Back to Journals » Drug Design, Development and Therapy » Volume 16
The Temporal Topography of Central Serous Chorioretinopathy in the Chinchilla Rabbits Induced by Intravenous Injection of Adrenaline: An in vivo Study
Authors Yan W, Long P, Zhang L, Chen M, Zhang Z, Chen T
Received 11 July 2022
Accepted for publication 9 September 2022
Published 23 September 2022 Volume 2022:16 Pages 3275—3283
DOI https://doi.org/10.2147/DDDT.S381957
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Weiming Yan1,2 *, Pan Long3 *, Lei Zhang4 *, Meizhu Chen,1 Zuoming Zhang,2 Tao Chen2
1Department of Ophthalmology, The 900th Hospital of Joint Logistic Support Force, PLA (Clinical Medical College of Fujian Medical University, Dongfang Hospital Affiliated to Xiamen University), Fuzhou, People’s Republic of China; 2Department of Clinical Aerospace Medicine, The Air Force Medical University, Xi’an, People’s Republic of China; 3Department of Ophthalmology, The General Hospital of Western Theatre Command, PLA, Chengdu, People’s Republic of China; 4Department of Ophthalmology, The Shaanxi Eye Hospital, Xi’an People’s Hospital (Xi’an Fourth Hospital), Affiliated Guangren Hospital, School of Medicine, Xi’an Jiaotong University, Xi’an, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Meizhu Chen, Email [email protected] Tao Chen Email [email protected]
Purpose: To explore the temporal topography of the chorioretinopathy in an animal model of central serous chorioretinopathy (CSC) induced by intravenous injection of adrenalin in the Chinchilla rabbits.
Methods: Ten Chinchilla rabbits received a daily intravenous injection of adrenaline at 0.04 mg/kg for 8 weeks. Fluorescence fundus angiography (FFA) and electroretinogram (ERG) were performed every week afterwards to see whether there was fluorescence leakage in the fundus and to evaluate the retinal function. Indocyanine green angiography (ICGA) and optical coherence tomography (OCT) were also conducted to detect the change of choroidal vessels. Finally, the eyes of the rabbits were enucleated to make the retinal sections for histological examination with hematoxylin–eosin (HE) staining.
Results: Within 8 weeks of the adrenalin administration, 7 out of the 10 rabbits showed different degrees of fluorescence leakage on FFA. The leakage was more obvious during 2– 3 weeks after the adrenalin administration. With the progress of disease, the leakage subsided gradually and a scar-like lesion formed. ICGA revealed the local choroidal ischemia and the dilated choroidal vessels. An obvious detachment of retina and an increased thickness of the choroid were found on OCT, which was most obvious 2 weeks after the adrenalin administration (P< 0.01). ERG revealed no obvious decline of the b-wave amplitude before and after the adrenalin administration (P> 0.05). A circumscribed retinal detachment, the depigmentation of retinal pigment epithelium and enlarged choroidal vessels were shown by the histological examination.
Conclusion: The temporal topography of the chorioretinopathy in the Chinchilla rabbits by intravenous injection of adrenaline somewhat mimicked that of the human CSC, which could enhance its application in the exploration for the pathogenesis and the therapeutic measures for human CSC.
Keywords: central serous chorioretinopathy, adrenaline, Chinchilla rabbits, choroid
Introduction
Central serous chorioretinopathy (CSC) is a vision-threatening ocular disease, leading to 5–10% vision loss caused by the foveal thinning and intraretinal cysts.1 CSC affects about 1 out of 10,000 individuals across the world. However, its recurrence and chronic evolution occur in 30–50% of all the cases.2 Dilation and leakage of choroidal vessels underneath the retina lead to the subretinal fluid accumulation and the retinal detachment in CSC.3 The reasons for CSC may be related to the emotional and mental stress, infections, injuries and other factors. As the glucocorticoids could induce and aggravate CSC, the relationship between adrenalin and CSC has also been explored in human.4–6
Recently, there has been an upsurge of interests in unraveling the pathological mechanism underlying the adrenalin-induced CSC in rabbits.7 Previously, we have successfully induced CSC in the Chinchilla rabbits8 by the similar methods as other works.9,10 Although the morphological alterations of this CSC model have been described, several basic issues remain to be addressed. For example, it was only roughly pointed out that the adrenalin-administrated retina showed the time-dependent pathological changes. Besides, the detailed retinal function pattern of this degeneration remains poorly characterized. The temporal tomography of the course of this CSC model has also not been fully clarified. The ambiguities would perplex the standardization of the constructive parameters and might act as impediments to a broader acceptance of this model to study human CSC.11
In the present study, we continued to systemically explore the temporal topography of CSC in the adrenalin-administered Chinchilla rabbits. Our work attempted to unveil more details about CSC in this animal model, and to provide the experimental basis for the subsequent explorations of the pathogenesis, prevention and control measures for human CSC.
