Back to Journals » Drug Design, Development and Therapy » Volume 15
The Therapeutic Effects of Curcumin in Early Septic Acute Kidney Injury: An Experimental Study
Authors Wang S , Zhao P, Zhang Y, Zhu L, Zhu J, Luo Y, Li Q
Received 4 August 2021
Accepted for publication 25 September 2021
Published 7 October 2021 Volume 2021:15 Pages 4243—4255
DOI https://doi.org/10.2147/DDDT.S332623
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Shuo Wang,1,2 Ping Zhao,1,2 Ying Zhang,2 Lianhua Zhu,2 Jianing Zhu,1,2 Yukun Luo,2 Qiuyang Li2
1Medical School of Chinese PLA, Chinese PLA General Hospital, Beijing, People’s Republic of China; 2Department of Ultrasound, The First Medical Center of Chinese PLA General Hospital, Beijing, People’s Republic of China
Correspondence: Yukun Luo; Qiuyang Li
Department of Ultrasound, The First Medical Center of Chinese PLA General Hospital, Fuxing Road 28#, Beijing, 100853, People’s Republic of China
Tel +86-10-5549-9056
Fax +86-10-6693-9533
Email [email protected]; [email protected]
Purpose: Sepsis is the leading condition associated with acute kidney injury (AKI) in the hospital and intensive care unit (ICU), sepsis-induced AKI (S-AKI) is strongly associated with poor clinical outcomes. Curcumin possesses an ability to ameliorate renal injury from ischemia-reperfusion, but it is still unknown whether they have the ability to reduce S-AKI. The aim of this study was to investigate the protective effects of curcumin on S-AKI and to assess its therapeutic potential on renal function, inflammatory response, and microcirculatory perfusion.
Methods: Male Sprague-Dawley (SD) rats underwent cecal ligation and puncture (CLP) to induce S-AKI and immediately received vehicle (CLP group) or curcumin (CLP+Cur group) after surgery. At 12 and 24h after surgery, serum indexes, inflammatory factors, cardiac output (CO), renal blood flow and microcirculatory flow were measured.
Results: Serum levels of creatinine (Scr), cystatin C (CysC), IL-6 and TNF-α were significantly lower in the CLP+Cur group than those in the CLP group (P < 0.05). Treatment with curcumin improved renal microcirculation at 24h by measurement of contrast enhanced ultrasound (CEUS) quantitative parameters [peak intensity (PI); half of descending time (DT/2); area under curve (AUC); P < 0.05]. In histopathological analysis, treatment with curcumin reduced damage caused by CLP.
Conclusion: Curcumin can alleviate S-AKI in rats by improving renal microcirculatory perfusion and reducing inflammatory response. Curcumin may be a potential novel therapeutic agent for the prevention or reduction of S-AKI.
Keywords: curcumin, acute kidney injury, sepsis, microcirculation, inflammation, contrast enhanced ultrasound
Introduction
Sepsis is a systemic inflammatory response syndrome caused by infection,1 and it is the leading condition associated with acute kidney infection (AKI) in the hospital and intensive care unit (ICU).2 The annual global incidence of sepsis-induced AKI (S-AKI) is approximately 6 million cases or nearly 1 per 1000 population,3 and for ICU patients, sepsis is found in 40% to 50% of patients with AKI.4,5 S-AKI is strongly associated with poor clinical outcomes. Early goal-directed therapy, such as fluid resuscitation and supportive treatment, can improve survival,6 however, an analysis of the Protocolized Care for Early Septic Shock (ProCESS) trial focused on renal outcomes found that the use of early goal-directed therapy or usual care did not influence new AKI development, severity of AKI, or renal recovery.7 It is becoming increasingly clear that restoring the macrocirculation is not always sufficient to adequately restore the microcirculation and preserve organ function.8 At the same time, inflammation associated with S-AKI is coupled to long-term outcomes, as elevated plasma concentrations of inflammatory factors have been associated with nonrecovery of renal function and death in critically ill patients receiving renal replacement therapy (RRT).9 Thus, there is a pressing need for the development of novel therapeutic approaches that can restore perfusion of the renal microcirculation and exert anti-inflammatory effects.10,11
Curcumin (Cur, C 21H 20O6, Figure 1), the major active component of Curcuma longa, was first isolated in 1870 and widely used as spice, flavor, and colorant in daily life.12 Several studies have evidenced the antioxidant, anti-inflammatory, anticarcinogenic, cardioprotective and nephroprotective effects of curcumin.13 The protective effects of curcumin on AKI are generally associated with its bifunctional antioxidant activity and anti-inflammatory activity.14–16 Curcumin could ameliorate kidney disease with either acute or chronic nephritis, and reduce activation of the NF-κB, MAPK, AKT and pBAD pathways either systemically, or within the inflamed kidneys.17 In addition, previous research found that curcumin could improve renal function during ischemia-reperfusion induced acute kidney injury, which protected the tubular epithelium from injury by modulating inflammatory processes, oxidative stress, and apoptosis.14 However, few studies have investigated the effects of curcumin on AKI induced by sepsis; whether or not curcumin can improve the renal microcirculation and exert anti-inflammatory effects is unclear. To investigate the therapeutic potential of curcumin, we established a septic rat model with AKI by cecal ligation and puncture (CLP), and then observed the changes in renal tissues and renal functions with or without curcumin intervention, and studied its effects on renal macro- and microcirculation and inflammatory reactions in septic rats.
