Back to Journals » International Journal of Nanomedicine » Volume 15
The Size-dependent Cytotoxicity of Amorphous Silica Nanoparticles: A Systematic Review of in vitro Studies
Authors Dong X, Wu Z, Li X, Xiao L, Yang M, Li Y, Duan J, Sun Z
Received 8 August 2020
Accepted for publication 29 September 2020
Published 18 November 2020 Volume 2020:15 Pages 9089—9113
DOI https://doi.org/10.2147/IJN.S276105
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Linlin Sun
Xuemeng Dong,1 Zehao Wu,1 Xiuping Li,1 Liyan Xiao,1 Man Yang,1,2 Yang Li,1,2 Junchao Duan,1,2 Zhiwei Sun1,2
1School of Public Health, Capital Medical University, Beijing 100069, People’s Republic of China; 2Beijing Key Laboratory of Environmental Toxicology, Capital Medical University, Beijing 100069, People’s Republic of China
Correspondence: Zhiwei Sun; Yang Li
Beijing Key Laboratory of Environmental Toxicology, Capital Medical University, Beijing 100069, People’s Republic of China
Tel/Fax +86 010 83911507
; +86 010 83911868
Email [email protected]; [email protected]
Abstract: With the increasing production and application of engineered amorphous silica nanoparticles (aSiNPs), people have more opportunities to be exposed to aSiNPs. However, the knowledge of its adverse health effects and related mechanisms is still limited, compared with the well-studied crystalline micron-sized silica. Since small differences in the physical–chemical properties of nanoparticles could cause significant differences in the toxic effect, it is important to distinguish how these variations influence the outcoming toxicity. Notably, particle size, as one of the essential characterizations of aSiNPs, is relevant to its biological activities. Thus, the aim of this systematic review was to summarize the relationship between the particle size of aSiNPs and its adverse biological effects. In order to avoid the influence of complicated in vivo experimental conditions on the toxic outcome, only in vitro toxicity studies which reported on the cytotoxic effect of different sizes aSiNPs were included. After the systematic literature retrieval, selection, and quality assessment process, 76 eligible scientific papers were finally included in this review. There were 76% of the studies that concluded a size-dependent cytotoxicity of aSiNPs, in which smaller-sized aSiNPs possessed greater toxicity. However, this trend could be modified by certain influence factors, such as the synthetic method of aSiNPs, particle aggregation state in cell culture medium, toxicity endpoint detection method, and some other experimental conditions. The effects of these influence factors on the size-dependent cytotoxicity of aSiNPs were also discussed in detail in the present review.
Keywords: silica nanoparticles, particle size, cytotoxicity, cell death, nanotoxicology, influence factors
Introduction
Silica particles often occur in crystalline and amorphous forms. Compared with crystalline silica, natural amorphous silica is generally considered as less harmful, since the toxicological potential of silica has so far been linked to its crystallinity.1 Synthetic amorphous silica nanoparticles (aSiNPs), an attractive engineering nanomaterial, was commonly referred to amorphous silicon dioxide (SiO2) with particle size ≤100 nm. Contrary to its micron-sized particles, aSiNPs possesses many excellent physical-chemical properties,2 and is penetrating into many aspects of people’s lives and productions.3 According to the Consumer Products Inventory (CPI), there were over 100 commercial products which contained aSiNPs, including foodstuffs, toothpastes, cosmetics, paints, electronic devices, even drugs and vitamins.4 Due to its multiple utilizations, aSiNPs has become the second largest engineering nanomaterial in terms of annual output.5 The large-scale production and widespread application of aSiNPs have increased the risk of human exposure. Thus, the adverse health impact of this kind of nanomaterial on human beings deserves more attention and should be carefully addressed.
The human body can be exposed to aSiNPs through several routes such as inhalation, oral ingestion, transdermal penetration and parenteral injection intentionally or unintentionally.6 In the last two decades, numerous studies in vivo and in vitro have evaluated the toxicity and biosafety of aSiNPs, and have demonstrated its potential adverse effects on human health. Several researches in vivo showed that after entering the experimental animal body, aSiNPs could distribute in almost all organs, and cause inflammation as well as tissue damage through direct or indirect ways.7,8 Further in vitro mechanism studies reported that aSiNPs could accumulate in cells by both active endocytotic pathway and passive diffusion, and distribute within endocytotic vesicles or freely in cytoplasma and organelles.9 Meanwhile, aSiNPs could exert cytotoxicity and genotoxicity in many kinds of cell lines, which was considered through the oxidative stress, endoplasmic reticulum stress, autophagy dysfunction or pro-inflammatory response induced by the particles.10,11
It is well accepted in nanotoxicology that physical–chemical properties of nanomaterials, such as particle size, surface area, morphology, porosity, aggregation state, crystallinity and other characterizations, should be taken into account when assessing the potential toxicity of nanomaterials in biological systems. As to aSiNPs, a large number of studies have confirmed that these properties could influence the adsorption, distribution, excretion as well as cellular internalization of the particles, which were further associated with the toxic effect in vivo and in vitro.12–14 Notably, particle size, as an essential characterization of aSiNPs, is relevant to its biological activities. In nanoscale, the extreme small size confers specific large surface area of nanoparticles, which makes the surface atoms or molecules increase exponentially. Therefore, aSiNPs of smaller size possesses larger surface area and higher surface reactivity, which may render it more active chemically and biologically.
The possible biological effect related to aSiNPs exposure of different particle sizes have been widely studied. Researchers indicated that smaller size could facilitate the cellular uptake, tissue penetration and systemic distribution of aSiNPs.13,15 In addition, plenty of studies also demonstrated the size dependent toxic effect of aSiNPs—the smaller the particle size was, the greater the toxicity produced.10,16 However, due to the complexity of experimental conditions and biological systems, there were still some researchers who reported that larger aSiNPs possessed higher toxicity or that no significant difference was observed between aSiNPs with different particle sizes.17,18 Herein, we provided a systematic literature review to summarize the relationship between the particle size of aSiNPs and its adverse biological effect. In order to avoid the influence of complicated experimental conditions in vivo, we only included in vitro studies of cell experiments to analyze the toxic intensity of aSiNPs with different sizes. We also explored the reasons why some studies did not conclude a size-dependent cytotoxicity of aSiNPs, and several influence factors of this trend were extracted and discussed in detail in this review.
Methodology
Inclusion Criteria
In this review, we would like to summarize the relationship between particle size of aSiNPs and its potential cytotoxicity. Firstly, the influence of other characteristics, such as crystallinity, shape, porosity, surface modification and so on, should be eliminated. Hence, only studies using amorphous non-mesoporous sphere silica nanoparticles without any surface modification were included. Secondly, compared with in vitro cell experiment, the internal environment of experimental animals is more complex and more difficult to control. As known, the ADME process and even the toxicity of aSiNPs can be influenced obviously by different exposure routes and complex internal environment, such as the presence of protein, the change of pH value, the difference of ionic strength and so on. Moreover, it was also difficult to trace the nanoparticles in vivo. Thus, in order to better summarize the size-dependent toxicity of aSiNPs and explore the influence factors of this trend, only in vitro studies assessing cytotoxicity of aSiNPs were included. Finally, we chose cell death as the toxicity endpoint to compare the toxic effect of aSiNPs with different particles, which due to the detection method of cell death is simple, convenient, and repeatable. Overall, according to the purpose of this review, studies should meet the following inclusion criteria otherwise they would be excluded:
- Use amorphous non-mesoporous sphere silica nanoparticles without any surface modification;
- Use different sizes of aSiNPs;
- Primary size of aSiNPs should be reported;
- In vitro cytotoxicity studies;
- Toxicological endpoints should include the detection of cell death or cell viability;
- Papers should be published in English.
Literature Search
A comprehensive literature search was performed to identify studies describing the cytotoxicity of aSiNPs based on the Office of Health Assessment and Translation (OHAT) approach for systematic review and evidence integration. We searched two databases for articles published and indexed from January 1, 2000 to July 30, 2020. In the PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and Web of Science (https://webofknowledge.com/) databases, the following search strategy was used: (TS = “Silica nanoparticle” OR TS = “SiO2 nanoparticle” OR TS = “silicon dioxide nanoparticle” OR TS = “silica nanomaterial” OR TS = “SiO2 nanomaterial” OR TS = “Nano silica” OR TS = “Nano SiO2”) AND (TS = “Cytotoxic*” OR TS = “Toxic*” OR TS = “Adverse effect”). Therefore, we could retrieve as many articles as possible concerning the toxic effect of aSiNPs. The article retrieval process was summarized in a flow chart as shown in Figure 1.
 |
Figure 1 Flow chart of systematic selection of articles. |
Quality Assessment and Information Extraction
Quality assessment of included studies was based on the guideline for assessing quality of in vitro studies as suggested by Samuel et al.19 The quality of each study was assessed from 11 items concerning the basic information and experimental design, which were scientific background description, study purpose description, study model justification, study design description, cell culture condition description, endpoint measurement description, endpoint outcome description, statistical method description, dose/concentration response consideration, result interpretation, and discussion, as well as research funding. Each item was scored 1 and the overall score was 11. Studies were graded as poor quality if they met ≤4 items, fair if they met 5 to 8 items, and good if they met ≥9 items.
Information extraction was performed independently by two reviewers using a predesigned data extraction form. For each study, the following data were extracted:
- Basic information of the article: first author and year published;
- ASiNPs characterization: primary particle size and aggregation state;
- Study design: cell type, exposure dose, and exposure time;
- Methodology: detection method of cell viability;
- Result: relationship between particle size and cytotoxicity.
