Back to Journals » Cancer Management and Research » Volume 13
The Strategy of Conditionally Replicating Adenovirus-Mediated PreS2 Mini-Antibody Expression Has Dual Effects of Inhibiting HBV Infection and Preventing Hepatocellular Carcinoma
Authors Ye Z, Zeng S, Xu P, Liu W, Wang S, Xia X, Su C, Guo M
Received 23 December 2020
Accepted for publication 14 February 2021
Published 24 February 2021 Volume 2021:13 Pages 1869—1876
DOI https://doi.org/10.2147/CMAR.S298331
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Ziheng Ye,1,* Su Zeng,1,* Peipei Xu,1 Wenfei Liu,1 Shoufei Wang,1 Xiaotian Xia,1 Changqing Su,2 Minggao Guo1
1Center of Thyroid and Parathyroid, Department of Thyroid, Parathyroid, Breast and Hernia Surgery, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, People’s Republic of China; 2Laboratory of Viral and Gene Therapy, Eastern Hepatobiliary Surgical Hospital and Institute, The Second Military Medical University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Minggao Guo Email [email protected]
Changqing Su Email [email protected]
Aim: To investigate the inhibitory effect of hepatitis B virus (HBV) preS2 mini-antibody (mPreS2) against HBV infection, HBV-associated liver injury and HBV-associated hepatic carcinogenesis.
Methods: A recombinant adenovirus vector with the human survivin promoter and mPreS2 gene, Ad5SVP-mPreS2, was constructed. Fluorescence microscopy examination and TCID 50 analysis were utilized to determine the specific proliferation of recombinant adenovirus in liver cancer cells. Western blot analysis was used to determine the mPreS2 expression levels. Enzyme-linked immunosorbent assay (ELISA) was used to examine HBsAg levels to evaluate the inhibitory effect of mPreS2 against HBV infection. The protective effects on hepatic function and preventive effects against hepatic carcinogenesis of Ad5SVP-mPreS2 were studied in diethylnitrosamine (DEN)-treated HBV transgenic Imprinting Control Region mice.
Results: The recombinant adenovirus regulated by the human survivin promoter proliferated exclusively in liver cancer cells rather than normal liver cells. The expression levels of mPreS2 were increased in liver cancer cells compared with normal liver cells, and mPreS2 could be used to recognize liver cells from HBV transgenic mice. ELISA showed that HBsAg levels were decreased in the group treated with Ad5SVP-mPreS2. Ad5SVP-mPreS2 had a protective effect on hepatic function in a DEN-induced liver injury model because of lower serum levels of alanine transaminase and aspartate transaminase. Additionally, HBV transgenic mice treated with Ad5SVP-mPreS2 had fewer and smaller cancerous nodes after induction with DEN than untreated mice.
Conclusion: Conditionally replicating adenovirus-mediated mPreS2 expression inhibited HBV infection and had an inhibitory effect on liver injury and hepatocellular carcinogenesis in HBV transgenic mice.
Keywords: hepatitis B virus, hepatocellular carcinoma, conditionally replicating adenovirus
Introduction
Hepatitis B and hepatitis B virus-associated hepatocellular carcinoma (HBV-HCC) are major challenges to human health, especially in developing countries. To prevent the development of HBV-HCC, the inhibition and treatment of HBV are becoming urgent tasks that are essential for the improvement in health conditions. The conventional and effective treatments for recurrent HBV infections in patients include immunization and antiviral therapies. However, immunoglobulin is expensive and has some limitations: it has an unsatisfactory neutralization effect and may carry pathogens.1 At the same time, gene therapy has become an important auxiliary measure for comprehensive treatment, which may provide a novel approach to the treatment of HBV and HBV-HCC.
Two HBV viral proteins, the hepatitis B X protein (HBx) and pre-S2 protein, play an important role in HBV infection and HCC oncogenesis.2,3 Notably, the preS2 mutant contributes to the development of HCC through the influence of the expression of transcription factors such as NF-κB, Ap-2, and Sp1, which may activate the Akt/mTOR and NF-κB/COX-2 signaling pathways.4 It is worth noting that the antigenicity of preS2 is stronger than that of HBsAg, with a robust T and B cell recognition cluster.5 During HBV infection, it can induce the host’s immune response and produce preS2 antibodies, which provide an important defense function for eliminating HBV and preventing virus from invading normal liver cells.6 Accordingly, preS2 antibody may be useful to inhibit the infection and duplication of HBV and exhibit antitumor activity in cancerous liver cells that persistently express HBx and preS2.