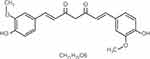 |
Figure 1 The chemical structure of curcumin. MW: 358.385, BP: 591.4°C, MP: 183°C, density (g/m3, 25/4°C): 0.93. |
Materials and Methods
Animals
Male Sprague-Dawley rats weighing 400g to 500g were housed in an animal facility with 12h light–dark cycle at 22–24°C. Rats were acclimated for 3–5 days before conducting experiments with free access to food and water. All rats were purchased from the Laboratory Animal Center of the First Medical Center of Chinese PLA General Hospital (Experimental Animal License: SCXK (Beijing) 2019–0010). This study was approved by the Institutional Animal Care and Use Committee of the Chinese PLA General Hospital (2020-X16-76), and it was carried out in accordance with the Code of Practice for the Housing and Care of Animals Used in Scientific Procedures published by Her Majesty’s Stationery Office.
CLP Model of Sepsis
A rat model of sepsis was established through cecal ligation and puncture (CLP) as described previously.18 Rats were anesthetized with 2% pentobarbital, and a 1 to 2cm midline incision was made along the linea alba of the abdominal muscle to isolate and exteriorize the cecum. Fifty percent of the cecum was ligated with a 4–0 silk suture, and the cecum was punctured twice with a 16-gauge needle about 1 cm from the distal end. An approximately 1mm column of fecal material was expressed. The cecum was then replaced to the peritoneal cavity and the abdomen was closed. In the sham group, rats underwent the same procedure but were neither ligated nor punctured. In the following surgery, all rats received 0.4 mL/kg of prewarmed saline and were placed on a heating pad. All post-surgery rats were given free access to food and water.
Animal Grouping
Forty-eight rats were randomly and equally divided into three groups (n = 16 for each group):
- CLP+Cur group (Cur), which the rats received intraperitoneal injection with 100mg/kg curcumin (Sigma, America) immediately after surgery.
- CLP group (CLP), which the rats received intraperitoneal injection with vehicle (2.5%DMSO) immediately after surgery.
- Sham group (Sham), which the rats underwent identical surgical procedure but without any injection.
The curcumin solution applied in the experimental animals was freshly prepared. Immediately before the study, 500 mg curcumin powder was dissolved with 2.5mL of 100% dimethyl sulfoxide (DMSO, Sigma, America), and supplemented into 10 mL corn oil (Aladdin, China).
Twelve hours after the surgery, half of the animals in each group (n = 8) were sacrificed to collect serum from inferior vena cava and renal tissue under anesthesia.
Twenty-four hours after the surgery, the other half of the animals in each group (n = 8) were sacrificed with the same method.
Assessment of Kidney Function
Kidney injury was assessed by measuring the levels of serum creatinine (Scr), urea and serum cystatin C (CysC). Rats were euthanized at indicated time points under anesthetic. Blood samples were collected from the inferior vena cava; serum was separated by centrifugation at 3000 revolutions/min for 15 min and then stored at −20°C until use. Scr, urea, and SysC were detected by automatic biochemistry analyzer (cobas 8000, Roche, Switzerland) according to manufacturer instructions. The results were recorded and analyzed.
