Back to Journals » Risk Management and Healthcare Policy » Volume 9
The SWEET SPOTS study: a real-world interpretation of the 2012 American Diabetes Association Position Statement regarding individualized A1C targets
Authors Bieszk N, Grabner M , Wei W, Bonine NG, Stephenson JJ
Received 8 July 2016
Accepted for publication 20 September 2016
Published 8 November 2016 Volume 2016:9 Pages 243—251
DOI https://doi.org/10.2147/RMHP.S116800
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Frank Papatheofanis
Nella Bieszk,1 Michael Grabner,2 Wenhui Wei,1 Nicole G Bonine,2 Judith J Stephenson,2
1Sanofi US, Inc., Bridgewater, NJ, 2HealthCore, Inc., Wilmington, DE, USA
Objective: To evaluate awareness of the 2012 American Diabetes Association (ADA) Position Statement among physicians and assess its effects on patient-centered glycated hemoglobin (A1C) goals in the management of type 2 diabetes (T2D).
Research design and methods: The Summarizing Real-World Individualized TrEatmEnT GoalS and Potential SuppOrT Systems in Type 2 Diabetes (SWEET SPOTS) study used the HealthCore claims database to identify T2D patients, stratified by risk, and their treating physicians to assess primary care physician and endocrinologist awareness of the 2012 ADA Position Statement. Physicians completed online surveys on A1C targets before and after receiving an educational intervention to review the position statement.
Results: Of 125 responding physicians (mean age 50.3 years, 12.8% endocrinologists) who were linked to 125 patient profiles (mean age 56.9 years, 42% female, mean A1C 7.2%), 92% were at least somewhat aware of the position statement prior to the intervention and 59% believed that the statement would impact how they set A1C targets. The educational intervention resulted in mostly less stringent goal setting for both lower and higher risk patients, but changes were not significant. The proportion of physician-assigned A1C targets within ADA-recommended ranges increased from 56% to 66% post-intervention (P<0.0001).
Conclusion: Physicians treating T2D are aware of the 2012 ADA Position Statement and believe that it may influence treatment goals. While patient-specific A1C targets were not significantly impacted, physicians indicated that they would make targets more or less stringent for lower and higher risk patients, respectively, across their practice. Further research into optimizing physician education regarding individualized A1C targets is warranted.
Keywords: type 2 diabetes, HealthCore claims database, patient-centered, individualized A1C targets
Introduction
The 2012 and 2015 American Diabetes Association (ADA) Position Statements on the standards of medical care in diabetes marked a significant change in the management of glycated hemoglobin (A1C) levels in adult patients with type 2 diabetes (T2D).1,2 Compared with previous position statements, the 2012 ADA Position Statement emphasized the individualization of A1C targets and suggested that more stringent targets (A1C 6.0%–6.5%) are needed for patients with a short duration of diabetes, long life expectancy, and no significant cardiovascular disease. Conversely, less stringent targets (A1C 7.5%–8.0%) are warranted for patients with a history of severe hypoglycemia, limited life expectancy, advanced micro- or macrovascular complications, and extensive comorbid conditions, and those with long-standing diabetes in whom the target A1C is difficult to attain despite diabetes self-management education, appropriate glucose monitoring, and effective doses of multiple glucose-lowering agents, including insulin. In addition to the ADA 2012 Position Statement, Ismail-Beigi et al3 proposed a specific algorithm for setting individualized A1C targets in patients with T2D. This approach to individualized therapy used 9 risk segments that took into account patient age, duration of T2D, and presence or absence and extent of micro- and macrovascular complications.
Understanding the real-world interpretation and implementation of individualized A1C targets and the impact that this interpretation has on treatment decisions is crucial for health care industries that are developing programs for T2D disease management, education, and adherence. This is particularly the case for patients with T2D who do not achieve optimal glucose control. Barriers to the implementation of individualized patient care – particularly the use of insulin to optimize glycemic control – include obstacles related to patients, physicians, and the health care system.4 Understanding physician awareness and beliefs and the path taken by physicians to access and become familiar with guidelines such as the ADA Position Statement 2012 on the way to developing such awareness are important starting points for recognizing and addressing physician-related barriers to the implementation of individualized patient care.
