Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 13
The Supportive Treatment of IgA Nephropathy and Idiopathic Nephrotic Syndrome: How Useful are Omega-3 Polyunsaturated Fatty Acids?
Authors Uwaezuoke SN , Muoneke UV , Mbanefo NR
Received 6 November 2019
Accepted for publication 12 February 2020
Published 26 February 2020 Volume 2020:13 Pages 27—35
DOI https://doi.org/10.2147/IJNRD.S237527
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Samuel N Uwaezuoke, Uzoamaka V Muoneke, Ngozi R Mbanefo
Pediatric Nephrology Firm, Department of Pediatrics, University of Nigeria Teaching Hospital, Ituku-Ozalla, Enugu, Nigeria
Correspondence: Samuel N Uwaezuoke
Pediatric Nephrology Firm, Department of Pediatrics, University of Nigeria Teaching Hospital, Ituku-Ozalla, Enugu 400001, Nigeria
Tel +234 8033248108
Email [email protected]
Abstract: IgA nephropathy (IgAN) is the most prevalent glomerular disease in young adults worldwide, while idiopathic nephrotic syndrome (INS) represents the most frequent manifestation of glomerular disease in childhood. Over the years, studies have speculated about the potential benefits of omega-3 polyunsaturated fatty acids (PUFAs) in improving morbidity in both forms of chronic kidney disease (CKD). The proposed mechanisms of action include reduction of proteinuria and modulation of dyslipidemia. Although in vitro and in vivo experimental studies report the suppressive effect of omega-3 PUFAs on inflammatory pathways linked with the progression of nephropathy, the evidence supporting their beneficial effect in IgAN and INS is still weak. Also, their ability to regulate levels of total cholesterol, low-density lipoprotein-cholesterol (LDL-C), and triglycerides (TG) suggests that they could delay both dyslipidemia-associated nephrotoxicity and atherosclerosis. Most of the clinical trials that were conducted on their therapeutic benefits in IgAN patients reported positive outcomes with low and high doses of omega-3 PUFAs. However, few of the trials noted inconclusive findings, with low-quality evidence suggesting potential improvements in surrogate renal function outcomes. If the beneficial effect of omega-3 PUFAs is predicated on their hypolipidemic action, much higher doses could be used in well-designed randomized-controlled trials (RCTs) to determine if they could produce better renal function outcomes and provide much stronger evidence of their therapeutic benefits in IgAN and INS. However, the current hypothetical mechanisms of action in these forms of CKD also include the effect of omega-3 PUFAs on renal inflammatory pathways and glomerular proteinuria. Perhaps, the unresolved therapeutic efficacy of these fatty acids in IgAN and INS suggests that their exact mechanisms of action are yet to be fully established. In this narrative review, we aim to appraise the current evidence of their potential therapeutic benefits in these diseases.
Keywords: IgA nephropathy, idiopathic nephrotic syndrome, omega-3 polyunsaturated fatty acids, renal function, therapeutics
Introduction
IgA nephropathy (IgAN) is regarded as the most prevalent glomerular disease in the second and third decades of life worldwide,1–4 while idiopathic nephrotic syndrome (INS) represents the most frequent manifestation of glomerular disease in childhood.5 Childhood INS is usually caused by any of these glomerulonephritides: minimal change nephropathy (MCN), focal segmental glomerulosclerosis (FSGS), membranoproliferative glomerulonephritis (MPGN), membranous nephropathy (MN) and mesangial proliferative glomerulonephritis (MesPGN).6 Both FSGS and MCN are the prevalent histopathologic lesions worldwide.5 A previous report indicates that the latter is the predominant lesion among preadolescent children in developed countries.7 Still, the prevalence of the former is rising in childhood in both developed and developing countries.6,8–12 Whereas the response of minimal-change nephrotic syndrome (MCNS) to corticosteroids appears predictable with a good prognosis, nephrotic syndrome due to FSGS is largely steroid-resistant with a poor prognosis, and may progress to end-stage kidney disease (ESKD). Alternative immunosuppressive drugs are also effective in FSGS.