Results
Search Result Description
We retrieved a list of 4038 and 1543 articles published in PubMed and Web of Science, respectively, using the approach described in the methodology section. As shown in Figure 1, after removing duplicates and screening the titles and abstracts, 185 relevant articles were left. Then, another 109 articles that did not meet the inclusion criteria were removed through full text retrieval. Finally, 76 eligible articles reporting the cytotoxicity of aSiNPs with different sizes were selected for this systematic review. All 76 papers had good quality rating and the quality assessment result was shown in Table 1. The information descriptions of included articles were manifested in Table 2, which contains article source, type of cell line, primary size of aSiNPs, exposure dosage, exposure time, detection method of cell death/cell viability, and relationship between particle size and cytotoxicity. As summarized in Figure 2, 76% of the studies concluded that smaller aSiNPs possessed greater cytotoxicity, while 17% of the studies obtained the opposite conclusion, and the remaining 7% studies did not show any significant difference in the toxicity of aSiNPs with different sizes.
 |  |  |
Table 1 Quality Assessment of Included Studies |
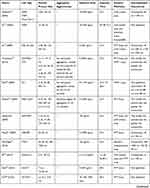 | 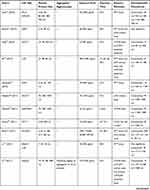 |  |  |  |  | 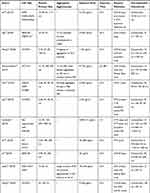 |
Table 2 In vitro Studies on Cytotoxicity of Amorphous Silica Nanoparticles (aSiNPs) with Different Sizes |
 |
Figure 2 Fan-shaped diagram of the relationship between cytotoxicity of aSiNPs and its particle size. |
Size-dependent Cytotoxicity of aSiNPs
Size-dependent Cytotoxicity of aSiNPs on Cell Lines from Respiratory System
Gonzalez et al20 examined the cytotoxic activity of aSiNPs with three different sizes in human alveolar carcinoma cell line (A549). The ED50 of 16 nm, 60 nm and 104 nm aSiNPs, determined by the MTT assay after 24 h exposure, was 45.9, 48.9, and 165.9 μg/mL, respectively, indicating a size-response cytotoxicity of aSiNPs. Fede et al21 also attempted to study the role of size in aSiNPs (9 and 18 nm) toxicity in A549 cells. Results obtained showed that two sizes of aSiNPs caused very similar cytotoxicity, while the smaller aSiNPs was more effective in inflammatory and apoptosis processes. In the study of Gonzalez et al,22 A549 cells were exposed to a series of different sizes of aSiNPs (ranged from 12–174 nm), and a size-dependency of toxicity was observed. The smallest aSiNPs (12 nm) induced the highest cytotoxicity, especially in the absence of serum. Tokgun et al23 demonstrated that among 6, 15, and 30 nm sizes of aSiNPs, 6 nm-sized nanoparticles had strongest cytotoxic effects on A549 cells, and this cytotoxicity came from dead receptor-mediated induction of apoptosis. Liu et al24 examined the cell viability of A549 cells after exposing to SiO2 particles at various concentrations (5, 10, 20, 30, 40, 60, 80, and 100 μg/mL). Results of CCK-8 assay showed that exposures of 10–20 nm aSiNPs resulted in significantly decreased viability of A549 cells, while 5 μm SiO2 particles did not affect the cell viability obviously. Leibe et al10 analyzed the effects of two aSiNPs on A549 cells, which were 12 nm aSiNPs produced by flame synthesis and 50 nm aSiNPs produced by Stöber synthesis. As observed by the LDH release assay, the cytotoxicity induced by pyrogenic 12 nm aSiNPs was more pronounced than colloidal 50 nm aSiNPs. In addition, both two kinds of aSiNPs reduced the number of viable cells and provoke cell death only in the absence, but not in the presence of serum.
Wottrich et al25 showed that aSiNPs (60 nm and 100 nm) induced dose-dependent and size-related reduction in cell viability of A549 cells and differentiated macrophage-like THP-1 cells. Meanwhile, smaller aSiNPs caused higher IL-6 and IL-8 release in co-culture system of A549 and macrophages (Mono Mac 6 or differentiated THP-1). In the research of Napierska et al,26 co-culture of A549 cells and differentiated THP-1 cells were also introduced to elucidate the toxicity and related mechanism of aSiNPs (2, 16, 60, and 104 nm). A size-dependent cytotoxic response to aSiNPs was observed in both cell lines, and TC50 increased with particle size. In addition, the release of pro-inflammatory mediators by endothelial cells after inhalation of aSiNPs could be a possible mechanism of its respiratory toxicity. Panas et al27 explored the toxic effects of 12 nm and 25 nm aSiNPs in A549 cells and RAW264.7 mouse macrophage cell line in the presence and absence of serum. Both two particles decreased cell viability of A549 and RAW264.7 cells, and induced pro-inflammatory significantly in the absence of serum. Cytotoxicity of 12 nm aSiNPs was more prominent than that of 25 nm. Conversely, the toxicity was completely suppressed in the presence of serum.
Li et al28,29 investigated the possible cytotoxicity of three sizes of aSiNPs (40, 60, and 200 nm) in human bronchial epithelial BEAS-2B cells. These particles inhibited the cell viability in a size-dependent manner, the smaller aSiNPs produced higher toxic effect. Låg et al30 studied the toxicity of 10 nm and 50 nm aSiNPs using two different human bronchial epithelial cell lines (BEAS-2B and HBEC3-KT). As measured by the LDH release assay, the cell viability was reduced approximately 50% after exposing to 50 μg/mL of 10 nm aSiNPs for 20 h in both cell types and 10–25% after exposing to 200 μg/mL 50 nm aSiNPs. Thus, 10 nm aSiNPs was far more cytotoxic than 50 nm aSiNPs. Kasper et al31 examined the cytotoxicity of different-sized aSiNPs (30, 70, and 300 nm) on lung epithelial cells line NCI H441 as well as endothelial cell line ISO-HAS-1, and illustrated a clearly increased toxicity of the smaller-sized aSiNPs. McCarthy et al32 studied the effects of exposing human lung adenocarcinoma cells (Calu-3) to aSiNPs of various sizes (10, 150, and 500 nm). Ten nanometer aSiNPs was found to induce cytotoxicity in a time and concentration-dependent way, while larger nanoparticles (150 and 500 nm) were not found to be cytotoxic even at high concentrations. Xu et al33 exposed human lung fibroblasts (HFL-Is) to 20 nm and 80 nm aSiNPs. Results of the cell viability, the ratio of apoptosis and the pathway of cell injury demonstrated that a size‐associated and a dose‐dependent toxicity on HFL-Is was induced by aSiNPs, smaller sizes and higher concentrations of aSiNPs seemed more cytotoxic. Guichard et al34 aimed to compare the cytotoxicity and genotoxicity of five representative manufactured aSiNPs samples (Pyr 20, Pyr 25/70, Pre 20, Col 15 and Col 40/80) in Chinese hamster lung fibroblasts (V79 cells). Results showed that the finer aSiNPs samples were more cytotoxic than the coarser samples. The cytotoxicity of aSiNPs in V79 cells can be ranked as follows: Col 15 (15 nm) >Pyr 20 (19 nm)=Pre 20 (19 nm) >Pyr 25/70 (25 and 70 nm)=Col 40/80 (38 and 79 nm).
Size-dependent Cytotoxicity of aSiNPs on Cell Lines from Cardiovascular System
Corbalan et al35 described that exposures of primary human umbilical vein endothelial cells (HUVECs) to aSiNPs (10, 50, 150, 500 nm) led to increased cell death. The smallest aSiNPs (10 nm) induced the highest cytotoxicity, while larger aSiNPs (50, 150, and 500 nm) showed very limited or even no cytotoxicity. Nemmar et al36 reported that exposures of HUVECs to aSiNPs (50 and 500 nm) showed a reduced cellular viability, and 50 nm aSiNPs led to more pronounced toxic effects than that with 500 nm aSiNPs. In the study of Wang et al,15 the potential of four sizes of aSiNPs (10, 25, 50, 100 nm) to induce the apoptosis of HUVECs was investigated. The results showed that all the four sizes of aSiNPs could significantly elicit apoptosis in HUVECs at the tested concentrations (1, 5, 25 mg/mL). Moreover, the apoptotic rates were increased with the elevating levels and decreasing sizes of administrative aSiNPs, showing both dose- and size-dependent effect relationships. In the research of Lee et al,16 HUVECs were treated with various concentrations (5–25 μg/mL) of aSiNPs (20, 30, 40, and 50 nm) for 24 h. Result of apoptosis and/or necrosis assessment showed that aSiNPs of 20 nm in size, but not those of 30 nm, 40 nm, and 50 nm in size, induced significant decreases in cellular viability.
Napierska et al2 reported that exposures to aSiNPs (14–335 nm) would result in cytotoxic damage and a decrease in cell survival in human umbilical vein endothelial cell line (EAHY926) in a dose-related manner. Concentrations leading to a 50% reduction in cell viability (TC50) declined gradually with the nanoparticle size decreased. Furthermore, the smaller particles also appeared to affect the exposed cells faster within just a few hours. The cytotoxicity of a series of aSiNPs (2–335 nm) in EAHY926 cell line and J774 differentiated monocyte-macrophage mouse cell line was investigated using MTT and WST1 assays, respectively. When cells were exposed to the same mass of aSiNPs, larger particles were less toxic compared to smaller ones.37 Napierska et al38 studied the cytotoxicity response of EAHY926 cells to aSiNPs of two sizes (16 and 60 nm). Results of MTT and LDH assays showed that cytotoxic effects could be detected after four hours exposure to 16 nm aSiNPs at the concentration of 50 μg/mL. However, no significant cytotoxicity was found for 60 nm aSiNPs at any dose and any time of exposure. Ye et al39 exposed rat embryonic ventricular myocardial cell line H9c2 (2–1) to 21 and 48 nm aSiNPs, and assessed cell viability, oxidative stress and cell cycle arrest. Consequently, both aSiNPs produced significant decreases in viabilities in dose-dependent manners. Furthermore, silica nanoparticles of smaller size possessed higher cytotoxicity.