The SWEET SPOTS (Summarizing Real-World Individualized TrEatmEnT GoalS and Potential SuppOrT Systems in Type 2 Diabetes) study was designed to determine physicians’ real-world interpretation of the 2012 ADA Position Statement regarding individualized A1C targets for patients with T2D. A survey was used to compare the A1C targets set by physicians for specific patients before and after the physicians participated in an educational intervention that reviewed the 2012 ADA Position Statement. The objectives of the study were to determine physician awareness of the 2012 ADA Position Statement, the routes by which physicians learned about the 2012 ADA Position Statement, physician perceptions of the impact that the 2012 ADA Position Statement has on A1C target setting, and patient A1C targets before and after the physician underwent an educational intervention.
Patients and methods
Study design
The SWEET SPOTS study utilized a unique pre–post study design to examine the impact of the 2012 ADA Position Statement on a real-world sample of physicians. An Internet-based study consisting of pre- and post-intervention surveys and an educational intervention was conducted among physicians with specialties in primary care (i.e., internal medicine, family/general practice, or geriatric medicine) and endocrinology, who were managing the care of patients with T2D identified from US administrative claims data in the HealthCore Integrated Research Database (HIRD).
Physicians were faxed an invitation to participate in an Internet-based study of physicians’ interpretations of the 2012 ADA Position Statement regarding T2D care. The invitation indicated that they were selected because of their experiences with diagnosing, treating, and managing patients with T2D. They were told that study participation consisted of completing a 15-minute pre-intervention survey, viewing a 5-minute educational intervention, and completing a 10-minute post-intervention survey and that the medical record of the patient referenced in the survey was required in order to complete the pre- and post-intervention surveys. The invitation also contained the link to the Internet-based survey. The target sample size was 180 completed physician surveys. All survey-related materials were approved by the New England Institutional Review Board. The surveys were conducted between November 2013 and September 2014. Patient written consent was not required since this was a physician survey. Physicians were invited to participate in the survey and they provided consent by clicking on the link and answering the questions. Physicians received an honorarium of US$200 for completion of the entire survey.
Patient selection strategy
Patients with commercial or Medicare Advantage health plans and physician office visits for T2D between October 31, 2012, and October 31, 2013, were identified from claims submitted to the HIRD. More specifically, patients with ≥1 inpatient or emergency department medical claim, ≥2 outpatient or office visit claims ≥30 days apart for T2D (International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes: 250.x0 or 250.x2), or ≥1 pharmacy claim for non-insulin and non-glucagon-like peptide (GLP)-1 receptor agonist anti-hyperglycemic medications were selected, and an index date was set that represented the patient’s most recent qualifying encounter with their selected treating physician.
Provider selection strategy
For each identified T2D patient, a corresponding treating physician was identified based on the provider information available from a longitudinal review of the patient’s medical and pharmacy claims data. In order to determine the primary provider of diabetes care, a hierarchical approach was applied among those physicians with primary care or endocrinology specialties. Thus, physicians identified from patients’ medical claims for ≥1 T2D office visit on different dates and ≥1 pharmacy claim for anti-hyperglycemic medications were selected first, followed by physicians with ≥1 T2D office visit (selecting the physician with the most office visits if there are multiple eligible physicians) and no pharmacy claims. Among patients without any office visits in the previous year, the prescribing physician identified as being responsible for the most recent pharmacy claim for anti-hyperglycemic medication was selected. If >1 physician was eligible at any step, information was retained for all, and 1 provider was randomly selected to be the treating physician for that patient. If multiple patients were connected to the same physician, a single patient was chosen based on a priority system involving claims information such as length of enrollment and availability of linked A1C results data.