Although IgAN was traditionally regarded as a single disease entity characterized by IgA deposition in the glomerular mesangium, it is now seen as a “converging point” for a diverse spectrum of endophenotypes and diseases given the heterogeneity in clinical presentation, histopathological response to IgA mesangial deposition and genetic links of the disease.13 IgAN may present with episodic macroscopic hematuria, acute kidney injury, nephrotic syndrome and a mixed nephrotic and nephritic picture. It runs a variable course which may progress and terminate in ESKD. Clinicopathologic parameters which can predict its poor prognosis include hypertension, elevated serum creatinine, severe proteinuria, severe glomerulosclerosis and interstitial fibrosis.14,15 Current treatment options for IgAN are mainly supportive (tonsillectomy, fish oil supplementation, dietary modification, renin-angiotensin system blockade, etc.), but immunosuppressive therapy is also an option in its management.16
Supportive treatment of IgAN and INS with fish oil-derived omega-3 polyunsaturated fatty acids (PUFAs) has elicited scientific interest over the years as both forms of CKD are associated with dyslipidemia. In- vitro and in-vivo experimental studies indicate the suppressive effect of omega-3 PUFAs on inflammatory pathways linked with the progression of nephropathy.17 Specifically, they reduce albuminuria, prevent deterioration in renal function, and reduce glomerulosclerosis and fibrin deposition in experimental animal models.18 Also, omega-3 PUFA can regulate the levels of total cholesterol, low-density lipoprotein-cholesterol (LDL-C), and triglycerides (TG) in nephrotic chronic kidney disease (CKD).19 Since CKD-related dyslipidemia may be complicated by atherosclerosis and nephrotoxicity,20 omega-3 PUFAs can also ameliorate CKD-related morbidity as well as delay the atherosclerotic process. Besides, their use in CKD-related dyslipidemia may obviate the need for hypolipidemic intervention with statins. Recent studies21,22 and guidelines14,23 underscore their potential benefits in IgAN when low and high doses were used, although there are conflicting results from some clinical trials.16 Thus, the use of omega-3 PUFAs as a supportive therapy in IgAN remains controversial. Nevertheless, their beneficial effect on lupus nephritis has also been reported.24 It is still unclear if much higher doses of omega-3 PUFAs could result in better renal function outcomes in patients with IgAN and INS given their hypolipidemic action at low and high doses. In this narrative review, we aim to appraise the current evidence of their potential therapeutic benefits in IgAN and INS.
Dyslipidemia Patterns in Nephrotic and Non-Nephrotic CKD
Given the possible modulatory action of omega-3 PUFAs on lipid profile,19 it is essential to appraise the changes that occur in lipid levels in nephrotic and non-nephrotic CKD. Persistent hyperlipidemia for prolonged periods is nephrotoxic and may result in chronic progressive glomerular and tubulointerstitial injury. Effective management of hyperlipidemia with hydroxyl-methyl-glutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) and recently with LDL apheresis in drug-resistant INS patients may prevent the progression of renal disease and, sometimes, resolution of INS symptoms. Hyperlipidemia (hypercholesterolemia alone or hypercholesterolemia with hypertriglyceridemia [HTG]) is a fundamental component of INS. Elevated levels of LDL-C (hypercholesterolemia) frequently occur in the syndrome. There are two mechanisms involved in nephrotic dyslipidemia: hepatic overproduction and deranged catabolism of apolipoprotein B (apo B)-containing lipoproteins.25 The mechanisms differ in INS patients with hypercholesterolemia alone and those with hypercholesterolemia and HTG (combined hyperlipidemia). The prospective comparative study by Vega et al found that INS patients with hypercholesterolemia generally had lower fractional catabolic rates of LDL apo B than normolipidemic healthy controls.26 These patients also had cholesterol-rich LDL particles with no overproduction of LDL apo B. In contrast, NS patients with combined hyperlipidemia had high fractional catabolic rates for LDL apo B compared to normolipidemic controls and cholesterol-poor LDL particles as well as markedly increased production rates for LDL.26 These observations suggest a clear difference in the metabolism of LDL between the two forms of nephrotic dyslipidemia. Also, there is a reduction in the catabolism of chylomicrons and VLDL in INS patients, which may equally explain the abnormalities in their lipid profile.27 The dyslipidemia patterns in patients with CKD vary with the different types of the disease. For instance, in nephrotic CKD, HDL-C is reduced whereas LDL-C and TG are markedly increased (very high triglyceride [VHTG]).28 In non-nephrotic CKD, HDL-C is decreased, LDL- C is normal or increased, while TG is high (HTG).28