Materials and Methods
Animals and the Adrenalin Administration
The Chinchilla rabbits (n=10, male, weight 2.0–3.0 kg) were obtained from the Laboratory Animal Centre of the Air Force Military Medical University (#2014270138S, Xi’an, China). Rabbits were given water and food alone ad libitum for 24 hours before the experiments. All animals were kept on a 12 h light/dark cycle, in a temperature of 20 ± 2 °C. All animal handles were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research, and were approved by the Animal Care and Use Committee of the 900th Hospital of Joint Logistic Support Force, PLA and the Air Force Military Medical University with an approval number of #P20191021-8.
Adrenalin (1 mL/mg, #H12020526, Jinyao pharmaceutical Co., LTD, Tianjin, China) was injected intravenously via the auricle vein once a day at about the same time of day for 8 weeks at an dose of 0.04mg/mL as previously reported.8 Fluorescence fundus angiography (FFA) and electroretinogram (ERG) were performed every week afterwards to see whether there was fluorescence leakage in the fundus and to evaluate retinal function. Besides, the indocyanine green angiography (ICGA), optical coherence tomography (OCT) and histological examination were conducted to deeply observe the characteristics of the retina and choroidal structure of the rabbits.
Fundus Fluorescence Angiography (FFA) and Indocyanine Green Angiography (ICGA)
The rabbits were fixed and placed on a platform, with the pupils dilated. The eyes were kept open with an eyelid opener. FFA and ICGA were performed almost the same way as what was done in clinic. In detail, the Fluorescein Sodium Injection (#H4402340, Guangzhou Baiyunshan Mingxing Pharmaceutical Co., Ltd., Guangzhou, China) and the Indocyanine Green for Injection (#H20073073, Dandong Pharmaceutical Co., Ltd., Liaoning, China) were injected into the auricle vein of the rabbits. Specifically, successive confocal in vivo angiography images were taken both at the early phase and the late phase (30 minutes) after the injection of the Fluorescein Sodium Injection and the Indocyanine Green for Injection. The simultaneous images of the FFA and ICGA were captured and analyzed using the Heidelberg Retina Angiography 2 (HRA2, Germany).
Optical Coherence Tomography (OCT) Examination
The rabbits were fixed and placed on a platform, with the pupils dilated. The eyes were kept open with an eyelid opener. The OCT examination was performed as previously reported.12 The cornea was covered with the Medical Sodium Hyaluronate Gel (#20153221764, Bausch & Lomb Freda, Jinan, China) and attached to the camera lens of the OCT Microscope Imaging System (OCT, OptoProbe, Canada) to capture the OCT images. In greater detail, the OCT images were taken with the optic papilla centered on the corresponding box in the screen by altering the position and angle of the rabbits’ eyes. The thickness of the choroidal vessels beneath the retina on the OCT images was measured and analyzed. After the examination, the Medical Sodium Hyaluronate Gel was rinsed off the cornea with the normal saline, and the Levofloxacin eye drops (#H20203122, Zhongshan Wanhan Pharmaceutical Co., Ltd, Guangzhou, China) were used three times a day for 3 days to avoid ocular infection.