In our previous study, the preS2 humanized antibody gene, which contained a full length of 2570 bp of light and heavy strand, was cloned into the type 5 adenoviral shuttle plasmid. This antibody could recognize hepatocytes of HBV transgenic Imprinting Control Region (ICR) mice and showed good affinity to the corresponding antigen, indicating that it might protect primary hepatocytes from HBV infection, inhibit HBV replication and protect hepatocyte function.7 However, the type 5 adenoviral shuttle plasmid lost its replication ability, and its antibody gene expression level was limited. Although the full-length antibody was more activated, its molecular weight was so large that it hardly permeated into tissue, which might damage its effect. To overcome these obstacles, this study constructed a treatment strategy in which liver cells could express preS2 mini-antibody (mPreS2) after transfection by conditionally replicating adenovirus vector containing the gene for humanized HBV-preS2 mini-antibody.
Materials and Methods
Cell Lines and Cell Culture
The Homo sapiens liver cancer cell lines HepG2 and Huh-7 and the normal liver cell line WRL-68 were provided by the Shanghai Institute for Biological Science, Chinese Academy of Sciences (Shanghai, China). The human embryo kidney cell line HEK293 was purchased from Microbix Biosystems Inc. (Toronto, Canada). All cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL, Grand Island, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Gibco BRL). Cells were maintained at 37°C in a humidified 5% CO2 incubator.
Construction of a Conditionally Replicating Adenovirus Vector Carrying the mPreS2 Gene
The preS2 mini-antibody gene was synthesized by overlap PCR according to the template of the humanized HBV preS2Ab gene, including the variable regions of the light and heavy chains and constant region 3 of heavy chain (CH3). The promotor gene of mouse cytomegalovirus (mCMV) was introduced upstream of the preS2 mini-antibody gene, which could activate the expression of the target gene. The whole expression cassette was cloned into the E3 region of a conditionally replicating adenovirus vector with the human survivin promoter. As a control, the EGFP reporter gene was cloned into a conditionally replicating adenovirus vector and a deficiently replicating adenovirus vector. All recombinant adenovirus vectors, including Ad5SVP-mPreS2, Ad5SVP-EGFP and Ad5-EGFP, were amplified in HEK293 cells.
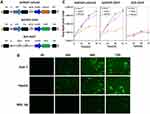
Identification of the Specific Proliferation of Conditionally Replicating Adenovirus Vector
HepG2, Huh-7 and WRL-68 cells were cultured in 6-well plates for 24 h and then subjected to serum-deprived medium. Ad5SVP-mPreS2, Ad5SVP-EGFP and Ad5-EGFP were added for infection according to the multiplicity of infection (MOI) gradient, and the expression levels of EGFP were observed under a fluorescence microscope at 0 h, 24 h, 48 h and 72 h after infection. The cell lysate solution was collected at 0 h, 24 h, 48 h and 72 h after infection, and 50% tissue culture infective dose (TCID 50) analysis was utilized to evaluate the copy levels of adenovirus in the liver cancer cell line and normal liver cell line.
Expression and Activity Experiment of mPreS2
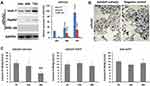
HepG2, Huh-7 and WRL-68 cells were cultured in 24-well plates for 24 h, and Ad5SVP-mPreS2 was added for infection with MOI=10 pfu/cell. The cell lysate solution was collected at 0 h, 24 h, 48 h and 72 h after infection, and Western blot analysis was utilized to determine the mPreS2 with HRP-conjugated rabbit anti-human IgG (1:1000, Cell Signaling Technology, USA).