HTG levels (200–499 mg/dl), reduced HDL-C, and varying concentrations of LDL-C are generally characteristic of CKD-related dyslipidemia.29 Although dyslipidemia is usually the traditional risk factor for CVD,30 dyslipidemia secondary to CKD is an independent risk factor for atherosclerosis.31 Shoji et al reported that moderate CKD and ESKD in children specifically disrupt the vaso-protective functions of HDL-C.32 Thus, nephrotic dyslipidemia (reduced HDL-C, increased LDL-C and VHTG [≥500 mg/dL]), results in nephrotoxicity and endothelial dysfunction. Endothelial dysfunction initiates the early development of atherosclerosis with subsequent formation of atherosclerotic plaques.33
Omega-3 PUFAs and Their Potential Therapeutic Role in Nephrotic CKD
Omega-3 PUFAs from dietary fish oil and other sources play a vital role in human physiology.34 Three types of omega-3 PUFAs are involved in human physiology, namely α-linolenic acid (from plant oils) and eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (commonly from marine oils). The therapeutic effect of omega-3 PUFAs in some renal diseases has remained a subject of speculation. While some studies indicate that supplementation with omega-3 PUFAs is not associated with a lower risk of all-cause mortality35,36 other authors report that omega-3 PUFAs can slow down the progression of renal diseases in human subjects and animal models.37 For instance, omega-3 PUFAs can delay the progression of IgA nephropathy,38–40 and can reduce proteinuria and attenuate renal injury in lupus nephritis, FSGS, and MN in experimental animal models.41–43 These fatty acids can also regulate hyperlipidemia in murine models of FSGS.42 With the nephrotoxicity associated with prolonged hyperlipidemia; the hypolipidemic effect of omega-3 PUFAs underscores their potential therapeutic benefits in FSGS. Interestingly, LDL apheresis has been used increasingly in clinical practice for the treatment of renal diseases presenting with nephrotic syndrome, particularly FSGS.44 A very recent study also reported induction of remission of FSGS recurrence in pediatric renal-transplant recipients following LDL apheresis.45 These findings suggest that supportive therapy with omega-3 PUFAs which regulates hyperlipidemia in nephrotic CKD may delay disease progression by reducing the associated nephrotoxicity.
The mechanism of action of omega-3 PUFAs in renal diseases may, however, be the competitive action of EPA and DHA against arachidonic acid (AA), which produces biologically less-effective prostaglandins and leukotrienes; these may slow down renal injury by reducing glomerular and interstitial inflammation, platelet aggregation and vasoconstriction.46 Specifically, omega-3 PUFAs (ie, EPA and DHA) can reduce biomarkers of inflammation such as C-reactive protein (CRP), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α).47–49 Thus, they exert anti–inflammatory action in renal diseases, improve their morbidity, and delay their progression, as was reported by some authors.17
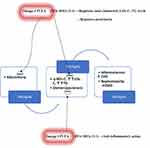
Some authors suggest that omega-3 PUFAs physiologically reduce blood TG levels but do not significantly alter LDL-C and HDL-C levels.50,51 Conversely, a recent review reports that they regulate levels of total cholesterol, LDL-C, and TG: especially at the ratio of 3:1 for EPA and DHA.52 Currently, the use of omega-3 PUFAs appears to be limited to the regulation of TG levels. In the recent recommendations from the American Heart Association (AHA), prescription of omega-3PUFAs at a dose of 4 g/d has consistently resulted in significant reductions in TG levels in patients with HTG or VHTG.53 In fact, omega-3 PUFAs (EPA+DHA or EPA-only) at a dose of 4 g/d (>3 g/d total EPA+DHA) have been suggested as an effective and safe monotherapy for reducing TG levels or as an adjunct to other hypolipidemic agents.53 Also, their ability to reduce glomerulosclerosis by anti–inflammatory action may retard the evolution of CKD to ESKD (Figure 1).
Finally, Grande et al concluded from their recent trials in animal models that omega-3 PUFA supplementation (particularly DHA) inhibits mesangial cell activation and proliferation in glomerulonephritis, reduces proteinuria and decreases histopathologic evidence of glomerular damage.54 They, therefore, suggested that DHA may have a suppressive effect in acute phases or relapses of glomerulonephritides by blocking the activation and proliferation of mesangial cells.54 Similarly, studies in murine models show that omega-3 PUFAs could suppress IL-6-induced production of abnormal IgA and deposition of IgA immune complexes in the mesangium,55,56 a finding which has been demonstrated in clinical trials involving human subjects with IgAN.
Clinical Trials of Omega-3 PUFAs in IgAN with Conclusive Results
As far back as three decades ago, clinical trials of omega-3 PUFAs in patients with IgAN had been conducted. In the randomized controlled trial (RCT) by Hamazaki et al,57 twenty patients with IgAN were evaluated. Ten received omega-3 PUFAs in the ratio of EPA (1.6 gram) and DHA (1 gram), while another ten who were used as controls were not given the fish-oil supplements. The patients were followed up for one year. The clinical outcomes showed that renal function improved in these patients.