Size-dependent Cytotoxicity of aSiNPs on Cell Lines from Digestive System
In the study of Ye et al,40 the normal human hepatic cell line (L-02) was exposed to 0.2, 0.4, and 0.6 mg/mL of aSiNPs (21, 48, and 86 nm) for 12, 24, 36, and 48 h. As indicated by MTT and LDH assays, 21 nm aSiNPs showed significantly higher cytotoxicity than the large sizes of aSiNPs. Xue et al41 exposed buffalo rat liver (BRL) cell line to nano- and microsized silica particles (20 nm and 0.5–6 μm). Both particles exhibited significant toxicity as determined by MTT, LDH leakage and mitochondrial membrane potential assessments. Moreover, greater effects were found for the silica nanoparticle samples than for the micrometer particle samples. Li et al42 compared the potential toxicity induced by aSiNPs with different sizes (19, 43, 68, and 498 nm) on cultured human hepatoma cell line (HepG2). Results of CCK-8 and LDH release assay suggested that the cytotoxicity of aSiNPs strongly depended on the particle size, and smaller particles possessed higher toxic effects. Kim et al13 studied the cytotoxicity of different sizes of aSiNPs (20, 30, 40, and 50 nm) in HepG2 cells and two other human carcinoma cell lines (A549 and SW480). The result of cell viability manifested that aSiNPs smaller than 30 nm in size were lethal to the cells, while the cytotoxicity of 40 and 50 nm aSiNPs was slight.
Tarantini et al43 introduced human intestinal cell line (Caco-2), which is a well characterized model of the intestinal epithelium and commonly used in toxicity studies. Following exposures to aSiNPs (15 and 55 nm), a statistically significant dose-dependent decrease in cell viability was observed in the 15 nm aSiNPs treated group, in contrast, 55 nm aSiNPs only affected viability of Caco-2 cells at the highest tested dose. Gehrke et al44 assessed the toxic effects of 12 nm, 40 nm, and 200 nm aSiNPs in human colon carcinoma cell line (HT29). Size-dependent cytotoxicity was confirmed using the SRB assay, WST-1 assay and LDH activity assay. Fritsch-Decker et al45 tested the effects of aSiNPs with nominal diameters of 70, 200, and 500 nm on the viability of human colon cancer epithelial cell line (HCT116). Administration of particles with increasing diameters but at the same mass concentration (10 or 50 µg/mL) resulted in an inverse relationship of particle size and cytotoxicity, larger particles were less toxic compared to smaller particles.
Size-dependent Cytotoxicity of aSiNPs on Cell Lines from Monocyte Macrophage System
Waters et al46 exposed RAW 264.7 cells to aSiNPs (7–500 nm) with mass concentrations from 0 to 1000 µg/mL, and observed the dose–response and size-dependent relationship for cytotoxicity. In addition, whole genome microarray analysis of the early gene expression changes induced by 10 and 500 nm aSiNPs manifested that the magnitude of changes for the majority of affected genes correlated more tightly with particle surface area than either particle mass or number. In other words, it was also identified that the changes of gene expression were particle size specific. Nabeshi et al47 investigated the cytotoxicity of 70, 300, and 1000 nm aSiNPs in RAW264.7 cells using WST-8 cell proliferation assay. As shown, 30 μg/mL of 300 and 1000 nm aSiNPs treatment for five days did not induce any toxic effect, while 70 nm aSiNPs treatment induced obvious cytotoxicity. Saikia et al12 exposed RAW 264.7 cells to 50 nm and 500 nm aSiNPs, and measured the LC50 values of each aSiNPs in the presence and absence of proteins. Results manifested that with the decrease of particle size, the cytotoxicity of aSiNPs increased by an increase in the surface area. Furthermore, the presence of the protein corona reduced the toxicity of both aSiNPs, which was probably due to the reduction of particle uptake efficiency.
Kim et al48 assessed the cytotoxicity of aSiNPs (15, 50, 100 nm) on human macrophage cells (U937) by the CCK-8 assay. As shown, the cell viability decreased depending on the particles size, and 15 nm particles triggered cell death more significantly than did 50 nm or 100 nm particles. The LD50 of 15 nm aSiNPs was 1.29 mg/mL and that of 50 nm aSiNPs was 1.5 mg/mL. In the study conducted by Premshekharan et al,49 the MTT assay was performed to explore the non-cytotoxic exposure levels of engineered 50 nm and 2 μm aSiNPs on differentiated THP-1 macrophages. For 2 μm silica particles, dosage of 50 μg/mL and below did not differ apparently from the control, whereas for 50 nm aSiNPs, doses of 10 μg/mL and below did not differ significantly from the control. Thus, 50 nm aSiNPs was more toxic than 2 μm particles. Kusaka et al50 addressed the relationship between the size of aSiNPs (30–10,000 nm) and the cytotoxicity as well as inflammatory activity in primary mouse macrophages (C57BL/6N mouse bone marrow-derived macrophages, BMDMs). The size-dependent induction of cytotoxicity and IL-1β secretion was observed, the toxic activity of 30–1000 nm aSiNPs was apparently higher than that of 3000 nm and 10,000 nm silica particles.
In the research of Mendoza et al,51 the effects of 10 or 100 nm aSiNPs on human peripheral blood mononuclear cells (PBMC) were examined. Ten nanometers of aSiNPs were more cytotoxic and induced more oxidative damage than 100 nm particles. Nakanishi et al52 examined LDH release as well as IL-1β and IL-18 production after incubating mouse bone marrow dendritic cells (mBMDCs) with aSiNPs of different sizes (30, 70, and 300 nm). An obviously size-dependent toxic and inflammation induction effect was confirmed, in which the greatest cytotoxicity was seen with 30 nm aSiNPs. Kojima et al53 further investigated the mechanism of inflammatory response in mouse Kupffer cell line (KUP5) induced by aSiNPs of 30, 70, and 300 nm. The cell viability decreased apparently with the particle size. In addition, 30 nm aSiNPs led to the greatest increase of IL-1β secretion, while the effect of 70 nm aSiNPs was similar to that of 300 nm particles.
Size-dependent Cytotoxicity of aSiNPs on Cell Lines from Epidermis
Yang et al54 evaluated the effects of aSiNPs (15, 30, and 365 nm) on cell viability, cell cycle and apoptosis in human epidermal keratinocyte cell line (HaCaT). The IC50 values of 15 nm, 30 nm and microsized silica particles were 23.0 μg/mL, 27.3 μg/mL and 34.8 μg/mL, respectively. Furthermore, the smaller SiO2 particle size was, the higher apoptotic rate the cells underwent. Gong et al55 explored the adverse effects of aSiNPs (15, 30, and 100 nm) on HaCaT cells as well. As observed in CCK-8 assay, exposure to aSiNPs decreased the cell viability in a concentration- and size-dependent manner, and the IC50 values of 24 h exposure were 19.4±1.3, 27.7±1.5 and 35.9±1.6 μg/mL for 15, 30, and 100 nm aSiNPs, respectively. The tendency of smaller nanoparticles showing higher toxicity was found. The nanoscale silica particles used by the two research groups were both 15 and 30 nm, and the IC50 values of each size of nanosilica were similar.
Nabeshi et al56 illustrated the size-dependent cytotoxicity of aSiNPs (70, 300, and 1000 nm) on HaCaT cells. Results of LDH release, intracellular ROS level and DNA oxidative damage indicated that the smallest particles produced greatest toxic effects. Then, they further studied the cytotoxic effects and inflammatory response caused by 30, 70, and 300 nm aSiNPs, and a clearly size-dependent manner of aSiNPs could be also concluded in cell viability and IL-6 production.57 Gong et al58 reported that exposure of HaCaT cells and HaCaT-shPARP-1 cells to 15 nm aSiNPs resulted in significantly decreased cell viability in a dose-dependent manner. Meanwhile, flow cytometric analysis showed the apoptotic rate in HaCaT-shPARP-1 cells induced by nanosized SiO2 (15 nm) was significantly higher than by microsized SiO2 (1–5 μm).
In the study of Yu et al,59 mouse keratinocytes cells (HEL-30) were exposed for 24 h to various concentrations (0–200 µg/mL) of aSiNPs with different sizes (30, 48, 118, and 535 nm). Results of LDH and MTT assay showed dose- and size-dependent cytotoxicity of 30 and 48 nm nanoparticles, while larger sizes of aSiNPs (118 and 535 nm) exhibited less toxicity compared with smaller nanoparticles. Vicente et al60 investigated the toxicological profile of aSiNPs (20, 70, 200, and 500 nm) in human keratinocytes (K17) and human dermal fibroblasts (HDF), and described that the ultra‐small particles (20 nm) was the most toxic aSiNPs, whereas particles larger than 70 nm demonstrated to be nontoxic for both dermal cells in a wide range of concentrations.
Size-dependent Cytotoxicity of aSiNPs on Cell Lines from Kidney or Nervous System
Wang et al61,62 showed that exposure to 20 or 50 nm aSiNPs at dosage levels between 20 and 100 µg/mL decreased cell viability of cultured human embryonic kidney cells (HEK293) in a dose-dependent manner. Median lethal dose (LD50) of 24 h exposure was 80.2±6.4 and 140.3±8.6 µg/mL for 20 and 50 nm aSiNPs, respectively. The same research group evaluated the neurotoxicity of these aSiNPs in pheochromocytoma cells (PC12) as well. As determined by MTT assay, the cell viability decreased as the function of both dose and time. The LD50 values obtained after 24 h exposure were 120±8 µg/mL for the 20 nm aSiNPs and 320±10 µg/mL for the 50 nm aSiNPs.63 Yuan et al64 studied the toxic effects of six sizes aSiNPs (20, 50, 80, 140, 280, and 760 nm) also using HEK293 cells and PC12 cells. Compared with microsized-SiO2 (140, 280, and 800 nm), nanosized-SiO2 (20, 50, and 80 nm) caused more severe cell damage, and it was obvious that the IC50 values of aSiNPs increased with the particle size. Kamikubo et al3 investigated the toxic effects of aSiNPs (10, 30, 100, 400, 1500 nm) on primary cultured rat hippocampal cells and HEK29 cells. As determined by the CellTiter-Glo 2.0 (CTG) Luminescent Cell Viability Assay system, aSiNPs with smaller sizes tended to cause higher toxicity on both types of cells at concentrations of 0.03–1 mg/mL. In addition, compared to HEK293 cells, rat hippocampal cells were more vulnerable to aSiNPs.