An HBV transgenic Imprinting Control Region (ICR) mouse provided by the Shanghai SLAC Laboratory Animal Center, Chinese Academy of Sciences (Shanghai, China), was sacrificed, and the liver was taken to prepare a hepatocyte suspension. The hepatocyte suspension was cultured in 6-well plates for 24 h, and the supernatants of HepG2 cells that were infected by Ad5SVP-mPreS2 we prepared before were added to 100 μL of each well. After incubation for 2 h, cells were collected and smeared, and HRP-conjugated rabbit anti-human IgG (1:1000) was used for incubation for 30 minutes. Finally, diaminobenzidine (Fuzhou Maixin Biotechnology Development Co., Fuzhou, China) was utilized as a chromogenic reagent, and photos were taken under a microscope.
Inhibitory Effects of Ad5SVP-mPreS2 Against Hepatitis B
The hepatocyte suspension we prepared before was cultured in 6-well plates for 24 h and then subjected to serum-deprived medium. Ad5SVP-mPreS2, Ad5SVP-EGFP and Ad5-EGFP were added for infection (MOI=50 pfu/cell), and the supernatants were collected at 24 h and 48 h after infection. An enzyme-linked immunosorbent assay (ELISA) kit (Shanghai Thermo Biotechnology Development Co., Shanghai, China) was used to determine hepatitis B surface antigen (HBsAg) levels in the supernatants.
Protective Effects of Ad5SVP-mPreS2 on Hepatic Function
A total of 20 HBV transgenic ICR mice (Shanghai SLAC), aged 4 weeks, were fed diethylnitrosamine (DEN) water (1:10,000) for 2 weeks to establish the model of liver injury. Then, all mice were evenly divided into 4 groups, and the virus groups (Ad5SVP-mPreS2, Ad5SVP-EGFP, and Ad5-EGFP) were given the corresponding adenovirus particles via tail vein injections; each mouse was injected with 2×108 pfu/100 μL adenovirus every other day for a total of 4 injections starting one week after the initial feeding. Mice in the nonvirus control group were given the same volume of normal saline simultaneously. The animals were fed for 2 weeks after the treatment and were sacrificed. Serum samples were collected and measured for alanine transaminase (ALT) and aspartate transaminase (AST). The liver tissues were prepared and subjected to hematoxylin/eosin (H&E) staining to observe the histological variation.
Preventive Effect of Ad5SVP-mPreS2 Against Hepatic Carcinogenesis
A total of 18 HBV transgenic ICR mice (Shanghai SLAC), aged 4 weeks, were fed DEN water (1:10,000) for 4 weeks to establish the model of HCC. Then, all mice were evenly divided into 2 groups, and the Ad5SVP-mPreS2 group was given the corresponding adenovirus particles via tail vein injections; each mouse was injected with 2.5×108 pfu/100 μL adenovirus once a week for a total of 4 injections. Mice in the blank control group were given the same volume of normal saline simultaneously. Three mice were sacrificed monthly in each group after the initial treatment. Serum samples were collected and measured for ALT and AST to evaluate hepatic function, and the liver tissues were sectioned at 0.5-cm intervals to observe the diameters of the cancerous nodes. The expression levels of E1a and Ki67 were assessed by immunohistochemistry (IHC) analysis with rabbit polyclonal antibodies (Abcam, Cambridge, USA).
Statistical Analysis
All data are presented as the mean ± SD. Statistical significance was calculated using unpaired Student’s t-tests. P < 0.05 was considered significant. All analyses were performed using the Statistical Package for Social Sciences for Windows (SPSS) version 25.0.
Results
The Specific Proliferation of Ad5SVP-mPreS2 in Liver Cancer Cells
As shown in Figure 1A, the human survivin promoter mediated the expression of Ad5SVP-mPreS2 with cancerization, and the mPreS2 expression cassette or EGFP gene was inserted after deletion of the E3 region. For liver cancer cells, fluorescence microscopy examination confirmed that the expression levels of EGFP protein in the Ad5SVP-EGFP group were much higher than those in the Ad5-EGFP group after infection with recombinant adenovirus vectors. Conversely, the two groups harboring the EGFP gene showed low expression levels of EGFP protein in normal liver cells (Figure 1B). Additionally, TCID50 analysis showed that with the extension of time, the viral titer of Ad5SVP-mPreS2 and Ad5SVP-EGFP in liver cancer cells increased gradually, up to more than 1 million times, while the viral titer remained below 50 times in normal liver cells. The viral titer of the Ad5-EGFP group was constantly low both in liver cancer cells and normal liver cells (Figure 1C).