Electroretinogram (ERG) Recording
ERG recording was carried out according to the method previously described13 with some adjustments. After the overnight dark adaption, the rabbits’ pupils were dilated with 0.5% Tropicamide-phenylephrine ophthalmic solution (#H20055546, Shenyang Xingji Co., Ltd, Shenyang, China). After the topical anesthesia with Oxybuprocaine (#J20100128, Shentian Pharmaceutical Co., Ltd, Japan), the cornea-contact electrode was placed on the center of the cornea of the rabbits. The reference electrode was inserted beneath the skin inside the inner pinna around the tested eye of the rabbits, while the ground electrode was inserted beneath the skin between the two ears. All operations were conducted under a dim red light to maximize the retinal sensitivity. A full-field (Ganzfeld) stimulation and a computer system (RETI port, Roland Consult GmbH, Brandenburg, Germany) were applied to record the ERG according to the ISCEV guidelines.14 Specifically, the stimulus was a brief white flash (3.0 cd·s/m2). The acquired signals were amplified and filtered to a bandpass of 1–300 Hz. A total of three Dark-adapted 3.0 ERG responses were recorded and averaged for the b-wave amplitude analysis, which is a major indicator of retinal function.
Histological Examination
After all the above examinations, the eyes of rabbits were enucleated after injecting a lethal dose of pentobarbital (Sigma-Aldrich, US). The eyes were fixed in 4% paraformaldehyde (Mediatech, Inc., Herndon, US) for 2 hours and then dissected to remove the front ocular section (the cornea and the lens). The tissues were then fixed for another 24 hours. The areas of lesions in each eye were marked on the sclera according to the lesions of the FFA and ICGA images. Three histological sections that crossed the lesions were attained and stained with hematoxylin and eosin. The histological images were then taken and analyzed through a digital imaging system (DP71, Olympus, Japan).
Statistical Analysis
All data attained in our research were expressed as mean ± standard error (SE) and analyzed by the one-way analysis of variance (ANOVA) using the SPSS software (version 16.0, Chicago, US). The Bonferroni test was applied for multiple comparisons. A p value of less than 0.05 was considered statistically significant.
Results
The Features of FFA in the Adrenalin-Administered Rabbits
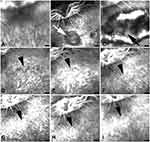
No fluorescence leakage spots were found under the fundus of the rabbits on the FFA examination before the adrenalin administration (Figure 1A). One week after the adrenalin injection, the FFA revealed multiple fluorescence leakage spots with a cloud-like shape inferior to the optic papilla (Figure 1B). At the second week, the lesion was enlarged with a large scale of mushroom-like leakage (Figure 1C). As time went on, the leakage subsided gradually and a scar-like lesion was then formed, which continued to be presented afterwards (Figure 1D–I). No other abnormalities were found. In total, 7 out of the 10 rabbits showed different degrees of fluorescence leakage on FFA within 8 weeks of the adrenalin administration.
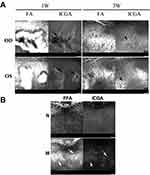
The Features of ICGA in the Adrenalin-Administered Rabbit
Typically, there was an obvious leakage of the indocyanine green on the ICGA in the corresponding area of the mushroom-like leakage of FFA after 1 week of the adrenalin injection. Localized choroidal ischemia and dilated choroidal vessels in the surrounding areas were found on the ICGA images (Figure 2A-1w). As the leakage of fluorescence on the FFA images was subsided 2 weeks later, a scar-like lesion left presented correspondingly on the ICGA images (Figure 2A-3w). The scar-like lesion on the ICGA continued to exist even after 30 min of the indocyanine green injection (Figure 2B).
The Features of OCT in the Adrenalin-Administered Rabbits
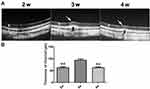
Typically, the choriocapillaris and choroidal vessels beneath the retina were dilated as showed on the OCT images from the adrenalin-administered rabbits, especially at the third week after the adrenalin injection (Figure 3A). Besides, the detachment of retina was also found 3 and 4 weeks after the adrenalin injection (Figure 3A). The thickness of choroid beneath the retina was increased especially 3 weeks after the adrenalin administration, compared to other timepoints (P<0.01) (Figure 3B).