A similar study by Alexopoulos et al administered a “very low dose” of PUFAs (0.85 gram EPA and 0.57 gram DHA) in fourteen patients with severe IgAN, while fourteen who served as controls received symptomatic treatment.58 Subjects were followed up for a more extended period of four years. The primary end-points used were an elevation of ≥ 50% in serum creatinine or a reduction of ≥ 50% in glomerular filtration rate (GFR) at the end of the study. There were statistically significant differences between the number of PUFA-treated and non-treated patients who had an increase of ≥ 50% in serum creatinine and between the number of both groups of patients whose GFR decreased by ≥ 50%. Thus, a minimal dose of PUFA was equally effective in retarding the deterioration in renal function among high-risk patients with IgAN, especially those with advanced renal disease.58
A group of Polish investigators also reported improvement in renal function (especially glomerular and tubular functions) as well as a reduction in the degree of proteinuria in patients with IgAN.59 The study assessed patients after one year of treatment with daily 0.54 gram EPA and 0.81 gram DHA. Again, the findings indicate the efficacy of “very low dose” of omega-3 PUFAs in improving disease morbidity.
In two collaborative RCTs,39,40 the suppressive effect of omega-3 PUFAs on the deterioration of renal function in IgAN patients was again demonstrated by Donadio et al. At the end of a 2-year daily treatment with 1.8 gram EPA and 1.2 gram DHA, the authors found that early and prolonged treatment with fish oil retarded disease progression for high-risk patients with IgAN. Specifically, disease course was substantially suppressed with the combination of DHA and EPA, particularly in patients who had nephrotic-range proteinuria, hypertension, and elevated serum creatinine.39 After a follow-up for over six years, reduction in the rate of decline in GFR and disease progression to ESKD were observed, although proteinuria was not significantly reduced.40 Remarkably, when low-dose omega-3 PUFAs (EPA 1.88 gram + DHA 1.47 gram/day) and high-dose omega-3 PUFAs (EPA 3.76 gram + DHA 2.94 gram/day) were administered, similar outcomes were documented.60
On the other hand, other investigators reported a substantial reduction of proteinuria in fifteen IgAN patients given omega-3 PUFAs for fifteen months.61 These patients had also been treated with renin-angiotensin system blockers (RASB). However, no reduction in proteinuria was seen in the control group who received only RASB. These outcomes suggest that omega-3 PUFAs could reduce IgAN-associated proteinuria without synergy with the renin-angiotensin system.
Clinical Trials of Omega-3 PUFAs in IgAN with Inconclusive Results
Despite the substantial evidence above establishing the therapeutic benefit of omega-3 PUFAs in human IgAN, few other clinical trials failed to document similar results. For instance, in a much earlier Australian study by Bennett and colleagues,62 daily 10 gram EPA did not alter the progression of disease in 37 biopsy-proven IgAN patients compared to the outcome in the untreated control.
Similarly, the clinical trial by Pettersson et al used fish oil containing a high dose of omega-3 PUFAs (6 gram/day; EPA 55%, DHA 30%) in the treatment of adult subjects with IgAN for six months. The results showed no therapeutic benefit in these patients over their counterparts in the control group who were treated with 6-gram corn oil per day for the same time duration.63
Finally, in an RCT by Hogg et al, 4 gram omega-3 PUFAs (1.88 gram EPA + 1.48 gram DHA) or placebo was added to oral steroid (prednisolone) and administered to 96 subjects with IgAN for two years.64 A comparison of the omega 3-PUFA-treated group with the placebo group showed that neither of the interventions slowed the deterioration in renal function. Thus, it would appear the beneficial effect of omega-3 PUFAs might not be dose-related given that these preceding studies used high and low doses of the fatty acids resulting in disparate outcomes.
Clinical Trials of Omega-3 PUFAs in IgAN and INS: Evidence from Systematic Reviews and Meta-Analyses
Despite the basis for the clinical benefits of omega-3 PUFAs in IgAN and INS, both anecdotal and published evidence show conflicting findings of their usefulness in these diseases. A recent report summarizing the published evidence on the subject underscores the controversial nature of their recommendation in these forms of CKD (Table 1). In this report, four systematic reviews and two non-randomized studies about the clinical effectiveness of omega-3 PUFA supplementation in reducing proteinuria in patients with IgAN or idiopathic steroid-resistant nephrotic syndrome (SRNS) were identified.65 In an evidence-based clinical practice guideline recommended for IgAN in 2014, it was suggested that the use of omega-3 PUFAs may improve renal outcomes in patients with IgAN. Thus, they may be considered as a therapeutic option despite the fact that only a weak scientific basis exists for their recommendation.66
 |
Table 1 Summary of Findings from Published Systematic Reviews and Meta-Analysis on the Therapeutic Benefits of Omega-3 Polyunsaturated Fatty Acids in IgA Nephropathy and Idiopathic Nephrotic Syndrome |
Firstly, the meta-analysis by Chou et al reviewed five randomized-controlled trials (RCTs) with a total of 233 patients.67 The authors evaluated the therapeutic efficacy of omega-3 PUFAs in IgA nephropathy by determining their dose-effect relationships on renal function and proteinuria. The significant findings were the absence of effect on glomerular filtration rate (GFR) but a substantial reduction in proteinuria in a non-dose dependent fashion. The authors concluded that omega-3 PUFAs had no impact in preserving renal function in IgA nephropathy even though proteinuria was reduced.67
Secondly, Reid et al reviewed a total of 56 RCTs on the use of non-immunosuppressive therapy for IgA nephropathy (7 RCTs focused on the use of fish oils).68 The outcome parameters that were assessed consisted of progression or improvement in renal disease (using proteinuria as a surrogate outcome) as well as all-cause mortality. Anti-hypertensive drugs - especially angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin-receptor blockers (ARBs) - reduced proteinuria but the all-cause mortality was not affected.