Patient profiling and risk segmentation
Following patient and provider selection, a claims-based profile was created for each patient that was used to assign patients to 1 of 9 risk segments and was shared with their physician during the survey. The patients were assigned to the 9 risk segments according to various combinations of age, duration of T2D, and presence or absence and extent of micro- and macrovascular complications, according to the metrics of Ismail-Beigi et al (Table 1).3
Educational intervention
The educational intervention consisted of a web-based slide show in a media-friendly format that took ~5 minutes to complete. All participating physicians were required to watch the intervention in its entirety after completing the pre-intervention survey and before completing the post-intervention survey. The slide show used ADA materials concerning the 2012 ADA Position Statement.5
Pre- and post-intervention surveys
During the pre-intervention survey, the physician was asked to obtain the medical chart of their identified patient in order to verify and provide updated information regarding the patient’s profile consisting of current age, gender, duration of T2D, the most recent A1C level, and body mass index. The profile information was shown as a reference on every screen of the pre- and post-intervention surveys.
Physicians were then asked to provide the A1C target for that specific patient and to identify patient and clinical characteristics most relevant to their decision making when setting the A1C goal and deciding on insulin and GLP-1 receptor agonist therapy for that specific patient. Finally, physicians answered a set of questions about themselves and their practice that included their gender, current age, years in practice, board certification, type of practice setting, number of T2D patients seen in a typical week, and whether they were aware of the 2012 ADA Position Statement.
In the post-intervention survey, the physician was asked again about the A1C target for their specific patient; if the A1C target was changed compared with the pre-intervention survey, the physician was asked about patient and clinical characteristics most relevant to their decision making in altering the A1C goal for that specific patient. Physicians were also asked to describe how A1C goal setting may change for patients in general across different risk segments in their practice.
Statistical analyses
Physician survey responses and the demographic and clinical characteristics of their T2D patients were described using mean and standard deviation (SD), medians, and absolute and relative frequencies for continuous and categorical variables. Changes in physician-assigned A1C goals before and after the educational intervention were assessed using non-parametric Wilcoxon signed rank sum tests. The proportion of A1C targets that were within the ranges recommended by Ismail-Beigi et al3 was calculated both pre- and post-intervention; changes in these proportions between the pre- and post-intervention surveys were assessed using a goodness-of-fit test. Statistical significance was defined as P<0.05. All analyses were performed using SAS® version 9.2 (SAS Institute, Cary, NC, USA).
Results
Physician characteristics and patient demographic and clinical characteristics
Of >8,500 physicians who met eligibility criteria and were faxed invitations, 125 completed the study (mean age 50.3 years; 21.6% female). Of these, 109 (87.2%) were primary care physicians (PCPs) and 16 (12.8%) were endocrinologists; 50 (40.0%) were in a solo practice, 73 (58.4%) were part of a group practice, and 2 (1.6%) had another arrangement. Duration of practice among physicians varied, with 21 (16.8%) physicians having practiced for <10 years, 53 (42.4%) for 10–20 years, and 51 (40.8%) for >20 years. The 125 physicians completed the pre- and post-intervention surveys for 125 of their patients with T2D (mean age 56.9 years; 41.6% female; mean A1C 7.2%).
The number of completed surveys was not distributed equally across risk segments, largely because fewer patients were available in higher risk segments requiring ages of >65 years. Therefore, in order to preserve statistical power, a post hoc analysis was performed in which the 9 risk segments were collapsed into 5 risk cohorts based on common A1C targets (Table 1).3 Patient demographics and clinical characteristics are presented overall and by risk cohort in Table 2.
Physician awareness of the 2012 ADA Position Statement
Before the educational intervention, 92% of all physicians reported they had some (83 of 125) or significant (32 of 125) awareness of the ADA 2012 Position Statement (Figure 1), including 91% of PCPs and 100% of endocrinologists. No awareness of the 2012 ADA Position Statement was reported by 8% of physicians (10 of 125).