Enzyme-Linked Immunosorbent Assay
The method of obtaining serum samples is the same as above. Serum levels of Interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and Interleukin-10 (IL-10) were measured using sandwich enzyme-linked immunosorbent assay according to manufacturer instructions (4Abio, China). Read plate at 450nm, then draw the standard line and analyze the data. Absorbance values at 450 nm were read and recorded by ELISA reader (Multiskan GO, Thermo, USA).
Assessment of Hemodynamics
Color Doppler ultrasound (CDFI) was fundamental for identifying vascularization, which can intuitively display the size and distribution of blood flow. Power Doppler (PD) is inherently more sensitive and can display flow in situations where CDFI is ineffective as it can display tissue perfusion in highly vascular organs such as the kidneys.19 PD and CDFI were used to measure renal hemodynamics at 12h and 24h after CLP by a high-resolution ultrasonic apparatus (M9t, Mindray, China) equipped with a linear array transducer (4–12 MHz). Velocity scale, wall filter, and gain settings were held constant. Then the hemodynamics of kidney was assessed according to the size and distribution of blood flow in CDFI and PD images. M-mode echocardiography, an effective and efficient way to record the necessary multiple cardiac cycles,20 was performed to measure cardiac output (CO). CO was calculated as the product of stroke volume and heart rate.
Assessment of Renal Microcirculation
Contrast-Enhanced Ultrasound (CEUS), a method for detecting renal microcirculation, was performed by a high-resolution ultrasonic apparatus (M9t, Mindray, China) equipped with a linear array transducer (4–12 MHz) at a low mechanical index (MI = 0.098). A maximum longitudinal scanning section that included the entire kidney was selected. Under contrast-enhanced mode, an intravenous infusion of 0.16mL/kg of SonoVue (Bracco, Italy) was administered to the caudal vein via bolus injection, followed by an immediate flush of 1 mL saline solution. SonoVue is a sulfur hexafluoride-filled microbubble encapsulated by a flexible phospholipid shell. The 2.5μm microbubbles cannot cross the endothelium; thus, the agent remains solely in the intravascular compartment.21 As soon as the contrast agent injection began, the CEUS process and the timer on the machine were started; real-time dynamic images were digitally stored at least 180s. Image depth, focus, gain, and frame rate were optimized and held constant for all further measurements.
CEUS images were quantitatively analyzed using the included software. Three similar-sized regions of interest (ROI) were drawn at the renal cortex and medulla, at the same approximate location and a similar depth while excluding interlobar and arcuate arteries. Signal time intensity curves (TIC) and blood perfusion parameters were automatically generated. The results of three ROIs at the renal cortex and medulla were averaged to minimize heterogeneity. CEUS parameters, arrival time (AT), time to peak (TTP), ascending slope (AS), peak intensity (PI), half of descending time (DT/2), descending slope (DS) and area under curve (AUC) were recorded and analyzed.
Histopathology Assessments
Kidneys were harvested and transversely incised in the center, fixed in 10% formalin, embedded in paraffin and then sectioned at 3μm thickness for staining with periodic acid-Schiff stain. Stained sections were observed by light microscopy (BX53F, OLYMPUS, Japan) at a magnification of 400X.
Statistical Analyses
Statistical analysis was performed using SPSS 26 (IBM Corporation, USA) and GraphPad Prism 8 software (GraphPad Software, USA). Results were evaluated using Shapiro–Wilk normality tests. For normally distributed data, the difference between experimental groups was shown using one-way ANOVA. Kruskal–Wallis tests were applied for data that did not show normal distribution. Tukey’s test was used in multiple comparisons and p < 0.05 was considered as significant. Data showing normal are given as mean ± standard error, those without normal distribution are given as M (IQR).
Results
Effects of Curcumin on Renal Function
The diagnosis of AKI is currently based on an increasing serum creatinine concentration. When creatinine ≥1.5 times baseline, AKI occured.22 As shown in Figure 2 and Table 1, at 12h and 24h after CLP, serum levels of Scr, urea and CysC were found significantly increased in the CLP group compared with the sham group (P < 0.05), indicating the development of AKI. Apart from that, at 12h after CLP, curcumin had no effect on the increase in all indicators; while at 24h after CLP, curcumin administration dramatically reduced Scr and CysC levels compared with CLP alone, there was a significant difference between the two groups (P < 0.05).
 |
Table 1 Serum Indicators of Renal Function and Inflammation at 24h After Surgery |
Effects of Curcumin on Inflammatory Cytokines
After sepsis by CLP, there was an initial increase in cytokines within the first few hours.23 The concentration of IL-6, IL-10 and TNF-α are shown in Figure 3 and Table 1 in order to investigate the inflammation induced by sepsis. At 12h and 24h after CLP, serum IL-6, TNF-α, IL-10 were significantly increased compared with Sham group (P < 0.05). At 24h after CLP, there was a significant difference on IL-6 and TNF-α, between CLP and CLP+Cur groups (P < 0.05), which suggested that curcumin treatment had an effect on the decrease in serum inflammation cytokines.