Thirdly, Miller et al evaluated 17 trials in their meta-analysis, but only five of them (4 RCTs and one non-RCTs) were conducted on patients with IgAN.69 Following the use of omega-3 PUFA supplementation, renal function (GFR) and renal damage (urine protein excretion) were the outcome parameters assessed by the authors. There was a reduction in urine protein excretion but no improvement in the GFR. Finally, in the meta-analytical study by Colquitt et al, two systematic reviews and eleven RCTs were evaluated.70 However, only one of the RCTs focused on the use of fish oil (tuna fish oil) in children with idiopathic SRNS (FSGS and MPGN). In assessing the efficacy of omega-3 PUFA supplementation, the trial evaluated renal function (urine protein, creatinine clearance, serum creatinine) and lipid profile (TGs, total cholesterol, HDL. LDL) of the patients. Interestingly, there were no statistically significant improvements in proteinuria, creatinine clearance, and serum creatinine as well as lipid profile when compared with the use of placebo.
Conclusions
Presently, there is little scientific evidence to recommend the use of omega-3 PUFAs in the supportive treatment of IgAN and INS. Most of the clinical trials were conducted on their benefits in IgAN patients in whom high and low doses were reportedly associated with improved morbidity. However, few of these studies reported inconclusive findings with limited low-quality evidence suggesting potential improvements in surrogate renal function outcomes. If the beneficial effect of omega-3 PUFAs is predicated on their hypolipidemic action, much higher doses could be used in well-designed RCTs to determine if they could produce better renal function outcomes and provide much stronger evidence about their therapeutic benefits in IgAN and INS. However, the current hypothetical mechanisms of action in these forms of CKD also include the effect of omega-3 PUFAs on renal inflammatory pathways and glomerular proteinuria. Perhaps, the unresolved therapeutic efficacy of these fatty acids in IgAN and INS suggests that their exact mechanisms of action are yet to be fully established.
Acknowledgment
The authors acknowledge the useful information obtained from the paper in reference 21 by Hirahashi J. Omega-3 Polyunsaturated Fatty Acids for the Treatment of IgA Nephropathy. J Clin Med. 2017;6(7):70.
Disclosure
The authors have no conflicts of interest to disclose.
References
1. Hwang S, Tsai J, Chen H. Epidemiology, impact and preventive care of chronic kidney disease in Taiwan. Nephrology (Carlton). 2010;15(Suppl 2):3–9. doi:10.1111/nep.2010.15.issue-s2
2. Chembo CL, Marshall MR, Williams LC, et al. Long-term outcomes for primary glomerulonephritis: New Zealand glomerulonephritis study. Nephrology (Carlton). 2015;20(12):899–907. doi:10.1111/nep.2015.20.issue-12
3. Murugapandian S, Mansour I, Hudeeb M, et al. Epidemiology of glomerular disease in Southern Arizona: a review of 10-year renal biopsy data. Medicine. 2016;95(18):e3633. doi:10.1097/MD.0000000000003633
