Back to Journals » Drug Design, Development and Therapy » Volume 14
The Skeletal Effects of Short-Term Triple Therapy in a Rat Model of Gastric Ulcer Induced by Helicobacter pylori Infection
Authors Ekeuku SO , Thong BKS , Quraisiah A , Annuar F , Hanafiah A , Nur Azlina MF, Chin KY
Received 16 October 2020
Accepted for publication 21 November 2020
Published 3 December 2020 Volume 2020:14 Pages 5359—5366
DOI https://doi.org/10.2147/DDDT.S287239
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Sophia Ogechi Ekeuku,1 Benjamin Ka Seng Thong,1,2 Adam Quraisiah,1 Fazalda Annuar,1 Alfizah Hanafiah,3 Mohd Fahami Nur Azlina,1 Kok-Yong Chin1
1Department of Pharmacology, Faculty of Medicine, Universiti Kebangsaan Malaysia, Cheras, Malaysia; 2Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 3Department of Medical Microbiology & Immunology Faculty of Medicine, Universiti Kebangsaan Malaysia, Cheras, Malaysia
Correspondence: Kok-Yong Chin
Department of Pharmacology, Faculty of Medicine, Universiti Kebangsaan Malaysia, Level 17, Preclinical Building, Jalan Yaacob Latif, Bandar Tun Razak, Cheras 56000, Kuala Lumpur, Malaysia
Tel +60 3-9145-9573
Email [email protected]
Purpose: Triple therapy is the standard therapy to eradicate Helicobacter pylori (H.pylori) infection. Chronic use of proton pump inhibitors (PPIs), a component of triple therapy, is associated with osteoporosis. However, the skeletal effects of short-term triple therapy containing PPI remain elusive. This study aims to determine the skeletal effect of short-term triple therapy in a rat model of gastric ulcer induced by H. pylori.
Methods: Three-month-old male Sprague Dawley rats were assigned to normal control, H. pylori-inoculated group (negative control) and H. pylori-inoculated group receiving triple therapy consisting of omeprazole [2.035 mg/kg body weight (b.w)], amoxicillin (102.80 mg/kg b.w) and clarithromycin (51.37 mg/kg b.w) (n=6/group). H. pylori infection developed for four weeks after inoculation, followed by two-week triple therapy. At the end of the treatment period, femoral bones of the rats were harvested for analysis. Bone mineral density and content of the femurs were determined using dual-energy X-ray absorptiometry, while bone strength was measured with a universal mechanical tester.
Results: Bone mineral content was significantly lower in the negative control group compared to the triple therapy group (p=0.014). Triple therapy decreased strain (vs negative control, p=0.002) and displacement of the femur (vs normal control, p=0.004; vs untreated control, p=0.005). No significant difference was observed in other parameters among the study groups (p> 0.05).
Conclusion: Short-term triple therapy increases bone mineral content but decreases bone strength of rats. Skeletal prophylaxis should be considered for patients on short-term triple therapy containing PPI.
Keywords: bone, gastric ulcer, omeprazole, osteoporosis, osteopenia, proton pump inhibitor
Introduction
Helicobacter pylori (H. pylori) is a type of Gram-negative bacteria known to cause peptic ulcer disease and gastric cancer.1 H. pylori infection is also associated with extra-gastroduodenal diseases, such as metabolic syndrome, cardiovascular diseases and neurodegenerative diseases due to alternation in the immune system and low-grade inflammation.2 Since bone cells are sensitive to pro-inflammatory cytokines,3 chronic H. pylori infection could predispose a person to bone loss and osteoporosis. The relationship between H. pylori infection and bone health in humans has been contradictory so far. A recent meta-analysis showed that H. pylori increases the risk of osteoporosis [odds ratio (OR) 1.61, 95% confidence interval (CI) 1.11–2.32] but is not associated with significant variation in bone mineral density.4 The limited evidence so far does not show a conclusive association between H. pylori infection and fracture risk.5 The effects of H. pylori infection on bone strength could be derived from destructive mechanical test, so it is suitable to be investigated in an in vivo model.