Effects of Curcumin on Hemodynamics
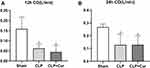
At 12h and 24h after CLP, CO was decreased compared with the sham group. These data indicate that curcumin treatment had no effect on the decrease of CO at either time point (Figures 4 and 5). As shown in Figure 6, the renal blood flow in CLP+Cur group is better than that in CLP group, the blood flow at 24h after CLP was better than that 12h after CLP. In the Sham group at 12h and 24h, segmental artery, interlobar artery and interlobular artery could be clearly displayed. In CLP and CLP+Cur group at 12h, the intensity of blood flow signal was weakened and the interlobular artery was not clear, which indicates the construction of renal blood flow. At 24h after surgery, the intensity of blood flow signal in CLP and CLP+Cur were increased compared with the two groups at 12h, and the signal of interlobular artery in CLP+Cur group at 24h was clearer than CLP group. These data indicate that curcumin has no effect on cardiac output, but can improve renal blood flow.
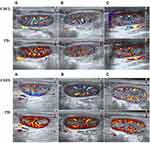
Effects of Curcumin on Renal Microcirculation
CEUS quantitative parameters reflect the perfusion sequence, time, intensity and distribution of renal microcirculation. 2–4 s after SonoVue bolus injection, renal blood vessels were enhanced, then the cortex and medulla developed; 1–2 min later, the medulla and cortex disappeared (Figure 7).
Renal microcirculation perfusion was significantly decreased compared with the sham group; curcumin treatment significantly increased perfusion of renal cortex at 24h after CLP. At 12h, cortical quantitative parameters of PI in CLP group were significantly decreased compared with the sham group; curcumin treatment significantly enhanced the level of PI compared with CLP. DS in CLP group was significantly decreased compared with the sham group, Curcumin treatment had no effect on the decrease of DS (Figure 8). At 24h, cortical levels of AUC, PI, DT/2 and DS in the CLP group were significantly decreased compared with the sham group, AS levels were significantly increased compared with the sham group. Curcumin treatment significantly increased levels of AUC, PI and DT/2 compared with the CLP group and had no effect on the increase of DS or the decrease of AS. Renal medullary levels of DT/2, DS, and AUC in CLP group were significantly decreased compared with the sham group. Curcumin treatment had no effect on the decrease of DT/2, DS, or AUC (Figure 9 and Table 2).
 |
Table 2 Quantitative Parameters of CEUS in Renal Cortex at 24h After Surgery |
Histopathological Results
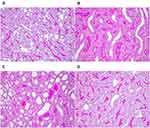
As seen in PAS-stained images, at 12 and 24h, kidney sections of the sham group revealed normal histological structure with malpighian bodies and tubular sections. Dilatation and unclear outline of renal tubules, shedding of brush margin, flattening and shedding of renal tubular epithelial cells were observed in kidney sections of the CLP group, parts of renal tubules were formed at 24h in CLP group. Images of CLP+Cur sections showed decreased damage compared to the CLP group at 24h (Figure 10).
Discussion
At present, the pathogenesis of S-AKI is not fully understood. Therefore, treatment remains reactive and nonspecific, and no available preventive therapies exist.3 In terms of mechanism, inflammatory responses and microcirculatory alterations were two major theories.3,24,25 Circulating toxins act on the endothelium, triggering a reduction in microcirculatory flow and interstitial infiltration of inflammatory cells;26 inflammatory mediators are released, which triggers tubular cell stress and injury.27 A dysregulated inflammatory response could be of great significance to organ dysfunction and poor outcome;3 microcirculatory alterations may play a key role in the development of organ injury.28 Previous studies showed that curcumin could protect renal function on AKI induced by ischemia/reperfusion (I/R);14 we studied the therapeutic potential of curcumin using a rat sepsis model induced by CLP to investigate whether curcumin could regulate inflammation response and microcirculatory flow in S-AKI.