4. D’Amico G. The commonest glomerulonephritis in the world: igA nephropathy. Q J Med. 1987;645:709–727.
5. Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet. 2003;362:629–639. doi:10.1016/S0140-6736(03)14184-0
6. Uwaezuoke SN, Ndu IK, Mbanefo NR. Prevalence rates of histopathologic subtypes associated with steroid resistance in childhood nephrotic syndrome in Sub-Saharan Africa: a systematic review. Int J Nephrol Renovasc Dis. 2019;12:167–176. doi:10.2147/IJNRD.S207372
7. International Study of Kidney Disease in Children. Nephrotic syndrome in children. Prediction of histopathology from clinical and laboratory characteristics at the time of diagnosis. Kidney Int. 1978;13:15–65. doi:10.1038/ki.1978.23
8. Gulati S, Sharma AP, Sharma RK, Gupta A. Changing trends of histopathology in childhood nephrotic syndrome. Am J Kidney Dis. 1999;34(4):646–650. doi:10.1016/S0272-6386(99)70388-4
9. Downie ML, Gallibois C, Parekh RS, Noone DG. Nephrotic syndrome in infants and children: pathophysiology and management. Paediatr Int Child Health. 2017;37(4):248–258. doi:10.1080/20469047.2017.1374003
10. Srivastava T, Simon SD, Alon US. High incidence of focal segmental glomerulosclerosis in nephrotic syndrome of childhood. Pediatr Nephrol. 1999;13(1):13–18. doi:10.1007/s004670050555
11. Bonilla-Felix M, Parra C, Dajani T, et al. Changing patterns in the histopathology of idiopathic nephrotic syndrome in children. Kidney Int. 1999;55(5):1885–1890. doi:10.1046/j.1523-1755.1999.00408.x
12. Filler G, Young E, Geier P, Carpenter B, Drukker A, Feber J. Is there really an increase in non-minimal change nephrotic syndrome in children? Am J Kidney Dis. 2003;42(6):1107–1113. doi:10.1053/j.ajkd.2003.08.010
13. Penfold RS, Prendecki M, McAdoo S, Tam FWK. Primary IgA nephropathy: current challenges and future prospects. Int J Nephrol Renovasc Dis. 2018;11:137–148. doi:10.2147/IJNRD.S129227
14. KDIGO. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl. 2012;2(2):209–217.
15. D’Amico G. Natural history of idiopathic IgA nephropathy: role of clinical and histological prognostic factors. Am J Kidney Dis. 2000;36:227–237. doi:10.1053/ajkd.2000.8966
16. Selvaskandan H, Cheung CK, Muto M, Barratt J. New strategies and perspectives on managing IgA nephropathy. Clin Exp Nephrol. 2019;23:577–588. doi:10.1007/s10157-019-01700-1
17. Fassett RG, Gobe GC, Peake JM, Coombes JS. Omega-3 polyunsaturated fatty acids in the treatment of kidney disease. Am J Kidney Dis. 2010;56(4):728–742. doi:10.1053/j.ajkd.2010.03.009
18. Clark WF, Parbtani A, Philbrick DJ, Holub BJ, Huff MW. Chronic effects of ω-3 fatty acids (fish oil) in a rat 5/6 ablation model. J Am Soc Nephrol. 1991;1:1343–1353.
19. Hall AV, Parbtani A, Clark WF, et al. Omega-3 fatty acid supplementation in primary nephrotic syndrome: effects on plasma lipids and coagulopathy. J Am Soc Nephrol. 1992;3:1321–1329.
20. Wheeler DC. Lipids - what is the evidence for their role in progressive renal disease? Nephrol Dial Transplant. 1995;10:14–16. doi:10.1093/oxfordjournals.ndt.a090837
21. Hirahashi J. Omega-3 polyunsaturated fatty acids for the treatment of IgA nephropathy. J Clin Med. 2017;6(7):E70. doi:10.3390/jcm6070070
22. Moriyama T, Kumon S, Kamiyama T, Karasawa K, Uchida K, Nitta K. The renoprotective effects of docosahexaenoic acid as add-on therapy in patients receiving eicosapentaenoic acid as a treatment for IgA nephropathy: a pilot uncontrolled trial. Intern Med. 2018;57(2):173–179. doi:10.2169/internalmedicine.9155-17
23. The Special Study Group of the Progressive Renal Dysfunction Research Group of the Ministry of Health Labor and Welfare. Evidence-Based Clinical Practice Guidelines for IgA Nephropathy 2017. Tokyo-igakusha, Tokyo; 2017.