The Features of ERR in the Adrenalin-Administered Rabbits
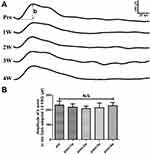
Typical Dark-adapted 3.0 ERG waveforms were found in the rabbits both before and after the adrenalin administration on the ERG examination (Figure 4A). The amplitude of the b-wave seemed to be slightly decreased after 2 weeks of the adrenalin injection. However, no statistical differences of the amplitude of the b-wave were found among all the timepoints (P > 0.05). (Figure 4B)
The Features of the Chorioretinopathy Histology in the Adrenalin-Administered Rabbits
Compared to that of the normal retina histology (Figure 5A), there was an obvious detachment of retina found in retinal sections from the rabbits with the adrenalin administration (Figure 5B–D). The structure of outer retina, mainly the outer segment of the photoreceptors, around the detached retina was disorganized. The degeneration of the retinal pigment epithelium (RPE) and an enlarged paracellular space between the RPE were also found. Furthermore, the choroidal vessels beneath the retina on the histological sections were dilated to some extent (Figure 5).
Discussion
The pathological mechanisms underlying the CSC are still not fully understood.15 Systemic and/or regional application of the glucocorticoids are the well-known risk factors for CSC, as the increased endogenous cortisol levels.16 It has been argued that the adult males with the “Type A” personality profile tend to be more affected by CSC.17 Psychological stress has also been reported as a contributing factor for CSC. In this sense, an increased level of adrenalin during stress might also be the direct cause of CSC.6 However, the role of adrenalin in the pathogenesis of CSC remains unknown.
Our study showed that the leakage of fluorescence on the FFA images was found after the adrenalin injection. The lesion on the FFA indicated the damage of the RPE conjunctions, which was also confirmed in our histological examination of retinal sections. The integrity of the retinal vessels on the FFA indicated that adrenalin might specifically affect the choroidal vessels. Our result was in consistency with previous reports supporting the theory that the elevated adrenalin level might contribute to the development of CSC. Yoshioka et al9 also successfully induced CSC shown by the FFA and histological examination in the pigmented rabbits via intravenous injection of adrenalin as we did. The complex of adrenaline, noradrenaline, histamine, insulin, catecholamines and glucocorticoids could also induce CSC in rabbits as shown in the work of Nagayoshi et al.18 Besides, the intravitreal injection of the glucocorticoid and/or corticosterone in the rat or mice eyes could also induce the choroid enlargement.19 These results consistently suggested that the stress plays an important role in the development of CSC.20
ICGA is the commonly used method for detecting the structure of the choroidal vessels, which could help to figure out the pathological changes of the choroidal vessels during CSC.21 The lesion of ICGA found in our study indicated that the choroidal vessels were damaged or affected by the adrenalin administration. Our histological results also revealed the enlargement of the choroidal vessels underneath those degenerated RPE. Besides, our OCT also revealed the choroidal vasculopathy shown by the substantial thickening of the choroid during CSC. In human CSC, the occurrence of choroidal thickening could represent as a precursor for the retinal detachment.11 It is gaining a common recognition that analyzing the thickness of the choroid may contribute to the diagnosis, treatment and prognosis of CSC.22
In addition to the changes found on the angiography and OCT, the depigmentation of RPE and the alteration of the paracellular permeability of the tight junctions were also revealed in our study. These changes were also pointed out in CSC by FFA and histological examination in vivo and in vitro.20 In fact, intravenous adrenalin injection may affect the choroidal venules as well as the choroidal arterioles and the choriocapillaris. This would result in the wide opening of their endothelial intercellular spaces. The histopathological changes may cause the damage of RPE due to the leakage of blood components from the choroid. The subsequent pathology could lead to the induction of CSC, which was in consistency with previous studies.7 However, as we previously reported, no chorioretinopathy was developed in albino rabbits even by repeated injections of a larger dose of adrenalin.8 The reasons might lie in the difference of RPE between the albino rabbits and the pigmented ones, which indicated that the effect of adrenalin on the choroidal vessels alone might not be the only cause for CSC. Instead, the original damage of adrenalin on RPE might also play a vital role in development of CSC.23,24 This idea might be of help to further clarify the original pathogenesis of CSC and the following sequence of events.