In the study of Ariano et al,65 the responses of the well differentiated neuronal cell line (GT1-7) to 50 and 200 nm aSiNPs were compared. The 50 nm aSiNPs affected cell proliferation in a dose-dependent way. In contrast, 200 nm aSiNPs did not show any toxicity even at relatively high concentrations. Kim et al66 determined the cytotoxicity of aSiNPs with mean sizes of either 20 nm or 100 nm in human glioblastoma cell line (U373MG). The results indicated that the 20 nm nanoparticles were more toxic than the 100 nm nanoparticles. Du et al67 compared the neurotoxicity of commercial 50, 100, and 300 nm Stöber aSiNPs using bEnd.3, N9 and HT22 cells. The result of MTS assay indicated that three aSiNPs induced size- and dose-dependent cytotoxicity in N9 cells, with smaller particles inducing higher cytotoxicity. In contrast, no alteration of cell viability was detected in bEnd.3 and HT22 cells. Passagne et al68 reported on the cytotoxicity of 20 nm and 100 nm aSiNPs in two renal proximal tubular cell lines (human HK-2 and porcine LLC-PK1). Results of a WST-1 assay indicated that both aSiNPs reduced cell viability in a dose- and time-dependent manner, and 20 nm aSiNPs was more cytotoxic than 100 nm aSiNPs.
Size-dependent Cytotoxicity of aSiNPs on Other Kinds of Cell Lines
Al-Rawi et al69 evaluated the possible size-dependent toxicity of aSiNPs (70, 200, and 500 nm) in human cervical carcinoma cell line (HeLa). In the presence of serum, all three sizes of aSiNPs did not show any negative effect on the viability of HeLa cells. However, compared to 200 nm and 500 nm aSiNPs, 70 nm aSiNPs diminished the cell viability apparently when the serum was absent. In the study of Vo et al,70 twelve adherent fish cell lines (RTgill-W1, RTgut-GC, RTL-W1, RTBrain, FHML2-6, FHMT-W1, GFB3C, GFSk-S1, GloFish, ZEB2J, EelBrain, and HEW) derived from six species (rainbow trout, fathead minnow, zebrafish, goldfish, haddock, and American eel) were used to investigate the toxic effects of aSiNPs (16, 24, and 44 nm). Toxicity produced by aSiNPs appeared to be size-, time-, temperature-, and dose-dependent as well as tissue-specific. Smaller particles (16 nm) were relatively more toxic than larger sized ones (24 and 44 nm).
Size-independent Cytotoxicity of aSiNPs
Size-independent Cytotoxicity of aSiNPs on Cell Lines from Respiratory System
Lin et al71 found that 15 nm or 46 nm aSiNPs could reduce A549 cell viability in a dose-dependent manner. Nevertheless, the cytotoxicity of 15 nm and 46 nm aSiNPs were not significantly different, since the similar hydrodynamic sizes of these two nanoparticles. Akhtar et al72 performed MTT assay and LDH activity assays to explore the viability of A549 cells exposed to two sizes (10 and 80 nm) of aSiNPs. Due to the agglomeration of particles in cell culture medium, the toxicity did not differ significantly between this two aSiNPs. In the study of Horie et al,73 aSiNPs (7, 25, and 34 nm) were dispersed directly in DMEM-FBS at concentrations of 10, 100, and 1000 μg/mL. There was no significant difference in the changes in mitochondrial activity of A549 and HaCaT cells induced by different sizes of particles. The authors found that the aSiNPs formed large secondary particles and accumulated onto cells by gravity sedimentation, therefore, the concentrations of the nanoparticles in dispersion did not correctly reflect the concentration to which the cells were exposed. In the research of Gualtieri et al,74 30 and 50 nm aSiNPs were examined for their cytotoxicity in BEAS-2B cells. However, little toxicity of both aSiNPs was generally observed, due to the formation of micrometer-sized agglomerates in the absence of bovine serum albumin in the culture medium.
Size-independent Cytotoxicity of aSiNPs on Cell Lines from Cardiovascular System
Bauer et al75 exposed HUVECs to aSiNPs with diameters ranging from 16 nm to 304 nm. As assessed by the MTT reduction method, mitochondrial activity significantly decreased upon incubation of aSiNPs with diameters between 212 nm and 304 nm. In contrast, aSiNPs with a diameter of 80 nm or less showed no cytotoxicity within the same particle concentrations. The authors observed that larger sized silica particles accumulated around the nucleus and blocked the cell migration obviously, but the reason why smaller sized silica nanoparticles did not show obvious toxicity was not clearly stated. Freese et al17 investigated the cytotoxicity of aSiNPs (30 and 70 nm) in HUVECs. As measured by the MTS-assay and LDH activity assay, the cell viability of HUVECs treated with 70 nm particles under stretch conditions was significantly decreased up to 77% (±12%). By comparison, 30 nm particles did not decrease the cell viability, owing to the tendency of agglomeration over time. Wang et al76 demonstrated that both 20 nm and 100 nm aSiNPs decreased the cell viability of HUVECs in a concentration-dependent manner from the minimal toxic concentration (50 μg/mL), whereas 100 nm aSiNPs was found to be more toxic than 20 nm aSiNPs. An in vitro sedimentation, diffusion and dosimetry (ISDD) model was introduced to estimate the aSiNPs sedimentations onto the cell surface, and results suggested that 100 nm aSiNPs reached to the cell surface more efficiently than 20 nm aSiNPs over time, larger aSiNPs thus have a higher effective exposure concentration than smaller particles.
Size-independent Cytotoxicity of aSiNPs on Cell Lines from Digestive System
The adverse effects of 7–50 nm aSiNPs on HepG2 cells was studied by Lu et al,77 whose results showed that the toxicity of 20 nm aSiNPs was much higher than that of 7 nm aSiNPs, while 50 nm aSiNPs did not have a significant cytotoxic effect at any concentration. In further comparison, it was found that 20 nm aSiNPs produced stronger oxidative damage than 7 nm aSiNPs. Docter et al78 reported that compared with untreated control Caco-2 cells, exposure to 30 nm aSiNPs reduced the cell vitality significantly. In contrast, low toxicity was observed upon treatment with 20 nm or 100 nm aSiNPs. The authors implied that the adverse effects triggered by 30 nm aSiNPs were significantly ameliorated upon formation of the protein corona, which they found was efficiently established on all aSiNPs studied. As a potential explanation, corona formation reduced cellular uptake of 30 nm aSiNPs, which was, however, not significantly affected by particle surface charge in their model.
Size-independent Cytotoxicity of aSiNPs on Cell Lines from Monocyte Macrophage System
Morishige et al79 evaluated the cytotoxicity of different-sized silica particles (30–1000 nm) on THP-1 cells. Silica particles with diameter of 300 and 1000 nm showed strong cytotoxicity which was depended on ROS level, whereas 30, 50, and 70 nm aSiNPs did not induce cell death obviously. Nishijima et al18 examined the effects of aSiNPs in THP-1 cells with a large overall size range (10, 30, 50, 70, 100, 300, and 1000 nm). The cytotoxicity in the dose range from12.5 to 200 μg/mL at six and twelve hours was hardly detected. Meanwhile, dose-dependent cytotoxicity was observed in all treated groups at 24 h, at a dose greater than 100 µg/mL, while the effect of particle size on the toxicity was not significant. Further assessment of inflammatory cytokines showed there was a bell-shaped size-specific effect, where the silica particles with a diameter of 50 nm induced the greatest secretion of IL-1β and TNF-α and silica particles with smaller or larger diameters had progressively less effect (overall size range, 10–1000 nm). Gazzano et al80 reported the toxicity of aSiNPs with different sizes (7, 40, and 1000 nm) on murine alveolar macrophages (MH-S). As manifested, except for 1000 nm silica particles, an increase in cytotoxic activity was shown on the other two samples. However, cytotoxic damage observed of 7 and 40 nm aSiNPs was similar, since the aggregation of 7 nm aSiNPs.
Size-independent Cytotoxicity of aSiNPs on Other Kinds of Cell Lines
Stępnik et al81 reported the potential toxic effects in murine (3T3-L1) and human (WI-38) fibroblast cell lines of commercially available aSiNPs. Results indicated that 3T3-L1 cells were more sensitive to aSiNPs than WI-38 cells, and 21 nm aSiNPs induced no, or marginal, cytotoxicity on WI-38 cells, whereas 30 nm aSiNPs was found to be cytotoxic at the higher concentrations. This could be the result due to the larger hydrodynamic diameter of 21 nm aSiNPs, as measured by dynamic light scattering (DLS), the hydrodynamic sizes of 21 nm and 30 nm aSiNPs in distilled water were 66±3 nm and 41±1 nm, respectively. Uboldi et al82 examined the cytotoxicity induced by 1–100 μg/mL of aSiNPs with a size diameter ranging from 16 nm to 300 nm, and showed that the viability of Balb/3T3 mouse fibroblasts was slightly impaired only after exposing to 80 nm aSiNPs. The authors implied that the absence of toxicity might be due to the huge aggregate formation of aSiNPs and the sensitivity of the cell line studied. Kim et al83 cultivated three types of cells, mouse embryonic fibroblasts (NIH/3T3), A549, and HepG2, with exposure of seven sizes aSiNPs (20, 40, 60, 80, 100, 150, 200 nm). The NIH/3T3 cells showed dose-dependent reduction in viability, and larger sized particles caused higher cytotoxicity. The following intracellular ROS measurement indicated that 100 and 200 nm aSiNPs induced the production of more ROS than 20 or 60 nm aSiNPs. For both the A549 and the HepG2 cells, there was no significant difference in viability reduction between different doses (≥50 μg/mL) and sizes except for 60 nm aSiNPs, owing to the preferential endocytosis of this nanoparticle observed. Park et al84 verified the developmental toxicity of four well-characterized aSiNPs (10, 30, 80, and 400 nm) in D3 embryonic stem cells. Addition of 80 nm and 400 nm aSiNPs to the cell culture medium did not affect the metabolic activity of D3 cells after exposure for either 24 h or 10 days. However, the treatments with 10 nm and 30 nm aSiNPs for 24 h remarkably increased the metabolic activity of D3 cells. In contrast, when the treatment was extended to 10 days, exposure to high concentration of the same particles caused a reduction in metabolic activity to 60% and 57%, respectively. This might be the result of a second burst of nanoparticles being dispersed following the cell culture medium change after three, five, and seven days, finally leading to toxic concentrations. The initial stimulation of metabolic activity after 24 h of exposure may be an adaptive response of the cell to the nanoparticles. Another research group evaluated the dermal toxicity of aSiNPs with different sizes (7 and 10–20 nm) on cultured HaCaT keratinocytes (CHKs). Both particles decreased cell viability in a dose-dependent manner. However, no difference in cytotoxicity was found between two sizes of aSiNPs, due to the agglomeration of particles and the small difference in hydrodynamic size.85 Kim et al86 exposed human neuronal cells (SH-SY5Y) to similar sizes of aSiNPs (16.9 nm and 15.3 nm). As revealed by MTT assay, there was no significant difference in cytotoxicity with small difference in particle size.