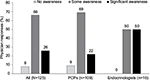  | Figure 1 Physician responses to the question “To what extent are you aware of the 2012 ADA Position Statement?”. Abbreviations: ADA, American Diabetes Association; PCP, primary care physician. |
How physicians learned about the 2012 ADA Position Statement
Before the educational intervention, physicians were asked how they had learned about the 2012 ADA Position Statement (Figure 2). Among PCPs, the most common sources of information were journal articles (71%) followed by continuing medical education (CME) courses (47%) and the ADA (32%). Hospital notices (7%) were the least common source of information. Among endocrinologists, the most common source of information was the ADA (69%), followed by specialty societies (56%) and journal articles (50%). Again, hospital notices (6%) were the least common source of information.
Physician beliefs: impact of the 2012 ADA Position Statement on A1C target setting
Following the educational intervention, an impact on A1C target setting was observed both at the individual patient level and at the practice level. At the individual patient level, 26% of all physicians, including 27% of PCPs and 19% of endocrinologists, indicated that they would change the A1C target for the patient whose profile was presented during the survey. At the practice level, 59% of all physicians, including 61% of PCPs and 50% of endocrinologists, believed that the 2012 ADA Position Statement would impact their A1C target setting (Figure 3), whereas 26% felt the statement would not impact how they set target A1C levels and 15% were not sure of the statement’s impact.
Effect of the educational intervention on A1C target setting
Although the educational intervention affected A1C target setting, the magnitude was less than expected, and the change in mean A1C target before and after the educational intervention was not significant. Before the intervention, the mean (SD) A1C target of profiled patients was 6.6% (0.89) for all physicians across all cohorts. After the intervention, the mean (SD) A1C target remained at 6.6% (0.35) across all cohorts. There were few differences between A1C targets set by PCPs and endocrinologists, among all physicians, and across all cohorts.
After the intervention, physicians changed the A1C targets for 27.2% of patients, while the targets for 72.8% of patients were unchanged. Among the patients whose targets were changed, the A1C target was increased (became less stringent) for 79.4% and the A1C target was decreased (became more stringent) for the remaining 20.6%. When looking at practice-wide effects, physicians generally indicated they would set more stringent A1C targets in lower risk patients and less stringent targets in higher risk patients (Figure 4). Overall, in the pre-intervention survey, 56% of A1C targets reported by physicians for their patients were within the 2012 ADA Position Statement recommended ranges. Following the educational intervention, this increased significantly to 83 of 125 (66%) A1C targets (P<0.0001; Figure 5).
Discussion
This interventional study examined the awareness and impact of the 2012 ADA Position Statement on A1C target setting by endocrinologists and PCPs. Although other diabetes therapeutic guidelines are available, the 2012 ADA Position Statement is widely used in clinical practice, and its updated version of 2015 does not differ significantly with regard to A1C targets and risk stratification. Barriers to the implementation of individualized patient care recognized in these statements include physician-related obstacles.4 Understanding physician awareness and the route of access to and opinions about the 2012 ADA Position Statement is thus an important starting point for recognizing and addressing such barriers. Awareness of the 2012 ADA Position Statement was generally high (92%), with most physicians who treat patients with T2D answering that they had either “some awareness” or “significant awareness” of the current guidelines even before the educational intervention.
This finding contrasts with the findings of Williamson et al,6 who conducted a survey of the familiarity of endocrinologists and family practitioners with diabetes guidelines. Williamson et al reported that most endocrinologists (64%–84%), but fewer family practitioners (22%–37%), reported that they were “very familiar” with the current guidelines (American Association of Clinical Endocrinologists 2011 Comprehensive Clinical Care Plan guidelines7 and 2012 ADA Standard of Care guidelines).1 However, the Williamson et al study differed from the current one in that it recruited nurse practitioners, physician assistants, certified diabetes educators, and retail and hospital pharmacists in addition to endocrinologists, PCPs, and internists. While awareness of the current guidelines was generally high in our study, 9% of PCPs who were treating T2D patients had no awareness of the recommendations, which is in line with other reports of suboptimal awareness of clinical guidelines.6,8 As noted by Williamson et al,6 this finding reinforces the need for education among some physicians treating T2D patients.