The diagnosis of AKI is currently based on an increase in serum creatinine concentration and/or a decrease in urine output.22 At 12 and 24h after CLP, the level of Scr conforms to the diagnostic criteria of AKI. At 24h after CLP, treatment with curcumin reduced serum levels of Scr and CysC, suggest that curcumin can protect against renal dysregulation of S-AKI and has better effect than at 12h after CLP. Though having a larger dataset such as NAGL and KIM-1 would offer further insight into kidney injury, the increase in Scr and urea was sufficient evidence that kidney was injured.
After CLP, proinflammatory cytokines TNF-α and IL-6 were released during the first few hours and competed with anti-inflammatory factors such as IL-10, which is closely related to the occurrence of renal injury.23 In our study, at 24h after CLP, treatment with curcumin inhibited IL-6, TNF-α. Benes et al11 showed that in contrast to AKI-free animals, the development of septic AKI was preceded by early and remarkable inflammatory response (elevated TNF-a, IL-6), which is consistent with the findings in our CLP groups. In our study, curcumin did not reduce the serum level of IL-10. It has been proposed that endogenous IL-10 serves as a protective function in models of endotoxic shock;29 thus, curcumin does not destroy this protective effect.
Previous studies showed that in humans and large animal models, including sheep and pigs, CO was usually increased during the initial stage of sepsis.30–32 In our study, CO and renal flow observing by CDFI and Power Doppler in CLP and CLP+Cur group were decreased compared with the sham group. We considered there may be a difference in CO between large animals and rodents. We established a severe sepsis animal model by adjusting the number of perforations and the location of ligation, which leads to septic shock in experimental animals, resulting in a decrease in blood flow. In our study, curcumin treatment has no effect on the decrease of CO.
Contrast-enhanced ultrasound (CEUS) is a new technique which enables the observation of blood perfusion and can provide real-time, noninvasive and relative quantitative estimates of renal microvascular perfusion independent of kidney function.33 It has been tested in critically ill patients, including S-AKI. When S-AKI occurred, there were profound heterogeneous changes in microcirculatory flow.3 ROIs were selected to draw a time intensity curve (TIC) and perform quantitative analysis. Arrival time (AT), ascending slope (AS), peak intensity (PI), time to peak (TTP), DT/2, descending slope (DS), and area under the curve (AUC) are the key observational parameters used in renal perfusion studies. PI is intensity parameter, which represents the maximum intensity of the curve. AT, TTP, DT/2 are time parameters. AT is the time when contrast agents arrive; TTP represents the time to maximum enhancement; DT/2 is the time from injection until decay to the half peak of enhancement. AS, DS, and AUC are time-intensity parameters, AS is the wash-in slope of the contrast agents, and reflects the perfusion velocity of contrast agents. DS is the wash-out slope of the contrast agents, and reflects the clearance velocity of the contrast agents; AUC is the area under the TIC, which is proportional to the total volume of blood flow in the ROI. In our study, cortical quantitative parameters of PI, DS at 12h; cortical parameters of AS, PI, DT/2, DS, AUC and medullary parameters of DT/2, DS, AUC at 24h are significantly different between the sham group and CLP group. The decrease in PI and AUC indicates a decrease in blood flow volume; when microcirculatory flow is decreased, the speed of perfusion and clearance is increased, and the time and PI are decreased. The quantitative parameters result of our study is consistent with it, which suggests that the renal microcirculatory flow decreases during S-AKI in rats, and further, the condition at 24h was more serious than at 12h. Previous study observed the decrease in cortical renal perfusion during S-AKI,34 which was consistent with our study and demonstrated the rationality of our model. Multiple mechanisms may lead to microcirculatory alterations, such as endothelial injury and activation of the coagulation cascade.35 Treatment with curcumin significantly increased the cortical quantitative parameters of PI at 12h, and PI, DT/2, AUC at 24h, indicating that curcumin significantly increased the renal microcirculation perfusion in CLP rats. From the results of the histopathological sections, it was clear that after curcumin intervention, damage in renal tubular epithelial cells was improved.