Discussion
As known, differences in the physical–chemical properties of nanoparticles could cause significant variation in its potential toxicity.87 The particle size, as an essential characterization of nanoparticles, could influence the particles’ biological reactivity obviously. In general, smaller sized nanoparticles possesses larger surface area as well as higher surface activity, which may make the particles more reactive in biological systems.2 Moreover, compared to larger sized nanoparticles, it is easier for smaller nanoparticles to penetrate the cell membrane and induce further damage inside the cells.42 However, due to the complexity of the biological system, the results of current reports on the size-dependent toxicity of aSiNPs are not completely consistent. In this systematic review, we explored the relationship between the particle size and cytotoxicity of aSiNPs in vitro, 76 scientific papers were included and the majority of studies tended to demonstrate that smaller sized aSiNPs possessed higher cytotoxicity. However, this size-dependent toxicity could also be influenced by other experimental factors, such as synthetic method of aSiNPs, aggregation state of aSiNPs, experimental conditions, toxicity endpoint detection method and so on.
Amorphous silica particles could be produced by thermal (pyrogenic) or wet routes (colloidal, precipitated and gel).87 According to different synthesis conditions and raw materials, the wet route for colloidal silica can be further classified into the Stöber method, Ludox method, and Lysine method, etc. It was reported silanol and siloxane were two main groups on the surface of aSiNPs, and the silanol group was more reactive than the siloxane group, moreover, the distribution of these active groups depended on the silica type and the method of synthesis.88 Thus, it could be proposed that different synthetic methods varied the surface properties of aSiNPs, which would eventually lead to diverse biological reactivity.89 However, Napierska et al26 used two types of silica nanoparticles produced by either Stöber process or Lys-Sil process, with four different diameters (2, 16, 60, and 104 nm), and found the cytotoxicity was related to the particle size directly, which indicated that compared with the synthetic method, the higher surface area of smaller particles played a pivotal role in the enhancement of surface reactivity. Similar results were obtained in the study of Napierska et al,2 in which little difference in toxicity was found between Ludox silica nanoparticles (diameters of 14 and 15 nm) and Stöber silica nanoparticles (16 nm). Guichard et al34 used three different types of aSiNPs, which were pyrogenic powder, precipitated powder and precipitated colloid, with the particle sizes ranging from 20–80 nm. The results also showed that the toxicity was strongly associated with the particle sizes. Nevertheless, the authors further observed that pyrogenic and colloidal manufactured aSiNPs of the same size were able to induce DNA damage, while no DNA damage was detected for precipitated particles. Thus, although the toxicity intensity of similar sizes silica particles synthesized in different ways was sometimes similar, the mechanism of toxicity was slightly different. Thomassen et al37 reported that the catalytic ability of hydroxyl radical formation varied with different synthesis methods of aSiNPs. Compared with the lysine method and Stöber method, aSiNPs synthesized using the Ludox method showed higher rate of hydroxyl radical formation and catalytic activity. In summary, the particle size and specific surface area of aSiNPs are still important factors affecting the toxic effects of aSiNPs, but the differences in particle surface chemistry caused by different synthesis methods would also affect the toxic pathways and mechanisms of the particles.
In some cases, aggregation of aSiNPs was observed in cell culture media, which could largely influence the cytotoxicity. Aggregation not only substantially alters the overall characteristics (such as size, shape and surface topology) but also potentially influences the biological outcomes of aSiNPs.23 In particular, smaller size of aSiNPs possess extremely large surface area and a huger number of particles in the same mass, consequently having more tendency to aggregate.60 By the influence of electron charge and Zeta potential, the suspensions of nanomaterials may be unstable and subsequently promote the aggregation of particles.84 Akhtar et al72 reported that the mean of hydrodynamic sizes of 10 nm and 80 nm aSiNPs in culture medium, were 352 nm and 380 nm, respectively. As a result, no significant difference in cytotoxicity between these two sizes of aSiNPs was detected. In the study of Lin et al,71 the hydrodynamic sizes of 15 nm and 46 nm aSiNPs in water suspension were 590±104 nm and 617±107 nm, respectively; and the decrease of cell viability of the group exposed to 15 nm aSiNPs was not significantly different from that of 46 nm aSiNPs. Due to aggregation, the size of nanoparticles was much larger than its original size which means only a small part of nonaggregated particles can penetrate the membrane of cells and produce cytotoxicity. Therefore, original particle size as well as hydrodynamic size should be mentioned in the experiment, which could facilitate the subsequent analysis and comparison of aSiNPs toxicity.
Differences in experimental conditions, such as exposure dose, exposure time, tested cell line, presence of serum, etc, have been proved to affect the toxicity of aSiNPs obviously. Lots of studies in this systematic review have demonstrated the dose- and time-dependent cytotoxic effects caused by aSiNPs. Tokgun et al23 reported that the viability of A549 cells was dose-dependently reduced by all four sizes aSiNPs (6, 15, 30, and 55 nm), examined at 24 h post-treatment. Wang et al61 exposed HEK293 cells to 20 or 50 nm aSiNPs and observed that the exposure at dosage levels between 20 and 100 μg/mL from 0 to 48 h decreased the cell viability in both dose- and time-dependent manner. In the study of Ye et al,40 L-02 cells were exposed to 0.2, 0.4, and 0.6 mg/mL of aSiNPs (21, 48, and 86 nm) for 12, 24, 36, and 48 h, similar toxicity trends were confirmed as well. It is well known that the number of nanoparticles accumulated around cells or internalized by cells rose gradually with the increased exposure dose, thus contributing more toxic effects on tested cells.40 In addition, the exposure time should be another influencing factor of experimental results. Since nanoparticles reached the cytosol of cells by penetration or endocytosis and it took time to induce cytotoxicity, the reduction in survival of cells showed gradually with time. Therefore, longer exposure time of aSiNPs usually causes higher toxicity, as irreparable cell damage accumulates kinetically.70
Studies have shown that the toxic reaction of different cell lines to aSiNPs was related to the cellular uptake rate of particles and the reaction mechanism. Du et al67 demonstrated that N9 cells (microglia) were more sensitive to the toxicity of aSiNPs than bEnd.3 cells (microendothelial cells) and HT22 cells (neurons), owing to the different uptake rate of aSiNPs. The authors observed that after crossing the blood–brain barrier, aSiNPs were mainly swallowed by N9 cells. However, HT22 cells, a representative model of neurons, were found to barely phagocytize aSiNPs and have minimal cellular uptake of particles. Further research showed that the uptake rate of aSiNPs was closely related to the cellular internalization mode of nanoparticles, and different cell types had different internalization processes. Vicente et al60 found human keratinocytes (K17) showed more intense toxic reactions to aSiNPs than human dermal fibroblasts (HDF), with higher uptake rate of nanoparticles. Moreover, phagocytosis had been identified as the main internalization mechanism for K17, while slower caveolae-mediated endocytosis for HDF. Different cell lines have different reaction mechanisms to aSiNPs, which is also one of the reasons influencing cytotoxicity. Oxidative stress is considered to be an important mechanism of nanomaterial-induced toxicity, leading to the elevated production of ROS, which are directly involved in the regulation of cell survival and death.90,91 Passagne et al68 used two proximal tubular cell lines (human HK-2 and porcine LLC-PK1) to investigate the toxic mechanism of aSiNPs. Compared with HK-2 cells, LLC-PK1 cells were found to be more susceptible to the toxic effects induced by aSiNPs, and the significantly increased intracellular ROS level was clearly related to the toxicity. After entering the cells, nanoparticles can also stimulate the cells to release cytokines, which are involved in inflammatory processes and further cell structure damage, even cell death.92 Låg et al30 found that after exposing to aSiNPs, the survival rate of BEAS-2B was lower than that of HBEC3-KT. Moreover, BEAS-2B cells showed much higher cytokine release than HBEC3-KT cells, including both IL-6 and CXCL8, which played a central role in inflammatory process induced by particle components. In addition to the above mechanisms, there are also other reaction mechanisms closely related to the toxicity of aSiNPs, such as autophagy dysfunction. The reaction mechanisms may vary from cell to cell, which requires special attention in the analysis of toxic effect of nanoparticles.