mPreS2 Mediated by Ad5SVP-mPreS2 Could Combine with Hepatocytes from HBV Transgenic Mice and Inhibit HBV Infection
Western blot analysis showed that the expression levels of mPreS2 were higher in Huh-7 and HepG2 cells than in WRL-68 cells, especially at 48 h and 72 h after Ad5SVP-mPreS2 infection (Figure 2A). After incubation with the supernatants of HepG2 cells that were infected by Ad5SVP-mPreS2, the hepatocytes derived from an HBV transgenic ICR mouse were combined with mPreS2 and were marked in smears (Figure 2B). Additionally, ELISA results showed that compared with the Ad5SVP-EGFP group and Ad5-EGFP group, the Ad5SVP-mPreS2 group had decreased HBsAg levels in the supernatant, which might indicate that mPreS2 could inhibit HBV infection and replication (Figure 2C).
Ad5SVP-mPreS2 Effectively Protected Against DEN-Induced Liver Injury in HBV Transgenic Mice
To investigate the effects of Ad5SVP-mPreS2 in vivo, we established a model of liver injury in HBV transgenic ICR mice. The ALT and AST levels in serum samples were measured, and we found that the Ad5SVP-mPreS2 group, compared with the Ad5-EGFP group and the nonvirus control group, had much lower levels of ALT and AST. Moreover, the Ad5SVP-EGFP group showed higher levels of ALT and AST, which were not significant (Figure 3A). Microscopic examination of liver tissue showed that hydropic degeneration and point necrosis could be found in the Ad5SVP-EGFP group, Ad5-EGFP group and nonvirus control group, especially in the Ad5SVP-EGFP group, in accordance with the ALT and AST measurements. However, in the Ad5SVP-mPreS2 group, most liver cells presented granular degeneration, and hydropic degeneration was rare (Figure 3B). All these data indicated that mPreS2 expressed by Ad5SVP-mPreS2 had positive effects on the protection of hepatic function in HBV mice.
Ad5SVP-mPreS2 Effectively Inhibited DEN-Induced HCC Carcinogenesis and Development in HBV Transgenic Mice
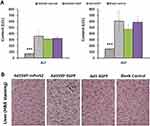
To further determine the preventive effect of Ad5SVP-mPreS2 against hepatic carcinogenesis, we established a model of HCC using DEN water. Serum samples for ALT and AST measurements confirmed that Ad5SVP-mPreS2 presented a more protective effect, although the serum levels of ALT and AST in the two groups were elevated gradually (Figure 4A).
For microscope examination, in the first month after the initial treatment, hydropic degeneration and point necrosis were present in liver cells in the blank control group, as well as moderate fibrosis in liver tissue. Conversely, the liver cells in the Ad5SVP-mPreS2 group only presented granular degeneration, and slight fibrous tissue hyperplasia could be found in liver tissue. In the second month, pseudolobule formation and laminar necrosis with inflammatory cell infiltration could be found in the blank control group, and the liver tissue in the Ad5SVP-mPreS2 group only presented slight-to-moderate fibrosis. In the third month, severe hepatic cirrhosis with fibrous septa and cancerous nodes of different numbers and various sizes were present in the blank control group. In contrast, the cancerous nodes in the Ad5SVP-mPreS2 group were both fewer in number and smaller (Figure 4B and C).
Finally, the IHC analysis showed that E1a protein which co-expressed with mPreS2, was only expressed in cancer cells rather than normal liver cells in the Ad5SVP-mPreS2 group, indicating the special expression of mPreS2 indirectly (Figure 4D). Additionally, the expression levels of Ki67 were much lower in the Ad5SVP-mPreS2 group, which indicated that the proliferation activity of cancer cells in the Ad5SVP-mPreS2 group was relatively low (Figure 4E).