With regard to education, there is no doubt that learning about and navigating treatment options to optimize patient care and tailor treatment to the individual have become a daunting task for clinicians.6 The present study revealed that different communication channels may be needed for the dissemination of educational diabetes information, such as ADA Position Statements, to different physician groups. For PCPs, delivery of information via journal articles and CME courses was the most common route of communication. For endocrinologists, the ADA, specialty societies, and journal articles were the most common. For both physician groups, hospital notices were the least common route.
In this study, the educational intervention affected goal-setting behavior, although to a lesser degree than was expected. The mean goal change was directionally consistent with expectations but was not significant. Out of 125 profiled patients, the A1C target was changed in 34 (27.2%) patients, and for most of these (79.4%), the target became less stringent (target A1C was increased). The direction of changes in A1C targets within individual risk cohorts was aligned with expectations, with targets for lower risk cohorts becoming more stringent and targets for higher risk cohorts becoming less stringent, and this was the same for both PCPs and endocrinologists. It is possible that the lack of significant changes in A1C target setting was due to the fact that many of the patients of the physicians who completed the study (i.e., responders) already had A1C values either in line with, or more aggressive than, the risk cohort to which they were assigned, with little scope for change. It is also possible that physicians were reluctant to change targets based on their knowledge of other non-modifiable factors associated with the particular patient. While mean goal changes were not significant, movements toward less, or more, stringent targets are in line with the individualization of A1C targets suggested by Inzucchi et al2 and Ismail-Beigi et al,3 which take into account comorbidities, patient age, duration of T2D, and presence or absence and extent of micro- and macrovascular complications. This finding suggests that the physicians understood and put into effect the 2012 ADA Position Statement.
The percentage of physician-assigned A1C targets that were within ADA-recommended risk-adjusted ranges increased significantly after the educational intervention. This finding reinforces our conclusion that the physicians understood and implemented the 2012 ADA Position Statement and suggests that education about guidelines can have a positive effect on real-world physician behavior. The findings of this study also increased understanding of the characteristics and opinions of the physicians managing patients with T2D in the real world, with most physicians having had some prior exposure to guidelines and having been in practice for a long time. The corollary to this finding is that the impact of guidelines may vary among physicians, for example, by duration of practice.
Direct comparison of other specific findings from this study with other surveys of physician beliefs and practices was not possible because of differences in study design and physician and patient samples. This study examined the impact of the 2012 ADA Position Statement using an educational intervention on a real-world sample of physicians viewing profiles of actual patients from their practices. Using actual patients allowed for more representative patient examples compared with generic patient profiles. As far as we are aware, this is the first study of its kind.
Limitations
The patients and their treating physicians in this study were identified on the basis of medical and pharmacy claims in a large US administrative claims database and may not be representative of all T2D patients and their treating physicians. Risk segments were assigned from claims, which may have resulted in misclassification due to incomplete information. Response rates for physician surveys tend to be lower than those for non-physician surveys because of the many challenges associated with contacting physicians individually and within medical groups or practices. We do not know how many of the fax invitations reached or did not reach their intended physician recipient. The response rate may also have been affected if physicians were not comfortable completing surveys about specific patients for whom they had to look up medical record information. Although the study’s low response rate raises concerns about potential non-response bias, studies have found physician surveys to be less affected by non-response than other types of surveys because the physician population tends to be more homogeneous with regard to knowledge, training, attitudes, and behaviors.9 The study did not explore the reasons why some physicians chose not to respond to the survey. It is possible that “overachievers” were more likely to complete the survey, that is, those physicians whose profiled patients were well under control. As in all survey studies, responders may behave differently from non-responders, and self-reports are subject to recall bias. Further, the number and percentage of endocrinologists were small, with endocrinologists accounting for just 16 of 125 (12.8%) physicians who completed the survey. Finally, we based our intervention on the 2012 ADA Position Statement, which emphasizes a personalized approach to the management of patients with T2D that is maintained in the more recent 2015 update. Although evidence-based guidelines are carefully developed to assist in clinical decision making, in the absence of randomized controlled trials, a position statement may clarify particular aspects of patient management and/or provide an expert opinion. Since a position statement is usually based on the clinical judgment and/or experience of the experts integrating the panel, it may be subject to bias.