24. Vieira SM, Pagovich OE, Kriegel MA. Diet, microbiota, and autoimmune diseases. Lupus. 2014;23:518–526. doi:10.1177/0961203313501401
25. Orth SR, Ritz E. The nephrotic syndrome. N Engl J Med. 1998;338(17):1202–1211. doi:10.1056/NEJM199804233381707
26. Vega GL, Toto RD, Grundy SM. Metabolism of low-density lipoproteins in nephrotic dyslipidemia: comparison of hypercholesterolemia alone and combined hyperlipidemia. Kidney Int. 1995;47:579–586. doi:10.1038/ki.1995.73
27. Chan MK, Persaud JW, Ramdial L, Varghese Z, Sweny P, Moorhead JF. Hyperlipidemia in untreated nephrotic syndrome, increased production, or decreased removal? Clin Chim Acta. 1981;117:317–323. doi:10.1016/0009-8981(81)90119-4
28. Scarpioni R, Ricardi M, Albertazzi V, Melfa L. Treatment of dyslipidemia in chronic kidney disease: effectiveness and safety of statins. World J Nephrol. 2012;1:184–194. doi:10.5527/wjn.v1.i6.184
29. Hager MR, Narla AD, Tannock LR. Dyslipidemia in patients with chronic kidney disease. Rev Endocrine Metabol Disord. 2016;18:29–40. doi:10.1007/s11154-016-9402-z
30. Tsimihodimos V, Dounousi E, Siamopoulos KC. Dyslipidemia in chronic kidney disease: an approach to pathogenesis and treatment. Am J Nephrol. 2008;28:958–973. doi:10.1159/000144024
31. Kaseda R, Jabs K, Hunley TE, et al. Dysfunctional high-density lipoproteins in children with chronic kidney disease. Metabolism. 2015;64:263–273. doi:10.1016/j.metabol.2014.10.020
32. Shoji T, Abe T, Matsuo H, et al. Chronic kidney disease, dyslipidemia, and atherosclerosis. J Atheroscler Thromb. 2012;19:299–315. doi:10.5551/jat.10454
33. McGill HC
34. Scorletti E, Byrne CD. Omega-3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease. Annu Rev Nutr. 2013;33(1):231–248. doi:10.1146/annurev-nutr-071812-161230
35. Rizos EC, Elisaf MS. Does supplementation with omega-3 PUFAs add to the prevention of cardiovascular disease? Curr Cardiol Rep. 2017;19(6):47. doi:10.1007/s11886-017-0856-8
36. Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308(10):1024–1033. doi:10.1001/2012.jama.11374
37. Donadio JV
38. Hogg RJ, Waldo B. Advances in treatment: immunoglobulin A nephropathy. Semin Nephrol. 1996;16:511–516.
39. Donadio JV
40. Donadio JV
41. Barcelli UO, Miyata J, Ito Y, et al. Beneficial effects of polyunsaturated fatty acids in partially nephrectomized rats. Prostaglandins. 1986;32:211–219. doi:10.1016/0090-6980(86)90126-7
42. Goldstein DJ, Wheeler DC, Sandstrom DJ, Kawachi H, Salant DJ. Fish oil ameliorates renal injury and hyperlipidemia in the Milan normotensive rat model of focal glomerulosclerosis. J Am Soc Nephrol. 1995;6:1468–1475.
43. Rahman MA, Sauter DC, Young MR. Effects of dietary fish oil on the induction of experimental membranous nephropathy in the rat. Lab Invest. 1991;64:371–376.
44. Raina R, Krishnappa V. An update on LDL apheresis for nephrotic syndrome. Pediatr Nephrol. 2019;34:1655. doi:10.1007/s00467-018-4061-9
45. Shah L, Hooper DK, Okamura D, et al. LDL-apheresis-induced remission of focal segmental glomerulosclerosis recurrence in pediatric renal transplant recipients. Pediatr Nephrol. 2019;34:2343. doi:10.1007/s00467-019-0426-6
46. Donadio JV
47. Li K, Huang T, Zheng J, Wu K, Li D. Effect of murine-derived ω-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6, and tumor necrosis factor α: a meta-analysis. PLoS One. 2014;9(2):e88103. doi:10.1371/journal.pone.0088103
48. Borges MC, Santos FM, Telles RW, Correia MI, Lanna CC. Polyunsaturated omega-3 fatty acids and systemic lupus erythematosus: what do we know? Rev Bras Reumatol. 2014;54:459–466. doi:10.1016/j.rbr.2013.12.002
49. Halade GV, Rahman MM, Bhattacharya A, Barnes JL, Chandrasekar B, Fernandes G. Docosahexaenoic acid-enriched fish oil attenuates kidney disease and prolongs acid-enriched fish oil attenuates kidney disease and prolongs the median and maximal life span of autoimmune lupus-prone mice. J Immunol. 2010;184:5280–5286. doi:10.4049/jimmunol.0903282
50. Wu L, Parhofer KG. Diabetic dyslipidemia. Metabolism. 2014;63(12):1469–1479. doi:10.1016/j.metabol.2014.08.010