Triple therapy is considered the first-line therapy in eradicating H. pylori. It consists of a proton pump inhibitor (PPI), clarithromycin and amoxicillin or metronidazole.6 PPIs suppress gastric acid secretion, creating a hypochlorhydric environment which does not favour the absorption of skeletal beneficial minerals like calcium, and vitamins like vitamin B.7 The associated increase in gastrin level also triggers histamine release which stimulates osteoclast formation.7 In our previous study, two-month pantoprazole treatment causes a significant trabecular microstructural deterioration in normal male rats, which is reversible by calcium supplementation.8 Others have demonstrated a significant decline in bone mineral density, increased bone resorption and decreased bone formation in rats treated with omeprazole for a few months.9–11 However, these models are not perfect as the animals are not infected with H. pylori. Besides, the data on short-term skeletal effects of triple therapy or PPI are very limited.
Thus, this study aims to determine the effects of 14-day triple therapy treatment on the bone of rat infected with H. pylori. The study is a secondary analysis of bone samples derived from a study that primarily aims to establish a rat model of H. pylori-induced gastric ulcer. We determined the bone health of the rats defined by densitometry and mechanical methods and hypothesised some degree of alternations associated with the infection and treatment. The information derived from this study will be important in deciding whether skeletal prophylaxis is necessary for patients on short-term triple therapy.
Materials and Methods
Bacteria Culture
H. pylori strain in the form of stock culture was obtained from the Department of Microbiology, Universiti Kebangsaan Malaysia Medical Centre. The bacteria were inoculated onto the Columbia blood agar and incubated microaerophilically with 10% oxygen gas, 10% carbon dioxide gas and 80% nitrogen gas for 5 days at 37°C.
Preparation of Treatment Agents
The triple agents were prepared by crushing clarithromycin (Noripharma, Selangor, Malaysia), amoxicillin (Duopharma, Selangor, Malaysia) and omeprazole (Y.S.P. Industries, Kuala Lumpur, Malaysia) into powder and dissolved in distilled water. The dose of drugs used in this experiment was converted from human suggested dosage using body surface conversion formula.6,12 The dose of clarithromycin [51.37 mg/kg (body weight) b.w], amoxicillin (102.80 mg/kg b.w) and omeprazole (2.035 mg/kg b.w) is equivalent to 500 mg, 1000 mg and 20 mg, respectively, for humans.
Treatment of Animals
Three-month-old Sprague Dawley male rats (n=18) weighted between 200 and 250 g were provided by Laboratory Animal Resource Unit, Universiti Kebangsaan Malaysia (Kuala Lumpur, Malaysia). The rats were housed at the Animal Laboratory, Department of Pharmacology, Faculty of Medicine, Universiti Kebangsaan Malaysia (Cheras, Malaysia) in ventilated plastic cages under standard condition (25±2°C, 14/10 hours dark/light cycle). The rats were given access to tap water and standard rat chow (Product No: 702P, Goldcoin Feedmills, Klang, Malaysia) ad libitum throughout the study period. The rats were randomised into three groups (n=6/group) after acclimatised for one week. Two groups (negative control and triple therapy group) were inoculated with H.pylori suspension (10⁸ CFU/mL) via oral gavage twice a day at an interval of four hours for seven consecutive days,13 and the rats were left for four weeks for gastric ulcer formation. Then, one of the infected groups was treated orally with triple therapy regimen consisting of omeprazole [2.035 mg/kg body weight (bw)], amoxicillin (102.80 mg/kg bw) and clarithromycin (51.37 mg/kg bw) for 14 days. The rest of the groups received distilled water to mimic stress due to oral gavage (Table 1).