Although the role of the curcumin is recognized in AKI, we have explored the protective effects on the circulation perfusion during treatment of S-AKI for the first time. In our study, we found that the curcumin has an excellent effect on anti-inflammation, which is consistent with previous studies.14,15 The mechanism of the anti-inflammation may be that curcumin reduced inflammatory processes via the semaphorin-plexin pathway and suppressed NF-κB mediating inflammation by activating JAK2/STAT3 signal pathway.14,15 When sepsis occurs, circulating toxins might act on the endothelium, which triggers reduced microcirculatory flow and interstitial infiltration of inflammatory cells.26 Curcumin is known to have an active biochemical impact on the endothelial protection, which may exert a protective effect on endothelium-based vascular regulation by regulating the intactness of microvascular endothelium,36 as a result, improving renal microcirculation. The evaluation of AKI needs to be based on the combination of serum renal function and hemodynamics.37 In our multiple evaluation of early S-AKI, curcumin exerted therapeutic effects in it.
Conclusion
In our study, we show that curcumin represents a new and promising effective treatment in S-AKI rat models. Treatment with curcumin ameliorates renal functions, improves both renal macro- and microcirculatory flow, reduces inflammatory response and prevents pathological changes in kidney. Further mechanism investigation is warranted, as curcumin may be a potentially effective treatment for S-AKI in humans.
Acknowledgments
This study was sponsored by the National Natural Science Foundation of China (NSFC) (Nos. 81971635 and 82001817).
Disclosure
The authors declare that there is no conflict of interest associated with this study.
References
1. Kirsi-Maija, Kaukonen, Michael, et al. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372(17):1629–1638.
2. Singbartl K, Kellum JA. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012;81(9):819–825. doi:10.1038/ki.2011.339
3. Peerapornratana S, Manrique-Caballero CL, Gómez H, et al. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96(5):1083–1099.
4. Bouchard J, Acharya A, Cerda J, et al. A prospective international multicenter study of AKI in the Intensive Care Unit. Clin J Am Soc Nephrol. 2015;10(8):1324–1331. doi:10.2215/CJN.04360514
5. Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411–1423. doi:10.1007/s00134-015-3934-7
6. Rivers EP, Coba V, Whitmill M. Early goal-directed therapy in severe sepsis and septic shock: a contemporary review of the literature. Curr Opin Anaesthesiol. 2008;21(2):128–140. doi:10.1097/ACO.0b013e3282f4db7a
7. Kellum JA, Chawla LS, Keener C, et al. The effects of alternative resuscitation strategies on acute kidney injury in patients with septic shock. Am J Respir Crit Care Med. 2016;193(3):281–287. doi:10.1164/rccm.201505-0995OC
8. Holthoff JH, Wang Z, Seely KA, et al. Resveratrol improves renal microcirculation, protects the tubular epithelium, and prolongs survival in a mouse model of sepsis-induced acute kidney injury. Kidney Int. 2012;81(4):370–378. doi:10.1038/ki.2011.347
9. Murugan R, Wen X, Shah N, et al. Plasma inflammatory and apoptosis markers are associated with dialysis dependence and death among critically ill patients receiving renal replacement therapy. Nephrol Dialysis Transplant. 2014;29(10):1854–1864. doi:10.1093/ndt/gfu051
10. Lundy DJ, Trzeciak S. Microcirculatory dysfunction in sepsis. Crit Care Nurs Clin North Am. 2011;25(4):721–731.
11. Benes J, Chvojka J, Sykora R, et al. Searching for mechanisms that matter in early septic acute kidney injury: an experimental study. Crit Care. 2011;15(5):R256. doi:10.1186/cc10517
12. Wang ZH, Deng LH, Chi CW, et al. A preclinical systematic review of curcumin for protecting the kidney with ischemia reperfusion injury. Oxid Med Cell Longev. 2020;2020(11):1–17. doi:10.1155/2020/8857906
13. Goel A, Aggarwal BB. Curcumin, the golden spice from Indian saffron is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer. 2010;62(7):919–930.
14. Kar F, Hacioglu C, Senturk H, et al. Curcumin and LOXblock-1 ameliorate ischemia-reperfusion induced inflammation and acute kidney injury by suppressing the semaphorin-plexin pathway. Life Sci. 2020;256:118016. doi:10.1016/j.lfs.2020.118016
15. Zhang J, Tang L, Li GS, Wang J. The anti-inflammatory effects of curcumin on renal ischemia-reperfusion injury in rats. Ren Fail. 2018;40(1):681–687.