Discussion
To our knowledge, the reason why the prevalence of HCC is so high in China is HBV infection.8,9 To inhibit HBV infection and prevent the carcinogenesis and development of HCC, we focused on these two aspects as follows. First, we transformed the full-length preS2 antibody into the preS2 mini-antibody (mPreS2), maintaining the variable regions of the light and heavy chains and CH3 region.10 Mini-antibody had better permeability because of its small molecular weight, and its Fc component could mediate antibody-dependent cell-mediated cytotoxicity (ADCC) with host immune cells.11,12 As a result, mPreS2 might present more effective inhibition of HBV infection and antitumor activity. In addition, mPreS2 did not need glycosylation after translation as a result of the deletion of the CH2 region and was suitable for high-volume production in yeast or E. coli systems,13 which might create the conditions for translational research and clinical application. Second, we utilized the conditionally replicating adenovirus vector with the human survivin promoter to carry and express mPreS2, aiming to enhance its targeting to cancer cells. In our study, we constructed a recombinant adenovirus vector named Ad5SVP-mPreS2 and verified its effect in vitro and in vivo. Excitingly, we found that Ad5SVP-mPreS2 could proliferate and express mPreS2 in liver cancer cells exclusively, inhibited HBV infection and presented promising antitumor activity, with minor damage to normal liver cells. The conditionally replicating adenovirus vector initiated its regulatory mechanism immediately and promoted adenovirus-specific proliferation when liver cells were cancerous. Not only could the extremely proliferative adenovirus dissolve the cancer cells, but it could also improve the copy number and the expression of antibody genes.
In recent years, targeted therapy of antitumor genes mediated by targeted adenovirus vectors has made remarkable achievements, which may open a new avenue for cancer therapy. Researchers from the University of Ottawa, Canada, and an American Cancer Drug Corporation named Jennerex announced the world’s first clinical trial of targeted gene therapy for cancer in Nature on September 1, 2011. In this research, a kind of tumor-targeting virus that was oncolytic was utilized for tumor treatment intravenously, and promising outcomes were gained.14 With the approval and marketing of oncolytic virus T-VEC, proliferative oncolytic virus has attracted great attention as a vector for gene therapy. The advantage of oncolytic viruses is that they can not only dissolve cancer cells but also express therapeutic genes carried by viral vectors more effectively.15–17 Moreover, oncolytic viruses can improve the microenvironment of tumor immunosuppression as a sensitizer to immunotherapy.18 Therefore, we went with flow cytometry and constructed our conditionally replicating adenovirus with the mPreS2 gene mediated by the human survivin promoter, and its targeting and accuracy were confirmed in previous studies.19,20
Conclusion
In summary, the treatment strategy we constructed is characterized by strict regulation, antiviral and antitumor efficacy and has better safety and efficacy in blocking HBV infection, protecting hepatic function and preventing hepatocyte cancerization, thus creating conditions for further clinical application of the product in the future.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Approval
Studies involving experiments with animals were approved by the animal experiment ethics committee of Shanghai Sixth People’s Hospital, China, and the animal care was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
Consent for Publication Statement
All of the authors have agreed to the content of the manuscript and all details of this work can be published.
Acknowledgments
We thank the other investigators and the staff of the present study for their valuable contributions. We thank AJE (www.aje.cn) for its linguistic assistance during the preparation of this manuscript.