 |
Table 1 Treatment Regimens of the Rats |
The rats were weighed weekly using a digital balance. They were euthanised after 14 days of treatment using ketamine/xylazine overdose. Their femoral bones were harvested for analysis. The bones were cleaned of soft tissues and weighted. A digital calliper was used to measure the length and diameter of the femurs. The stomach of the rats was processed into paraffinised histological slides and stained with haematoxylin and eosin (H&E) to determine the absence/presence of gastric lesions, and Giemsa to determine the absence/presence of H.pylori. This study was performed in accordance with the Principles and Guidelines for Ethical Use of Laboratory Animals, Universiti Kebangsaan Malaysia, Malaysian Code of Practice for the Care and Use of Animals for Scientific Purpose (2015), and Malaysian Animal Welfare Act (Act 772). The protocol of this study was reviewed and approved by Universiti Kebangsaan Malaysia Animal Ethics Committee (approval code: FAR/PP/2016/ISA/28-SEPT./798-JAN.-2017-DEC.-2018).
Bone Densitometry
The left femurs of the rats were scanned at high resolution using dual-energy X-ray absorptiometry (Hologic Discovery QDR Wi densitometer, Hologic, MA, USA) to evaluate bone mineral content (BMC) and bone mineral density (BMD). All DXA scans were analysed using the manufacturer’s software (Hologic QDR-1000 System). The short-term in vivo coefficient of error of the machine is 1.4%.14
Bone Mechanical Strength Test
Three points bending test was performed on the femur to measure its mechanical strength. The test was conducted with a precision universal tester (Autograph AG-10kNG; Shimadzu, Kyoto, Japan) and Trapezium X material testing operation software. The femurs were supported by rounded edge-free notches with a 10 mm gap in between. A blunt-end aluminium roller stamp was lowered gradually (5 mm/min) until the strength of 1 N was achieved. The test was halted automatically when a loss of force >20 N or a linear change of 2 mm was detected. The Trapezium X software received the data and analysed the load (N), displacement (mm), stiffness (N/mm), stress (N/mm2), strain (%) and Young modulus (N/mm2).
Statistical Analysis
Shapiro–Wilk test was used to determine the normality of data. Comparison of body weight adopted a time × treatment design, thus was interpreted using mixed-design analysis of variance (ANOVA) with small effect analysis. Normally distributed parameters which involved end-point measurement were analysed using one-way ANOVA with post hoc pairwise comparison (Tukey’s or Dunnett T3). Data with skewed distribution were analysed using Kruskal Wallis test and Bonferroni test for pairwise analysis. All data were displayed as mean ± standard error of the mean. A p-value <0.05 was considered as statistically significant. Statistical analysis was conducted using Statistical Package for Social Sciences version 26 (IBM, Armonk, USA).
Results
There was a time effect on body weight (p<0.001) but the interaction between time and treatment was not significant (p=0.572). All groups experienced a significant increase in body weight with time (p<0.05). However, there was no significant difference in body weight among the groups at all time points (p>0.05) except during week 5 of the experiment, in which the body weight of the negative control group was lower than the triple therapy group (p=0.032) (Figure 1).
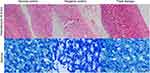
H. pylori infection for 4 weeks caused lesions on the gastric mucosa. Triple therapy eradicated the bacteria and preserved gastric mucosa integrity in infected rats. The H&E and Giemsa stained photomicrographs are presented in Figure 2.
The physical indices of femoral bone are depicted in Figure 3. The femoral weight of triple therapy group was significantly higher than the normal group and negative control group (p=0.016). Besides, the femoral area was significantly lower in the negative control group compared to the normal control group (p=0.01). Femoral length (p=0.006), area (p=0.002) and diameter (p=0.005) were significantly higher in the triple therapy group compared to the negative control group. No significant difference in femoral bone mineral density was detected among the study groups (p>0.05). However, BMC of the negative control group was significantly lower than the triple therapy group (p=0.014) (Figure 4).
No significant difference was observed in force, strain and stiffness among the study groups. However, the triple therapy group exhibited significantly lower strain (p=0.002) and displacement (p=0.005) than the normal group in displacement. The displacement of the triple therapy group was also lower compared to the negative control group (p=0.004) (Figure 5).