The last experimental condition we would like to discuss was the presence of serum in aSiNPs suspension. Due to its high surface reactivity, aSiNPs always absorbs the serum protein leading to the formation of protein shield or protein corona surrounds the particles. It has been well established that the presence of protein corona resulted in surface modification, which further contributed to changes in Zeta potential, aggregation state, cellular uptake, even toxicity.12 Lesniak et al93 reported that in the absence of serum, an increased amount of aSiNPs associated with A549 cells was detected and hence an enhanced uptake was postulated. Leibe et al10 analyzed the effects of two sizes of aSiNPs on A549 cells, and found that the cytotoxicity induced by 12 nm aSiNPs was more pronounced than that of 50 nm aSiNPs. In addition, both aSiNPs reduced cell viability only in the absence, but not in the presence of serum. Similar phenomenon that serum protein or protein corona reduced the toxicity of aSiNPs was also confirmed by several other investigations.27,69 From a mechanistic point of view, researchers suggested different hypotheses of this phenomenon: (1) the formation of protein corona reduced the surface energy of bare aSiNPs; (2) the protein corona decreased the cellular uptake of aSiNPs and thereby the cytotoxic effects were induced to a lesser extent compared to serum free conditions; (3) the presence/absence of protein led to different cellular targeting of aSiNPs.12,22 Moreover, for risk assessment with the possibility of threshold dose effects, it is essential to apply the most sensitive conditions. Therefore, serum free condition in addition to serum condition was advised for hazard identification because it demonstrated a higher sensitivity.22
Detection method of toxicity endpoint may also influence the results’ consistency in different research. The most common endpoint of cytotoxicity was cell viability reduction, which could be reflected by the decrease of mitochondrial activity of tested cells and the increase of lactate dehydrogenase (LDH) release in culture medium. Mitochondria are the energy center of cells, and the mitochondrial activity is closely related to the cell viability. LDH release is an indicator of plasma membrane rupture, which is proportional to the number of cells damaged or lysed.2 Several researchers studied the cellular uptake of aSiNPs and pointed out that the transport of smaller particles was dominated by diffusion while larger particles were mainly transported by sedimentation or endocytosis,13 which means smaller aSiNPs could easily penetrate cells and induce oxidative reaction to mitochondrial, while larger aSiNPs tended to firstly induce membrane injury through mechanical friction or oxidative damage.94 Moreover, it was reported that nanoparticles, owing to their high reactivity, would possibly absorb or interact with the experimental components, leading to inaccurate or false results provided by some classical toxicity assays, including MTT and LDH activity assay.95 Therefore, in order to ensure the accuracy of experimental results, different toxicity endpoint detection methods should be combined when detecting and comparing the toxic effects induced by aSiNPs with wide range of sizes.
The abovementioned were the main influence factors of size-dependent cytotoxicity of aSiNPs in vitro, which should be taken into consideration when performing relevant studies.
Conclusion
In the present review, most of the included studies tended to demonstrate that smaller sized aSiNPs with larger surface areas produced higher cytotoxicity. However, there were also some studies that did not reach similar conclusions. As for the influence factors of size-dependent cytotoxicity of aSiNPs in vitro, we mainly expounded from the following four aspects: (1) The synthetic method of aSiNPs could influence the distribution of active groups on the surface of aSiNPs, and further affect the toxic pathways or mechanisms. (2) Aggregation of particles would alter the overall characteristics (size, shape, surface topology, etc) and the cytotoxicity of aSiNPs significantly. Thus, both the original particle size and hydrodynamic size of nanoparticles should be measured in toxicology study. (3) In terms of the influence of different experimental conditions, we mainly discussed exposure dose, exposure time, tested cell line and the presence of serum. A majority of studies reported both dose- and time-dependent cytotoxicity of aSiNPs, since higher exposure dose and longer exposure time could increase the cellular uptake of nanoparticles and the interaction duration between particles and cells. The sensitivity of different cell lines to aSiNPs mainly depends on different cellular uptake rate of particles and different reaction mechanisms to the particles. In the presence of serum, aSiNPs could absorb the serum protein and form protein corona, which inhibited its cellular toxicity in most cases. (4) Detection method of toxicity endpoint could influence the toxic effect of aSiNPs from two aspects. Firstly, aSiNPs with different particle sizes might produce toxicity in different subcellular structures. Secondly, nanoparticles owing to its high reactivity would possibly absorb or interact with the experimental components, leading to inaccurate or false results. Therefore, two or more kinds of toxicity endpoint detection methods should be combined in nanotoxicology study.
In addition to the above influence factors, the current studies on toxicity of aSiNPs still have some limitations, which should also be taken into account in future experimental research. Most included investigations in this review did not use a specific guideline to carry out their experiments, causing some diversity of experimental details and difficulties in quantitative comparison between studies. Thus, it is recommended to apply a certain guideline, which can largely improve the comparability of different studies and the control of systematic bias.96 Another insufficiency was that some researchers used very high dosage in their toxicity and mechanism studies, which was far beyond the actual exposure dose of occupational population or general population. Hence, it is inappropriate and not recommended to use extremely high dosage in a nanotoxicology study in vitro. Lastly, although adverse effects of aSiNPs observed in vitro might indicate toxicity in vivo, follow-up investigations are needed to confirm this relationship. Moreover, evidence on population exposure data and potential effect of aSiNPs in human beings is still insufficient and needs to be explored further. Therefore, it is of great significance to conduct more studies on the toxicity of aSiNPs both in vitro and in vivo, and related adverse effects on human health should get more attention.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81930091, 91943301, and 81602875) and National Key Research and Development Program of China (2017YFC0211600, 2017YFC0211602, 2017YFC0211606).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Turci F, Pavan C, Leinardi R, et al. Revisiting the paradigm of silica pathogenicity with synthetic quartz crystals: the role of crystallinity and surface disorder. Part Fibre Toxicol. 2016;13(1):32. doi:10.1186/s12989-016-0136-6
2. Napierska D, Thomassen LC, Rabolli V, et al. Size-dependent cytotoxicity of monodisperse silica nanoparticles in human endothelial cells. Small. 2009;5(7):846–853. doi:10.1002/smll.200800461
3. Kamikubo Y, Yamana T, Hashimoto Y, Sakurai T. Induction of oxidative stress and cell death in neural cells by silica nanoparticles. ACS Chem Neurosci. 2019;10(1):304–312. doi:10.1021/acschemneuro.8b00248
4. Vance ME, Kuiken T, Vejerano EP, et al. Nanotechnology in the real world: redeveloping the nanomaterial consumer products inventory. Beilstein J Nanotechnol. 2015;6:1769–1780. doi:10.3762/bjnano.6.181
5. WHO. Guidelines on Protecting Workers from Potential Risks of Manufactured Nanomaterials. Geneva: World Health Organization; 2017.
6. Yazdimamaghani M, Moos PJ, Dobrovolskaia MA, Ghandehari H. Genotoxicity of amorphous silica nanoparticles: status and prospects. Nanomedicine. 2019;16:106–125. doi:10.1016/j.nano.2018.11.013
7. Al Faraj A, Alotaibi B, Shaik AP, Shamma KZ, Al Jammaz I, Gerl J. Sodium-22-radiolabeled silica nanoparticles as new radiotracer for biomedical applications: in vivo positron emission tomography imaging, biodistribution, and biocompatibility. Int J Nanomedicine. 2015;10:6293–6302. doi:10.2147/IJN.S93523
8. Guo M, Xu X, Yan X, Wang S, Gao S, Zhu S. In vivo biodistribution and synergistic toxicity of silica nanoparticles and cadmium chloride in mice. J Hazard Mater. 2013;260:
9. Li Y, Yu Y, Duan J, et al. The internalization, distribution, and ultrastructure damage of silica nanoparticles in human hepatic L-02 cells. Part Part Syst Charact. 2016;33(9):664–674. doi:10.1002/ppsc.201600043
10. Leibe R, Hsiao IL, Fritsch-Decker S, et al. The protein corona suppresses the cytotoxic and pro-inflammatory response in lung epithelial cells and macrophages upon exposure to nanosilica. Arch Toxicol. 2019;93(4):871–885. doi:10.1007/s00204-019-02422-9
11. Li Y, Ma R, Liu X, et al. Endoplasmic reticulum stress-dependent oxidative stress mediated vascular injury induced by silica nanoparticles in vivo and in vitro. NanoImpact. 2019;14:100169. doi:10.1016/j.impact.2019.100169
12. Saikia J, Yazdimamaghani M, Hadipour Moghaddam SP, Ghandehari H. Differential protein adsorption and cellular uptake of silica nanoparticles based on size and porosity. ACS Appl Mater Interfaces. 2016;8(50):34820–34832. doi:10.1021/acsami.6b09950
13. Kim W, Kim WK, Lee K, et al. A reliable approach for assessing size-dependent effects of silica nanoparticles on cellular internalization behavior and cytotoxic mechanisms. Int J Nanomedicine. 2019;14:7375–7387. doi:10.2147/IJN.S224183
14. Murugadoss S, van den Brule S, Brassinne F, et al. Is aggregated synthetic amorphous silica toxicologically relevant? Part Fibre Toxicol. 2020;17(1):1. doi:10.1186/s12989-019-0331-3
15. Wang W, Zeng C, Feng Y, et al. The size-dependent effects of silica nanoparticles on endothelial cell apoptosis through activating the p53-caspase pathway. Environ Pollut. 2018;233:218–225. doi:10.1016/j.envpol.2017.10.053
16. Lee K, Lee J, Kwak M, et al. Two distinct cellular pathways leading to endothelial cell cytotoxicity by silica nanoparticle size. J Nanobiotechnology. 2019;17(1):24. doi:10.1186/s12951-019-0456-4
17. Freese C, Schreiner D, Anspach L, et al. In vitro investigation of silica nanoparticle uptake into human endothelial cells under physiological cyclic stretch. Part Fibre Toxicol. 2014;11:68. doi:10.1186/s12989-014-0068-y
18. Nishijima N, Hirai T, Misato K, et al. Human scavenger receptor A1-mediated inflammatory response to silica particle exposure is size specific. Front Immunol. 2017;8:379. doi:10.3389/fimmu.2017.00379
19. Samuel GO, Hoffmann S, Wright RA, et al. Guidance on assessing the methodological and reporting quality of toxicologically relevant studies: a scoping review. Environ Int. 2016;92:630–646. doi:10.1016/j.envint.2016.03.010
20. Gonzalez L, Thomassen LC, Plas G, et al. Exploring the aneugenic and clastogenic potential in the nanosize range: A549 human lung carcinoma cells and amorphous monodisperse silica nanoparticles as models. Nanotoxicology. 2010;4:382–395. doi:10.3109/17435390.2010.501913
21. Fede C, Millino C, Pacchioni B, et al. Altered gene transcription in human cells treated with Ludox® silica nanoparticles. Int J Environ Res Public Health. 2014;11(9):
22. Gonzalez L, Lukamowicz-Rajska M, Thomassen LC, et al. Co-assessment of cell cycle and micronucleus frequencies demonstrates the influence of serum on the in vitro genotoxic response to amorphous monodisperse silica nanoparticles of varying sizes. Nanotoxicology. 2014;8(8):876–884. doi:10.3109/17435390.2013.842266
23. Tokgun O, Demiray A, Kaya B, et al. Silica nanoparticles can induce apoptosis via dead receptor and caspase 8 pathway on A549 cells. Adv Food Sci. 2015;37:65–70.