51. Weintraub HS. Overview of prescription omega-3 fatty acid products for hypertriglyceridemia. Postgrad Med. 2014;126(7):7–18. doi:10.3810/pgm.2014.11.2828
52. Constantin MM, Nita JE, Olteanu R, et al. Significance and impact of dietary factors on systemic lupus erythematosus pathogenesis (Review). Exp Ther Med. 2019;17:1085–1090. doi:10.3892/etm.2018.6986
53. Skulas-Ray AC, Wilson PWF, Harris WS, et al. Omega-3 fatty acids for the management of hypertriglyceridemia-A Science Advisory from the American Heart Association. Circulation. 2019;140:e673–e691. doi:10.1161/CIR.0000000000000709
54. Grande JP, Walker HJ, Holub BJ, et al. Suppressive effects of fish oil on mesangial cell proliferation in vitro and in vivo. Kidney Int. 2000;57:1027–1040. doi:10.1046/j.1523-1755.2000.00930.x
55. Dong W, Sell JE, Pestka JJ. Quantitative assessment of mesangial immunoglobulin A (IgA) accumulation, elevated circulating IgA immune complexes, and hematuria during vomitoxin-induced IgA nephropathy. Fundam Appl Toxicol. 1991;17:197–207. doi:10.1016/0272-0590(91)90251-X
56. Pestka JJ, Zhou HR. Interleukin-6-deficient mice refractory to IgA dysregulation but not anorexia induction by vomitoxin (deoxynivalenol) ingestion. Food Chem Toxicol. 2000;38:565–575. doi:10.1016/S0278-6915(00)00041-7
57. Hamazaki T, Tateno S, Shishido H. Eicosapentaenoic acid, and IgA nephropathy. Lancet. 1984;1:11017–11018. doi:10.1016/S0140-6736(84)92355-9
58. Alexopoulos E, Stangou M, Pantzaki A, Kirmizis D, Memmos D. Treatment of severe IgA nephropathy with omega-3 fatty acids: the effect of a “very low dose” regimen. Ren Fail. 2004;26:453–459. doi:10.1081/JDI-200026763
59. Sulikowsa B, Niewegłowski T, Manitius J, Lysiak-Szydłowska W, Rutkowski B. Effect of 12-month therapy with omega-3 polyunsaturated acids on glomerular filtration response to dopamine in IgA nephropathy. Am J Nephrol. 2004;24:474–482. doi:10.1159/000080670
60. Donadio JV
61. Ferraro PM, Ferraccioli GF, Gambaro G, Fulignati P, Costanzi S. Combined treatment with renin-angiotensin system blockers and polyunsaturated fatty acids in proteinuric IgA nephropathy: a randomized controlled trial. Nephrol Dial Transplant. 2009;24:156–160. doi:10.1093/ndt/gfn454
62. Bennett WM, Walker RG, Kincaid-Smith P. Treatment of IgA nephropathy with eicosapentanoic acid (EPA): a two-year prospective trial. Clin Nephrol. 1989;31:128–131.
63. Pettersson EE, Rekola S, Berglund L, et al. Treatment of IgA nephropathy with omega-3-polyunsaturated fatty acids: a prospective, double-blind, randomized study. Clin Nephrol. 1994;41:183–190.
64. Hogg RJ, Lee J, Nardelli N, et al. Clinical trial to evaluate omega-3 fatty acids and alternate-day prednisone in patients with IgA nephropathy: report from the Southwest Pediatric Nephrology Study Group. Clin J Am Soc Nephrol. 2006;1:467–474. doi:10.2215/CJN.01020905
65. Omega-3 Fatty Acids for Proteinuria Due to Nephrotic Syndrome: A Review of Clinical Effectiveness and Cost-Effectiveness [Internet]. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health;2016 February 5. KEY FINDINGS. Available from https://www.ncbi.nlm.nih.gov/books/NBK350634/.
66. Yuzawa Y, Yamamoto R, Takahashi K, et al. Evidence-based clinical practice guidelines for IgA nephropathy 2014. Clin Exp Nephrol. 2016;20:511–535. doi:10.1007/s10157-015-1223-y
67. Chou HH, Chiou YY, Hung PH, Chiang PC, Wang ST. Omega-3 fatty acids ameliorate proteinuria but not renal function in IgA nephropathy: a meta-analysis of randomized controlled trials. Nephron Clin Pract. 2012;121(1–2):c30–5. doi:10.1159/000341929
68. Reid S, Cawthon PM, Craig JC, Samuels JA, Molony DA, Strippoli GF. Non-immunosuppressive treatment for IgA nephropathy. Cochrane Database Syst Rev. 2011;16(3):CD003962. doi:10.1002/14651858.cd003962.pub2
69. Miller ER
70. Colquitt JL, Kirby J, Green C, Cooper K, Trompeter RS. The clinical effectiveness and cost-effectiveness of treatments for children with idiopathic steroid-resistant nephrotic syndrome: a systematic review. Health Technol Assess. 2007;11(21):iii–x.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.