Discussion
The present study demonstrated that H. pylori infection did not affect bone mineral density/content and mechanical strength but significantly decreased femoral bone area compared to the normal control group. Triple therapy increased femoral weight, length, diameter and BMC, but reduced strain and displacement significantly compared to the negative control group.
Epidemiological studies on the relationship between H. pylori infection and osteoporosis have yielded heterogeneous findings so far, whereby positive15,16 and negligible associations17,18 have been reported. A recent meta-analysis showed that H. pylori infection was associated with osteoporosis [odds ratio (95% confidence interval): 1.39 (1.13–1.71)] but not significantly with BMD at the hip, lumbar and femur.4 The inflammatory and immune reactions due to H. pylori infection are proposed as the mechanism altering bone turnover and resulting in osteoporosis.18 In our study, H. pylori infection did not significantly affect femoral bone mineral density/content and strength except the femoral bone area. This finding might be due to the short period of infection.
Our study showed that two-week triple therapy increased BMC but not BMD compared to the negative control group. Since areal BMD is the mineral content in a given area of bone while BMC is the unadjusted mineral content,19 the significant difference observed might be related to the reduced femoral area observed in the negative control. The observation might not have significant biological importance since the mineral content per area did not significantly differ between groups. However, triple therapy significantly reduced displacement and strain of bone compared to the negative control. Changes in these two parameters reflect reduced ductility or the ability of the bone to deform to prevent fracture.20 Therefore, short-term triple therapy might predispose bone fracture.
There is no evidence associating the use of amoxicillin or clarithromycin with bone health so far. On the other hand, omeprazole is a stomach acid suppressor used to prevent stomach ulcer caused by H. pylori infection.6 PPIs have been reported to induce bone loss through two main mechanisms, namely hypergastrinemia and hypochlorhydria.7 The irreversible binding of PPI to H+/K+-ATPase of parietal cells reduces hydrogen ions and increases gastric pH.21 This event reduces somatostatin secretion from D-cells and increases gastrin secretion from G-cells, which in turn, stimulates hypersecretion of histamine from enterochromaffin-like cells.22 The increased circulating histamine stimulates differentiation of osteoclast precursors and bone resorption activity.23 Hypochlorhydria (reduced stomach acid) can reduce the intestinal absorption of minerals essential for bone health, like calcium. This event will trigger the secretion of parathyroid hormone to mobilise calcium storage in the bone through the resorption process,24,25 subsequently causing bone loss and increased fracture risk.26
Considering all evidence together, we suggest that short-term triple therapy containing PPI might reduce bone strength without altering BMD. Since bone strength is determined by factors beyond bone mass, such as bone microarchitecture/geometry,27 properties of organic matrix and mineral crystal, an in-depth investigation into the skeletal actions of PPI on these factors is warranted. Human cross-sectional studies demonstrated that chronic PPI use was associated with reduced trabecular BMD but not other bone geometry indices.28 A clinical trial showed that 8-week pantoprazole treatment increased deoxypyridinoline urine level associated with osteoclast activity in the subjects.29 In preclinical studies, PPI could decrease collagen production in lung and liver, but no studies have been conducted on bone.30
Several limitations were identified in our study. The primary aim of the study is to establish a rat model of H. pylori-induced gastric ulcer. Thus, the period of H. pylori infection and triple therapy treatment was not optimised to produce skeletal effects. Prolonging the infection or triple therapy might result in more severe bone changes. Characterisation of the cellular and molecular changes of the bone induced by H.plylori infection and triple therapy was not performed. These aspects would be explored in future studies. The photomicrographs show evidence of mucosal exfoliation resulted from tissue processing, so the readers should interpret the images with cautions.
Conclusion
Short-term H. pylori infection did not alter bone health significantly, but a two-week course of triple therapy consisting of clarithromycin, amoxicillin and omeprazole lowers bone ductability without affecting the femoral BMD in the rats. Cellular and mechanistic changes of the bone need to be characterised to understand the effects of triple therapy on bone health. In line with our observation, patients on short-term triple therapy containing PPI should consider prophylaxis such as calcium and vitamin D supplements to protect bone health.