Conclusion
Most physicians treating patients with T2D were aware of the 2012 ADA Position Statement. A small percentage of PCPs treating patients with T2D had no awareness of these recommendations, a finding that highlights the need for education among some PCPs. With regard to education, different communication channels may be needed for the dissemination of educational diabetes information, such as ADA Position Statements, to different physician groups. Goal-setting behavior was influenced by the educational intervention, with greater individualization of targets in line with current ADA recommendations. Although physicians did not change the A1C target they had set for most of their patients, those who did change A1C targets were more likely to set less stringent targets across all risk segments. The percentage of physicians whose A1C targets for their patients were within ADA-recommended, risk-adjusted ranges increased after the educational intervention. The impact of the 2012 ADA Position Statement may vary depending on physician characteristics, and research investigating the long-term effects on clinical practice and patient outcomes would be valuable.
Acknowledgments
This study was funded by Sanofi US, Inc. The authors received writing/editorial support in the preparation of this manuscript from Pim Dekker, PhD, of Excerpta Medica, funded by Sanofi US, Inc.
Author contributions
NB proposed and codeveloped the concept, codeveloped the analysis plan, interpreted the results of the analyses, reviewed manuscript drafts, and provided comments. MG codeveloped the concept, codeveloped the analysis plan, provided input into the study objectives, design, and methodology, performed the analysis, interpreted the results of the analyses, prepared the study report, reviewed manuscript drafts, and provided comments. WW codeveloped the concept, interpreted the results of the analyses, reviewed manuscript drafts, and provided comments. NGB provided input into the study objectives, design, and methodology, performed the analysis, interpreted the results of the analyses, prepared the study report, reviewed manuscript drafts, and provided comments. JJS codeveloped the concept, codeveloped the analysis plan, provided input into the study objectives, design, and methodology, performed the analysis, interpreted the results of the analyses, prepared the study report, reviewed manuscript drafts, and provided comments.
Disclosure
NB and WW are employees of Sanofi US, Inc. MG and JJS are employees of HealthCore, Inc., under contract with Sanofi US, Inc., at the time the study was conducted. NGB was an employee of HealthCore, Inc., under contract with Sanofi US, Inc., at the time the study was conducted. The authors report no other conflicts of interest in this work.
References
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012;55(6):1577–1596. | ||
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58(3):429–442. | ||
Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med. 2011;154(8):554–559. | ||
Sorli C, Heile MK. Identifying and meeting the challenges of insulin therapy in type 2 diabetes. J Multidiscip Healthc. 2014;7:267–282. | ||
American Diabetes Association Diabetes Pro. Professional Resources Online. Management of Hyperglycemia in Type 2 Diabetes: A Patient-Centered Approach. Available from: http://professional.diabetes.org/slidelibrary/management-hyperglycemia-type-2-diabetes-patient-centered-approach. Accessed June 29, 2016. | ||
Williamson C, Glauser TA, Burton BS, Schneider D, Dubois AM, Patel D. Health care provider management of patients with type 2 diabetes mellitus: analysis of trends in attitudes and practices. Postgrad Med. 2014;126(3):145–160. | ||
Handelsman Y, Mechanick JI, Blonde L, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract. 2011;17(Suppl 2):1–53. | ||
Beaser RS, Okeke E, Neighbours J, Brown J, Ronk K, Wolyniec WW. Coordinated primary and specialty care for type 2 diabetes mellitus, guidelines, and systems: an educational needs assessment. Endocr Pract. 2011;17(6):880–890. | ||
Kellerman SE, Herold J. Physician response to surveys: a review of the literature. Am J Prev Med. 2001;20(1):61–67. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