16. Avila-Rojas SH, Tapia E, Briones-Herrera A, et al. Curcumin prevents potassium dichromate (K2Cr2O7)-induced renal hypoxia. Food Chem Toxicol. 2018;121:472–482. doi:10.1016/j.fct.2018.09.046
17. Wu T, Marakka TB, Ye Y, et al. Curcumin attenuates both acute and chronic immune nephritis. Int J Mol Sci. 2020;21(5):1745. doi:10.3390/ijms21051745
18. Hubbard WJ, Choudhry M, Schwacha MG, et al. Cecal ligation and puncture. Shock. 2005;24(Suppl 1):52–57. doi:10.1097/01.shk.0000191414.94461.7e
19. Murphy KJ, Rubin JM. Power Doppler: it’s a good thing. Semin Ultrasound CT MR. 1997;18(1):13–21. doi:10.1016/S0887-2171(97)90034-2
20. Feigenbaum H. Role of M-mode technique in today’s Echocardiography. J Am Soc Echocardiogr. 2010;23(3):240–257. doi:10.1016/j.echo.2010.01.015
21. Ayache B, de Jong N. WFUMB safety symposium on echo-contrast agents: nature and types of ultrasound contrast agents. Ultrasound Med Biol. 2007;33(2):187–196. doi:10.1016/j.ultrasmedbio.2006.07.008
22. Kellum JA, Lameire N, Aspelin P, et al. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2(1):1–38.
23. Anderberg SB, Luther T, Frithiof R. Physiological aspects of Toll-like receptor 4 activation in sepsis-induced acute kidney injury. Acta Physiol. 2017;219(3):573–588. doi:10.1111/apha.12798
24. Kellum JA, Prowle JR. Paradigms of acute kidney injury in the intensive care setting. Nat Rev Nephrol. 2018;14(4):217–230. doi:10.1038/nrneph.2017.184
25. Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380(9843):756–766. doi:10.1016/S0140-6736(11)61454-2
26. Verma SK, Molitoris BA. Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin Nephrol. 2015;35(1):96–107.
27. Gomez H, Ince C, Backer DD, et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41(1):3–11. doi:10.1097/SHK.0000000000000052
28. Post EH, Kellum JA, Bellomo R, et al. Renal perfusion in sepsis: from macro- to microcirculation. Kidney Int. 2017;91(1):45–60. doi:10.1016/j.kint.2016.07.032
29. Luo CJ, Zhang FJ, Zhang L, et al. Mesenchymal stem cells ameliorate sepsis-associated acute kidney injury in mice. Shock. 2014;41(2):123–129. doi:10.1097/SHK.0000000000000080
30. Maiden MJ, Otto S, Brealey JK, et al. Structure and function of the kidney in septic shock: a prospective controlled experimental study. Am J Respir Crit Care Med. 2016;194(6):692–700. doi:10.1164/rccm.201511-2285OC
31. Lankadeva YR, Kosaka J, Iguchi N, et al. Effects of fluid bolus therapy on renal perfusion, oxygenation, and function in early experimental septic kidney injury. Crit Care Med. 2018;47(1):e36–e43. doi:10.1097/CCM.0000000000003507
32. Kosaka J, Lankadeva YR, May CN, et al. Histopathology of septic acute kidney injury: a systematic review of experimental data. Crit Care Med. 2016;44(9):e897–903. doi:10.1097/CCM.0000000000001735
33. Wang L, Mohan C. Contrast-enhanced ultrasound: a promising method for renal microvascular perfusion evaluation. J Transl Int Med. 2016;4(3):104–108. doi:10.1515/jtim-2016-0033
34. Anatole H, Nicolas G, Samy F, et al. Acute kidney injury is associated with a decrease in cortical renal perfusion during septic shock. Crit Care. 2018;22(1):161. doi:10.1186/s13054-018-2067-0
35. Backer DD, Donadello K, Taccone FS, et al. Microcirculatory alterations: potential mechanisms and implications for therapy. Ann Intensive Care. 2011;1(1):1–8. doi:10.1186/2110-5820-1-27
36. Xia J, Wang H, Zhang QM, et al. The therapeutic effect of curcumin in male albino rats and its putative mechanisms on cerebral microvascular flow. Brain Res. 2016;1642:131–135. doi:10.1016/j.brainres.2016.03.022
37. Zhu JN, Zhang Y, Li XM, et al. Doppler-based renal resistive index for prediction of acute kidney injury in critically ill patients: a systematic review and meta-analysis. Adv Ultrasound Diagn Ther. 2021;03:183–196.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.