Acknowledgments
We would like to thank Mr Mohd Mustazil Mohd Noor, Mr Azlan Mohd Arlamsyah, Ms Juliana Abdul Hamid and Mr Fadhlullah Zuhair Japar Sidik, as well as all laboratory technicians of the Department of Pharmacology, Faculty of Medicine, Universiti Kebangsaan Malaysia for their help and support.
Funding
We would like to acknowledge the Ministry of Higher Education Malaysia (FRGS/1/2016/SKK08/UKM/02/5) and Universiti Kebangsaan Malaysia (FF-2018-422) for financial support. Dr Sophia Ogechi Ekeuku is a postdoctoral researcher funded by Universiti Kebangsaan Malaysia through FPR-1.
Disclosure
The authors report no conflicts of interest in this work.
References
1. White JR, Winter JA, Robinson K. Differential inflammatory response to Helicobacter pylori infection: etiology and clinical outcomes. J Inflamm Res. 2015;8:137–147.
2. Tsay F-W, Hsu P-I. H. pylori infection and extra-gastroduodenal diseases. J Biomed Sci. 2018;25(1):65. doi:10.1186/s12929-018-0469-6
3. Mohamad NV, Soelaiman IN, Chin KY. Are oxidative stress and inflammation mediators of bone loss due to estrogen deficiency? A review of current evidence. Endocr Metab Immune Disord Drug Targets. 2020;20(9):1478–1487. doi:10.2174/1871530320666200604160614
4. Wang T, Li X, Zhang Q, et al. Relationship between Helicobacter pylori infection and osteoporosis: a systematic review and meta-analysis. BMJ Open. 2019;9(6):e027356.
5. Chen L-W, Chen F-P, Hsieh C-W, Kuo S-F, Chien R-N. Analysis of the associations among Helicobacter pylori infection, adiponectin, leptin, and 10-year fracture risk using the fracture risk assessment tool: a cross-sectional community-based study. PLoS One. 2017;12(4):e0175365. doi:10.1371/journal.pone.0175365
6. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112(2):212–239. doi:10.1038/ajg.2016.563
7. Thong BKS, Ima-Nirwana S, Chin KY. Proton pump inhibitors and fracture risk: a review of current evidence and mechanisms involved. Int J Environ Res Public Health. 2019;16(9):1571. doi:10.3390/ijerph16091571
8. Chin KY, Thong BKS, Kamalulloh RF, et al. Effects of calcium and annatto tocotrienol supplementation on bone loss induced by pantoprazole in male rats. Drug Des Devel Ther. 2020;14:2561–2572. doi:10.2147/DDDT.S260565
9. Yanagihara GR, de Paiva AG, Neto MP, et al. Effects of long-term administration of omeprazole on bone mineral density and the mechanical properties of the bone. Rev Bras Ortop. 2015;50(2):232–238. doi:10.1016/j.rbo.2014.05.012
10. Takasugi S, Shioyama M, Kitade M, Nagata M, Yamaji T. Effects of proton pump inhibitor administration and intake of a combination of yogurt and galactooligosaccharides on bone and mineral metabolism in rats. Nutrients. 2016;8(10):653. doi:10.3390/nu8100653
11. Hasanin AH. Impact of omeprazole on bone remodeling in normal and ovariectomised Wistar rats. Eur Rev Med Pharmacol Sci. 2014;18(13):1948–1956.