24. Liu W, Hu T, Zhou L, et al. Nrf2 protects against oxidative stress induced by SiO2 nanoparticles. Nanomedicine (Lond). 2017;12(19):2303–2318. doi:10.2217/nnm-2017-0046
25. Wottrich R, Diabaté S, Krug HF. Biological effects of ultrafine model particles in human macrophages and epithelial cells in mono- and co-culture. Int J Hyg Environ Health. 2004;207(4):353–361. doi:10.1078/1438-4639-00300
26. Napierska D, Thomassen LC, Vanaudenaerde B, et al. Cytokine production by co-cultures exposed to monodisperse amorphous silica nanoparticles: the role of size and surface area. Toxicol Lett. 2012;211(2):98–104. doi:10.1016/j.toxlet.2012.03.002
27. Panas A, Marquardt C, Nalcaci O, et al. Screening of different metal oxide nanoparticles reveals selective toxicity and inflammatory potential of silica nanoparticles in lung epithelial cells and macrophages. Nanotoxicology. 2012;7(3):259–273. doi:10.3109/17435390.2011.652206
28. Li Q, Hu H, Jiang L, Zou Y, Duan J, Sun Z. Cytotoxicity and autophagy dysfunction induced by different sizes of silica particles in human bronchial epithelial BEAS-2B cells. Toxicol Res (Camb). 2016;5(4):1216–1228. doi:10.1039/c6tx00100a
29. Li Y, Duan J, Chai X, et al. Microarray-assisted size-effect study of amorphous silica nanoparticles on human bronchial epithelial cells. Nanoscale. 2019;11(47):22907–22923. doi:10.1039/c9nr07350g
30. Låg M, Skuland T, Godymchuk A, Nguyen THT, Pham HLT, Refsnes M. Silica nanoparticle-induced cytokine responses in BEAS-2B and HBEC3-KT cells: significance of particle size and signalling pathways in different lung cell cultures. Basic Clin Pharmacol Toxicol. 2018;122(6):620–632. doi:10.1111/bcpt.12963
31. Kasper J, Hermanns MI, Bantz C, et al. Interactions of silica nanoparticles with lung epithelial cells and the association to flotillins. Arch Toxicol. 2013;87(6):1053–1065. doi:10.1007/s00204-012-0876-5
32. McCarthy J, Inkielewicz-Stępniak I, Corbalan JJ, Radomski MW. Mechanisms of toxicity of amorphous silica nanoparticles on human lung submucosal cells in vitro: protective effects of fisetin. Chem Res Toxicol. 2012;25(10):2227–2235. doi:10.1021/tx3002884
33. Xu Z, Chou L, Sun J. Effects of SiO2 nanoparticles on HFL-I activating ROS-mediated apoptosis via p53 pathway. J Appl Toxicol. 2012;32(5):358–364. doi:10.1002/jat.1710
34. Guichard Y, Fontana C, Chavinier E, et al. Cytotoxic and genotoxic evaluation of different synthetic amorphous silica nanomaterials in the V79 cell line. Toxicol Ind Health. 2016;32(9):1639–1650. doi:10.1177/0748233715572562
35. Corbalan JJ, Medina C, Jacoby A, Malinski T, Radomski MW. Amorphous silica nanoparticles trigger nitric oxide/peroxynitrite imbalance in human endothelial cells: inflammatory and cytotoxic effects. Int J Nanomedicine. 2011;6:2821–2835. doi:10.2147/IJN.S25071
36. Nemmar A, Albarwani S, Beegam S, et al. Amorphous silica nanoparticles impair vascular homeostasis and induce systemic inflammation. Int J Nanomedicine. 2014;9:2779–2789. doi:10.2147/IJN.S52818
37. Thomassen LC, Aerts A, Rabolli V, et al. Synthesis and characterization of stable monodisperse silica nanoparticle sols for in vitro cytotoxicity testing. Langmuir. 2010;26(1):328–335. doi:10.1021/la902050k
38. Napierska D, Rabolli V, Thomassen LC, et al. Oxidative stress induced by pure and iron-doped amorphous silica nanoparticles in subtoxic conditions. Chem Res Toxicol. 2012;25(4):828–837. doi:10.1021/tx200361v
39. Ye Y, Liu J, Chen M, Sun L, Lan M. In vitro toxicity of silica nanoparticles in myocardial cells. Environ Toxicol Pharmacol. 2010;29(2):131–137. doi:10.1016/j.etap.2009.12.002
40. Ye Y, Liu J, Xu J, Sun L, Chen M, Lan M. Nano-SiO2 induces apoptosis via activation of p53 and Bax mediated by oxidative stress in human hepatic cell line. Toxicol in Vitro. 2010;24(3):751–758. doi:10.1016/j.tiv.2010.01.001
41. Xue Y, Chen Q, Ding T, Sun J. SiO₂ nanoparticle-induced impairment of mitochondrial energy metabolism in hepatocytes directly and through a Kupffer cell-mediated pathway in vitro. Int J Nanomedicine. 2014;9:2891–2903. doi:10.2147/IJN.S60661
42. Li Y, Sun L, Jin M, et al. Size-dependent cytotoxicity of amorphous silica nanoparticles in human hepatoma HepG2 cells. Toxicol in Vitro. 2011;25(7):1343–1352. doi:10.1016/j.tiv.2011.05.003
43. Tarantini A, Lanceleur R, Mourot A, et al. Toxicity, genotoxicity and proinflammatory effects of amorphous nanosilica in the human intestinal Caco-2 cell line. Toxicol in Vitro. 2015;29(2):398–407. doi:10.1016/j.tiv.2014.10.023
44. Gehrke H, Frühmesser A, Pelka J, et al. In vitro toxicity of amorphous silica nanoparticles in human colon carcinoma cells. Nanotoxicology. 2013;7(3):274–293. doi:10.3109/17435390.2011.652207
45. Fritsch-Decker S, An Z, Yan J, et al. Silica nanoparticles provoke cell death independent of p53 and BAX in human colon cancer cells. Nanomaterials (Basel). 2019;9(8):1172. doi:10.3390/nano9081172
46. Waters KM, Masiello LM, Zangar RC, et al. Macrophage responses to silica nanoparticles are highly conserved across particle sizes. Toxicol Sci. 2009;107(2):553–569. doi:10.1093/toxsci/kfn250
47. Nabeshi H, Yoshikawa T, Akase T, et al. Effect of amorphous silica nanoparticles on in vitro RANKL-induced osteoclast differentiation in murine macrophages. Nanoscale Res Lett. 2011;6(1):464. doi:10.1186/1556-276X-6-464
48. Kim S, Jang J, Kim H, Choi H, Lee K, Choi IH. The effects of silica nanoparticles in macrophage cells. Immune Netw. 2012;12(6):296–300. doi:10.4110/in.2012.12.6.296
49. Premshekharan G, Nguyen K, Zhang H, Forman HJ, Leppert VJ. Low dose inflammatory potential of silica particles in human-derived THP-1 macrophage cell culture studies - Mechanism and effects of particle size and iron. Chem Biol Interact. 2017;272:160–171. doi:10.1016/j.cbi.2017.05.004
50. Kusaka T, Nakayama M, Nakamura K, Ishimiya M, Furusawa E, Ogasawara K. Effect of silica particle size on macrophage inflammatory responses. PLoS One. 2014;9(3):e92634. doi:10.1371/journal.pone.0092634
51. Mendoza A, Torres-Hernandez JA, Ault JG, Pedersen-Lane JH, Gao D, Lawrence DA. Silica nanoparticles induce oxidative stress and inflammation of human peripheral blood mononuclear cells. Cell Stress Chaperones. 2014;19(6):777–790. doi:10.1007/s12192-014-0502-y
52. Nakanishi K, Tsukimoto M, Tanuma S, Takeda K, Kojima S. Silica nanoparticles activate purinergic signaling via P2X7 receptor in dendritic cells, leading to production of pro-inflammatory cytokines. Toxicol in Vitro. 2016;35:
53. Kojima S, Negishi Y, Tsukimoto M, Takenouchi T, Kitani H, Takeda K. Purinergic signaling via P2X7 receptor mediates IL-1β production in Kupffer cells exposed to silica nanoparticle. Toxicology. 2014;321:13–20. doi:10.1016/j.tox.2014.03.008
54. Yang X, Liu J, He H, et al. SiO2 nanoparticles induce cytotoxicity and protein expression alteration in HaCaT cells. Part Fibre Toxicol. 2010;7:1. doi:10.1186/1743-8977-7-1
55. Gong C, Tao G, Yang L, Liu J, He H, Zhuang Z. The role of reactive oxygen species in silicon dioxide nanoparticle-induced cytotoxicity and DNA damage in HaCaT cells. Mol Biol Rep. 2012;39(4):
56. Nabeshi H, Yoshikawa T, Matsuyama K, et al. Amorphous nanosilica induce endocytosis-dependent ROS generation and DNA damage in human keratinocytes. Part Fibre Toxicol. 2011;8:1. doi:10.1186/1743-8977-8-1
57. Nagakura C, Negishi Y, Tsukimoto M, et al. Involvement of P2Y11 receptor in silica nanoparticles 30-induced IL-6 production by human keratinocytes. Toxicology. 2014;322:61–68. doi:10.1016/j.tox.2014.03.010
58. Gong C, Yang L, Zhou J, Guo X, Zhuang Z. Possible role of PAPR-1 in protecting human HaCaT cells against cytotoxicity of SiO2 nanoparticles. Toxicol Lett. 2017;280:213–221. doi:10.1016/j.toxlet.2017.07.213
59. Yu K, Grabinski C, Schrand A, et al. Toxicity of amorphous silica nanoparticles in mouse keratinocytes. J Nanoparticle Res. 2009;11:15–24. doi:10.1007/s11051-008-9417-9