Funding
This study was funded by The National Natural Science Foundation of China (81672373).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Zhang Q, Li ZQ, Liu H, Yang JH. Adenovirus-expressed preS2 antibody inhibits hepatitis B virus infection and hepatic carcinogenesis. World J Gastroenterol. 2012;18:349–355. doi:10.3748/wjg.v18.i4.349
2. Tan A, Yeh SH, Liu CJ, Cheung C, Chen PJ. Viral hepatocarcinogenesis: from infection to cancer. Liver Int. 2008;28:175–188. doi:10.1111/j.1478-3231.2007.01652.x
3. Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64(1):S84–S101. doi:10.1016/j.jhep.2016.02.021
4. Teng CF, Wu HC, Shyu WC, Jeng LB, Su IJ. Pre-S2 mutant-induced mammalian target of rapamycin signal pathways as potential therapeutic targets for hepatitis B virus-associated hepatocellular carcinoma. Cell Transplant. 2017;26:429–438. doi:10.3727/096368916X694382
5. Ochoa-Callejero L, Otano I, Vales A, et al. Identification of CD4+ and CD8+ T cell epitopes of woodchuck hepatitis virus core and surface antigens in BALB/c mice. Vaccine. 2010;28:5323–5331. doi:10.1016/j.vaccine.2010.05.043
6. Krawczyk A, Ludwig C, Jochum C, et al. Induction of a robust T- and B-cell immune response in non- and low-responders to conventional vaccination against hepatitis B by using a third generation PreS/S vaccine. Vaccine. 2014;32(39):5077–5082. doi:10.1016/j.vaccine.2014.06.076
7. Guo M, Kang B, Zheng Q, et al. An anti-preS2 antibody protects human hepatocytes from hepatitis B virus infection. Acta Gastroenterol Belg. 2009;72:306–311.
8. Sandhu HS, Roesel S, Sharifuzzaman M, Chunsuttiwat S, Tohme RA. Progress toward hepatitis B control - South-East Asia Region, 2016–2019. MMWR Morb Mortal Wkly Rep. 2020;69:988–992. doi:10.15585/mmwr.mm6930a2
9. Teufel A. Bioinformatics and database resources in hepatology. J Hepatol. 2015;62:712–719. doi:10.1016/j.jhep.2014.10.036
10. Desplancq D, Rinaldi A-S, Stoessel A, et al. Single-chain Fv fragment antibodies selected from an intrabody library as effective mono- or bivalent reagents for in vitro protein detection. J Immunol Methods. 2011;369(1–2):42–50. doi:10.1016/j.jim.2011.04.001
11. Krah S, Kolmar H, Becker S, Zielonka S. Engineering IgG-like bispecific antibodies-an overview. Antibodies. 2018;7:28. doi:10.3390/antib7030028
12. Zauli G, Corallini F, Zorzet S, Grill V, Marzari R, Secchiero P. In vivo anti-lymphoma activity of an agonistic human recombinant anti-TRAIL-R2 minibody. Invest New Drugs. 2012;30(1):405–407. doi:10.1007/s10637-010-9519-y
13. Chang X, Cui H, Feng J, et al. Preparation of humanized ovarian carcinoma anti-idiotypic minibody. Hybrid Hybridomics. 2003;22(2):109–115. doi:10.1089/153685903321948030
14. Breitbach CJ, Burke J, Jonker D, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477(7362):99–102. doi:10.1038/nature10358
15. Chaurasiya S, Fong Y, Warner SG. Optimizing oncolytic viral design to enhance antitumor efficacy: progress and challenges. Cancers. 2020;12(6):1699. doi:10.3390/cancers12061699
16. Li X, Su Y, Sun B, et al. An artificially designed interfering lncRNA expressed by oncolytic adenovirus competitively consumes OncomiRs to exert antitumor efficacy in hepatocellular carcinoma. Mol Cancer Ther. 2016;15(7):1436–1451. doi:10.1158/1535-7163.MCT-16-0096
17. Zhang Y, Fang L, Zhang Q, et al. An oncolytic adenovirus regulated by a radiation-inducible promoter selectively mediates hSulf-1 gene expression and mutually reinforces antitumor activity of I131-metuximab in hepatocellular carcinoma. Mol Oncol. 2013;7:346–358. doi:10.1016/j.molonc.2012.10.007
18. Hemminki O, Dos Santos JM, Hemminki A. Oncolytic viruses for cancer immunotherapy. J Hematol Oncol. 2020;13(1):84. doi:10.1186/s13045-020-00922-1
19. Ang L, Guo L, Wang J, Huang J, Lou X, Zhao M. Oncolytic virotherapy armed with an engineered interfering lncRNA exhibits antitumor activity by blocking the epithelial mesenchymal transition in triple-negative breast cancer. Cancer Lett. 2020;479:42–53. doi:10.1016/j.canlet.2020.03.012
20. Su C. Survivin in survival of hepatocellular carcinoma. Cancer Lett. 2016;379(2):184–190. doi:10.1016/j.canlet.2015.06.016
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.