12. Nair A, Morsy MA, Jacob S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev Res. 2018;79(8):373–382. doi:10.1002/ddr.21461
13. Werawatganon D. Simple animal model of Helicobacter pylori infection. World J Gastroenterol. 2014;20(21):6420–6424. doi:10.3748/wjg.v20.i21.6420
14. Subramaniam S, Mohamad NV, Chan CY, Ima Nirwana S, Kok Yong C. Calculating in-vivo short-term precision error of dual-energy X-ray absorptiometry in human and animal: a technical report. Med Health. 2020;15:70–77. doi:10.17576/MH.2020.1501.06
15. Asaoka D, Nagahara A, Shimada Y, et al. Risk factors for osteoporosis in Japan: is it associated with Helicobacter pylori? Ther Clin Risk Manag. 2015;11:381–391. doi:10.2147/TCRM.S80647
16. Lin S-C, Koo M, Tsai K-W. Association between Helicobacter pylori infection and risk of osteoporosis in elderly Taiwanese women with upper gastrointestinal diseases: a retrospective patient record review. Gastroenterol Res Pract. 2014;2014:814756. doi:10.1155/2014/814756
17. Kakehasi AM, Mendes CMC, Coelho LGV, Castro LP, Barbosa AJA. The presence of Helicobacter pylori in postmenopausal women is not a factor to the decrease of bone mineral density. Arq Gastroenterol. 2007;44(3):266–270. doi:10.1590/S0004-28032007000300016
18. Pan B-L, Huang C-F, Chuah S-K, Chiang J-C, Loke -S-S. Relationship between Helicobacter pylori infection and bone mineral density: a retrospective cross-sectional study. BMC Gastroenterol. 2018;18(1):54. doi:10.1186/s12876-018-0780-4
19. Chaudhary NK, Timilsena MN, Sunuwar DR, Pradhan PMS, Sangroula RK. Association of lifestyle and food consumption with bone mineral density among people aged 50 years and above attending the hospitals of Kathmandu, Nepal. J Osteoporos. 2019;2019:1536394. doi:10.1155/2019/1536394
20. Forestier-Zhang L, Bishop N. Bone strength in children: understanding basic bone biomechanics. Arch Dis Child. 2016;101(1):2–7. doi:10.1136/archdischild-2015-308597
21. Dammann HG, Burkhardt F, Wolf N. The effects of oral rabeprazole on endocrine and gastric secretory function in healthy volunteers. Aliment Pharmacol Ther. 1999;13(9):1195–1203. doi:10.1046/j.1365-2036.1999.00545.x
22. Dacha S, Razvi M, Massaad J, Cai Q, Wehbi M. Hypergastrinemia. Gastroenterol Rep. 2015;3(3):201–208. doi:10.1093/gastro/gov004
23. Biosse-Duplan M, Baroukh B, Dy M, de Vernejoul M-C, Saffar J-L. Histamine promotes osteoclastogenesis through the differential expression of histamine receptors on osteoclasts and osteoblasts. Am J Pathol. 2009;174(4):1426–1434. doi:10.2353/ajpath.2009.080871
24. Goltzman D, Mannstadt M, Marcocci C. Physiology of the calcium-parathyroid hormone-vitamin D axis. Front Horm Res. 2018;50:1–13.
25. Kroll MH. Parathyroid hormone temporal effects on bone formation and resorption. Bull Math Biol. 2000;62(1):163–188.
26. Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care. 2008;35(2):215–vi. doi:10.1016/j.pop.2008.01.007
27. Fonseca H, Moreira-Gonçalves D, Coriolano H-JA, Duarte JA. Bone quality: the determinants of bone strength and fragility. Sports Med. 2014;44(1):37–53.
28. Maggio M, Lauretani F, Ceda GP, et al. Use of proton pump inhibitors is associated with lower trabecular bone density in older individuals. Bone. 2013;57(2):437–442. doi:10.1016/j.bone.2013.09.014
29. Jo Y, Park E, Ahn SB, et al. A proton pump inhibitor’s effect on bone metabolism mediated by osteoclast action in old age: a prospective randomised study. Gut Liver. 2015;9(5):607–614. doi:10.5009/gnl14135
30. Ghebre YT. Proton pump inhibitors and osteoporosis: is collagen a direct target? Front Endocrinol (Lausanne). 2020;11:473. doi:10.3389/fendo.2020.00473
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.