEs, oxidative stress and inflammatory pathways. Curr Protein Pept Sci. 2019;20(1):92–110. doi:10.2174/1389203719666180928123449
62. Neelam K, Goenadi CJ, Lun K, Yip CC, Au Eong KG. Putative protective role of lutein and zeaxanthin in diabetic retinopathy. Br J Ophthalmol. 2017;101(5):551–558. doi:10.1136/bjophthalmol-2016-309814
63. Muriach M, Bosch-Morell F, Alexander G, et al. Lutein effect on retina and hippocampus of diabetic mice. Free Radic Biol Med. 2006;41(6):979–984. doi:10.1016/j.freeradbiomed.2006.06.023
64. Hozawa A, Jacobs DR, Steffes MW, Gross MD, Steffen LM, Lee DH. Relationships of circulating carotenoid concentrations with several markers of inflammation, oxidative stress, and endothelial dysfunction: the Coronary Artery Risk Development in Young Adults (CARDIA)/Young Adult Longitudinal Trends in Antioxidants (YALTA) study. Clin Chem. 2007;53(3):447–455. doi:10.1373/clinchem.2006.074930
65. Simo R, Stitt AW, Gardner TW. Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia. 2018;61(9):1902–1912. doi:10.1007/s00125-018-4692-1
66. Barber AJ, Baccouche B. Neurodegeneration in diabetic retinopathy: potential for novel therapies. Vision Res. 2017;139:82–92. doi:10.1016/j.visres.2017.06.014
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