60. Vicente S, Moia C, Zhu H, Vigé X. In vitro evaluation of the internalization and toxicological profile of silica nanoparticles and submicroparticles for the design of dermal drug delivery strategies. J Appl Toxicol. 2017;37(12):1396–1407. doi:10.1002/jat.3507
61. Wang F, Gao F, Lan M, Yuan H, Huang Y, Liu J. Oxidative stress contributes to silica nanoparticle-induced cytotoxicity in human embryonic kidney cells. Toxicol in Vitro. 2009;23(5):808–815. doi:10.1016/j.tiv.2009.04.009
62. Manganelli S, Leone C, Toropov AA, Toropova AP, Benfenati E. QSAR model for predicting cell viability of human embryonic kidney cells exposed to SiO₂ nanoparticles. Chemosphere. 2016;144:995–1001. doi:10.1016/j.chemosphere.2015.09.086
63. Wang F, Jiao C, Liu J, Yuan H, Lan M, Gao F. Oxidative mechanisms contribute to nanosize silicon dioxide-induced developmental neurotoxicity in PC12 cells. Toxicol in Vitro. 2011;25(8):1548–1556. doi:10.1016/j.tiv.2011.05.019
64. Yuan H, Gao F, Zhang Z, et al. Study on controllable preparation of silica nanoparticles with multi-sizes and their size-dependent cytotoxicity in pheochromocytoma cells and human embryonic kidney cells. J Health Sci. 2010;56:632–640. doi:10.1248/jhs.56.632
65. Ariano P, Zamburlin P, Gilardino A, et al. Interaction of spherical silica nanoparticles with neuronal cells: size-dependent toxicity and perturbation of calcium homeostasis. Small. 2011;7(6):766–774. doi:10.1002/smll.201002287
66. Kim JE, Kim H, An SS, Maeng EH, Kim MK, Song YJ. In vitro cytotoxicity of SiO2 or ZnO nanoparticles with different sizes and surface charges on U373MG human glioblastoma cells. Int J Nanomedicine. 2014;9(Suppl2):235–241. doi:10.2147/IJN.S57936
67. Du Q, Ge D, Mirshafiee V, et al. Assessment of neurotoxicity induced by different-sized Stöber silica nanoparticles: induction of pyroptosis in microglia. Nanoscale. 2019;11(27):12965–12972. doi:10.1039/c9nr03756j
68. Passagne I, Morille M, Rousset M, Pujalté I, L’azou B. Implication of oxidative stress in size-dependent toxicity of silica nanoparticles in kidney cells. Toxicology. 2012;299(2–3):112–124. doi:10.1016/j.tox.2012.05.010
69. Al-Rawi M, Diabaté S, Weiss C. Uptake and intracellular localization of submicron and nano-sized SiO₂ particles in HeLa cells. Arch Toxicol. 2011;85(7):813–826. doi:10.1007/s00204-010-0642-5
70. Vo NT, Bufalino MR, Hartlen KD, Kitaev V, Lee LE. Cytotoxicity evaluation of silica nanoparticles using fish cell lines. Vitro Cell Dev Biol Anim. 2014;50(5):427–438. doi:10.1007/s11626-013-9720-3
71. Lin W, Huang YW, Zhou XD, Ma Y. In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol Appl Pharmacol. 2006;217(3):252–259. doi:10.1016/j.taap.2006.10.004
72. Akhtar MJ, Ahamed M, Kumar S, et al. Nanotoxicity of pure silica mediated through oxidant generation rather than glutathione depletion in human lung epithelial cells. Toxicology. 2010;276(2):95–102. doi:10.1016/j.tox.2010.07.010
73. Horie M, Nishio K, Kato H, et al. Evaluation of cellular effects of silicon dioxide nanoparticles. Toxicol Mech Methods. 2014;24(3):196–203. doi:10.3109/15376516.2013.879505
74. Gualtieri M, Skuland T, Iversen TG, et al. Importance of agglomeration state and exposure conditions for uptake and pro-inflammatory responses to amorphous silica nanoparticles in bronchial epithelial cells. Nanotoxicology. 2012;6(7):700–712. doi:10.3109/17435390.2011.604441
75. Bauer AT, Strozyk EA, Gorzelanny C, et al. Cytotoxicity of silica nanoparticles through exocytosis of von Willebrand factor and necrotic cell death in primary human endothelial cells. Biomaterials. 2011;32(33):8385–8393. doi:10.1016/j.biomaterials.2011.07.078
76. Wang DP, Wang ZJ, Zhao R, et al. Silica nanomaterials induce organ injuries by Ca2+-ROS-initiated disruption of the endothelial barrier and triggering intravascular coagulation. Part Fibre Toxicol. 2020;17(1):12. doi:10.1186/s12989-020-00340-8
77. Lu X, Qian J, Zhou H, et al. In vitro cytotoxicity and induction of apoptosis by silica nanoparticles in human HepG2 hepatoma cells. Int J Nanomedicine. 2011;6:1889–1901. doi:10.2147/IJN.S24005
78. Docter D, Bantz C, Westmeier D, et al. The protein corona protects against size- and dose-dependent toxicity of amorphous silica nanoparticles. Beilstein J Nanotechnol. 2014;5:1380–1392. doi:10.3762/bjnano.5.151
79. Morishige T, Yoshioka Y, Inakura H, et al. Cytotoxicity of amorphous silica particles against macrophage-like THP-1 cells depends on particle-size and surface properties. Pharmazie. 2010;65(8):596–599.
80. Gazzano E, Ghiazza M, Polimeni M, et al. Physicochemical determinants in the cellular responses to nanostructured amorphous silicas. Toxicol Sci. 2012;128(1):158–170. doi:10.1093/toxsci/kfs128
81. Stępnik M, Arkusz J, Smok-Pieniążek A, et al. Cytotoxic effects in 3T3-L1 mouse and WI-38 human fibroblasts following 72 hour and 7 day exposures to commercial silica nanoparticles. Toxicol Appl Pharmacol. 2012;263(1):89–101. doi:10.1016/j.taap.2012.06.002
82. Uboldi C, Giudetti G, Broggi F, Gilliland D, Ponti J, Rossi F. Amorphous silica nanoparticles do not induce cytotoxicity, cell transformation or genotoxicity in Balb/3T3 mouse fibroblasts. Mutat Res. 2012;745(1–2):11–20. doi:10.1016/j.mrgentox.2011.10.010
83. Kim IY, Joachim E, Choi H, Kim K. Toxicity of silica nanoparticles depends on size, dose, and cell type. Nanomedicine. 2015;11(6):1407–1416. doi:10.1016/j.nano.2015.03.004
84. Park MV, Annema W, Salvati A, et al. In vitro developmental toxicity test detects inhibition of stem cell differentiation by silica nanoparticles. Toxicol Appl Pharmacol. 2009;240(1):108–116. doi:10.1016/j.taap.2009.07.019
85. Park YH, Kim JN, Jeong SH, et al. Assessment of dermal toxicity of nanosilica using cultured keratinocytes, a human skin equivalent model and an in vivo model. Toxicology. 2010;267(1–3):178–181. doi:10.1016/j.tox.2009.10.011
86. Kim YJ, Yu M, Park HO, Yang SI. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by silica nanomaterials in human neuronal cell line. Mol Cell Toxicol. 2010;6:336–343. doi:10.1007/s13273-010-0045-y
87. Murugadoss S, Lison D, Godderis L, et al. Toxicology of silica nanoparticles: an update. Arch Toxicol. 2017;91(9):2967–3010. doi:10.1007/s00204-017-1993-y
88. OECD. Series on the safety of manufactured nanomaterials no. 71: silicon dioxide: summary of the dossier. OECD. 2016.
89. Napierska D, Thomassen LC, Lison D, Martens JA, Hoet PH. The nanosilica hazard: another variable entity. Part Fibre Toxicol. 2010;7(1):39. doi:10.1186/1743-8977-7-39
90. Fu PP, Xia Q, Hwang HM, Ray PC, Yu H. Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal. 2014;22(1):64–75. doi:10.1016/j.jfda.2014.01.005
91. Petrache Voicu SN, Dinu D, Sima C, et al. Silica nanoparticles induce oxidative stress and autophagy but not apoptosis in the MRC-5 cell line. Int J Mol Sci. 2015;16(12):29398–29416. doi:10.3390/ijms161226171
92. Voicu SN, Balas M, Stan MS, et al. Amorphous silica nanoparticles obtained by laser ablation induce inflammatory response in human lung fibroblasts. Materials (Basel). 2019;12(7):1026. doi:10.3390/ma12071026
93. Lesniak A, Fenaroli F, Monopoli MP, Åberg C, Dawson KA, Salvati A. Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano. 2012;6(7):5845–5857. doi:10.1021/nn300223w
94. Faklaris O, Joshi V, Irinopoulou T, et al. Photoluminescent diamond nanoparticles for cell labeling: study of the uptake mechanism in mammalian cells. ACS Nano. 2009;3(12):3955–3962. doi:10.1021/nn901014j
95. Monteiro-Riviere NA, Inman AO, Zhang LW. Limitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell line. Toxicol Appl Pharmacol. 2009;234(2):222–235. doi:10.1016/j.taap.2008.09.030
96. Faria M, Björnmalm M, Thurecht KJ, et al. Minimum information reporting in bio-nano experimental literature. Nat Nanotechnol. 2018;13(9):777–785. doi:10.1038/s41565-018-0246-4
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