Full-field electroretinogram (ffERG or simply ERG) reflects the function of the whole retina.14 Usually, the localized lesion of CSC would not be large enough to cause the change of whole retinal function reflected by ERG.25 Our ERG data confirmed that no obvious change of the whole retinal function occurred during the chorioretinopathy in the rabbits induced by the adrenalin injection. Localized retinal dysfunction during CSC in human is commonly measured via the multifocal electroretinogram (mfERG).26,27 The mfERG could record the focal response of every stimuli ted element in the packing matrix and reveal the subtle dysfunction especially in the posterior retina. Patients with CSC usually showed reduced amplitudes and/or delayed peak times, especially around the macular regions of the mfERG data. The mfERG changes are closely associated with the apparent retinal morphological lesions in CSC.28 As the rabbits could not fix at a stable direction as human does, the mfERG of the rabbits with CSC-like chorioretinopathy could not be conducted smoothly and accurately to evaluate the local retinal function.
Conclusion
Our study revealed that the chorioretinopathy in the Chinchilla rabbits induced by the intravenous injection of adrenaline somewhat mimicked the morphological and functional features of the human CSC. This CSC animal model might be of help in the exploration of the pathogenesis and the therapeutic measures for the human CSC.
Data Sharing Statement
The authors declared the data were available on request by contacting the author of Weiming Yan (Email: [email protected]).
Ethics Approval and Consent to Participate
The study was approved by the Animal Care and Use Committee of the 900th Hospital of Joint Logistic Support Force, PLA and the Air Force Military Medical University with an approval number of #P20191021-8. We confirmed that the study was reported in accordance with ARRIVE guidelines.
Funding
This work was supported by the grants from the Pilot projects of Fujian province (Grant number: 2020Y0076, 2020J05282) and the Postdoctoral Science Foundation of the Fuzhou General Hospital in the Nanjing Military Area Command (Grant number: 48678).
Disclosure
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
1. Lotery A. Can we classify central serous chorioretinopathy better? Yes we can. Eye. 2022;36(3):487. doi:10.1038/s41433-021-01786-6
2. Fusi-Rubiano W, Saedon H, Patel V, et al. Oral medications for central serous chorioretinopathy: a literature review. Eye. 2020;34(5):809–824. doi:10.1038/s41433-019-0568-y
3. Pujari A, Surve A, Azad SV, et al. Optical coherence tomography angiography in central serous chorioretinopathy: the current clinical role and future perspectives. Surv Ophthalmol. 2022;67(1):68–82. doi:10.1016/j.survophthal.2021.05.003
4. Sun J, Tan J, Wang Z, et al. Effect of catecholamine on central serous chorioretinopathy. J Huazhong Univ Sci Technolog Med Sci. 2003;23(3):313–316. doi:10.1007/BF02829525
5. Garg SP, Dada T, Talwar D, et al. Endogenous cortisol profile in patients with central serous chorioretinopathy. Br J Ophthalmol. 1997;81(11):962–964. doi:10.1136/bjo.81.11.962
6. Liu B, Deng T, Zhang J. Risk factors for central serous chorioretinopathy: a systematic review and meta-analysis. Retina. 2016;36(1):9–19. doi:10.1097/IAE.0000000000000837
7. Iwaki Y, Sugita A, Mochizuki M, et al. [Histopathologic study of experimental chorioretinopathy induced in pigmented rabbits by intravenous adrenalin injection]. Nippon Ganka Gakkai Zasshi. 1992;96(1):74–84. Japanese.
8. Yan W, Chen T, Ren Z, et al. 肾上腺素对不同品系兔脉络膜视网膜病变的诱导作用 [Induction of chorioretinopathy by adrenaline injection in different strains of rabbits]. Chin J Exp Ophthalmol Chinese. 2018;36(1):23–27.
9. Yoshioka H. 中枢性漿液性脈絡網膜症の病因 [The etiology of central serous chorioretinopathy]. Nippon Ganka Gakkai Zasshi. 1991;95(12):1181–1195. Japanese.
10. Piccolino FC. Central serous chorioretinopathy: some considerations on the pathogenesis. Ophthalmologica. 1981;182(4):204–210. doi:10.1159/000309115
11. Daruich A, Matet A, Dirani A, et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82–118. doi:10.1016/j.preteyeres.2015.05.003
12. Yan W, Long P, Chen T, et al. A natural occurring mouse model with Adgrv1 mutation of usher syndrome 2C and characterization of its recombinant inbred strains. Cell Physiol Biochem. 2018;47(5):1883–1897. doi:10.1159/000491068
13. Yan W, Long P, Wei D, et al. Protection of retinal function and morphology in MNU-induced retinitis pigmentosa rats by ALDH2: an in-vivo study. BMC Ophthalmol. 2020;20(1):55. doi:10.1186/s12886-020-1330-8
14. Robson AG, Frishman LJ, Grigg J, et al. ISCEV standard for full-field clinical electroretinography (2022 update). Doc Ophthalmol. 2022;144(3):165–177. doi:10.1007/s10633-022-09872-0
15. Liew G, Quin G, Gillies M, et al. Central serous chorioretinopathy: a review of epidemiology and pathophysiology. Clin Exp Ophthalmol. 2013;41(2):201–214. doi:10.1111/j.1442-9071.2012.02848.x
16. Nicholson BP, Atchison E, Idris AA, et al. Central serous chorioretinopathy and glucocorticoids: an update on evidence for association. Surv Ophthalmol. 2018;63(1):1–8. doi:10.1016/j.survophthal.2017.06.008
17. Genovese G, Meduri A, Muscatello M, et al. Central serous chorioretinopathy and personality characteristics: a systematic review of scientific evidence over the last 10 years (2010 to 2020). Medicina. 2021;57(6):628. doi:10.3390/medicina57060628
18. Nagayoshi K. アドレナリン静注による脈絡膜網膜症の実験的研究 [Experimental study of choroidoretinopathy by intravenous injection of Adrenaline]. Nippon Ganka Gakkai Zasshi. 1971;75(8):1720–1727. Japanese.
19. Zhao M, Celerier I, Bousquet E, et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012;122(7):2672–2679. doi:10.1172/JCI61427
20. Sibayan SA, Kobuch K, Spiegel D, et al. Epinephrine, but not dexamethasone, induces apoptosis in retinal pigment epithelium cells in vitro: possible implications on the pathogenesis of central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2000;238(6):515–519. doi:10.1007/PL00007893
21. Yoshioka H, Katsume Y. Experimental central serous chorioretinopathy. III: ultrastructural findings. Jpn J Ophthalmol. 1982;26(4):397–409.
22. Kaye R, Chandra S, Sheth J, et al. Central serous chorioretinopathy: an update on risk factors, pathophysiology and imaging modalities. Prog Retin Eye Res. 2020;79:100865. doi:10.1016/j.preteyeres.2020.100865
23. Su Y, Zhang X, Gan Y, et al. Characteristics and associated factors of flat irregular pigment epithelial detachment with choroidal neovascularization in chronic central serous chorioretinopathy. Front Med. 2021;8:687023. doi:10.3389/fmed.2021.687023
24. Gunna NT, Rani PK, Rani PK. Bullous central serous chorioretinopathy and retinal pigment epithelium sequelae postblunt trauma. BMJ Case Rep. 2020;13(9):e235882. doi:10.1136/bcr-2020-235882
25. Moschos M, Brouzas D, Koutsandrea C, et al. Assessment of central serous chorioretinopathy by optical coherence tomography and multifocal electroretinography. Ophthalmologica. 2007;221(5):292–298. doi:10.1159/000104758
26. Wittstrom E, Ekvall S, Schatz P, et al. Morphological and functional changes in multifocal vitelliform retinopathy and biallelic mutations in BEST1. Ophthalmic Genet. 2011;32(2):83–96. doi:10.3109/13816810.2010.535890
27. Hoffmann MB, Bach M, Kondo M, et al. ISCEV standard for clinical multifocal electroretinography (mfERG) (2021 update). Doc Ophthalmol. 2021;142(1):5–16. doi:10.1007/s10633-020-09812-w
28. Vajaranant TS, Szlyk JP, Fishman GA, et al. Localized retinal dysfunction in central serous chorioretinopathy as measured using the multifocal electroretinogram. Ophthalmology. 2002;109(7):1243–1250. doi:10.1016/S0161-6420(02)01065-5
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.