Back to Journals » Cancer Management and Research » Volume 12
The Significance of CXCL1 and CXCL8 as Well as Their Specific Receptors in Colorectal Cancer
Authors Łukaszewicz-Zając M , Pączek S , Mroczko P , Kulczyńska-Przybik A
Received 10 June 2020
Accepted for publication 14 August 2020
Published 14 September 2020 Volume 2020:12 Pages 8435—8443
DOI https://doi.org/10.2147/CMAR.S267176
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Marta Łukaszewicz-Zając,1 Sara Pączek,1 Piotr Mroczko,2 Agnieszka Kulczyńska-Przybik3
1Department of Biochemical Diagnostics, Medical University of Bialystok, Bialystok, Poland; 2Department of Criminal Law and Criminology, Faculty of Law, University of Bialystok, Bialystok, Poland; 3Department of Neurodegeneration Diagnostics, Medical University of Bialystok, Bialystok, Poland
Correspondence: Marta Łukaszewicz-Zając
Department of Biochemical Diagnostics, Medical University of Bialystok, Poland ul. Waszyngtona 15 a, Bialystok 15-269, Poland
Tel/Fax +48 85 7468785
Email [email protected]
Abstract: Every year, almost 2 million people develop colorectal cancer (CRC), which makes it the fourth most common malignancy worldwide. It is also estimated that approximately 48% of CRC patients will die from the disease. Thus, noninvasive and accurate methods for early detection and prevention of CRC are sorely needed. It is suggested that C-X-C motif ligand 1 (CXCL1) and C-X-C motif ligand 8 (CXCL8) as well as their cognate receptors can mediate tumor growth, proliferation, survival, neoangiogenesis and metastasis of malignant cells, including CRC. However, little is known about the clinical significance of these proteins as potential biomarkers for CRC. Therefore, in our review, we performed a comprehensive literature search using the PubMed database to identify original articles that investigated whether CXCL1 and CXCL8 and their receptors play a role in CRC pathogenesis. In summary, our review highlighted the potential significance of CXCL1/CXCR2 and CXCL8/CXCR1,-2 in the diagnosis and progression of CRC as well as indicated their potential therapeutic significance. However, given the non-specific nature of analyzed chemokines and a small number of studies concerning the assessment of blood concentration of these proteins in CRC patients, investigations need to be continued in the future before selected chemokines could be established as biomarkers for CRC.
Keywords: biochemical diagnosis, chemokines, colorectal cancer, tumor biomarkers
Colorectal Cancer – General Characteristics and Current Diagnosis
It has been proven that chronic inflammation is one of the key risk factors for colorectal cancer (CRC). This malignancy develops via a multistep process including various genetic and morphological changes.1 Every year almost 2 million people develop CRC, which makes it the fourth most common malignancy worldwide. Furthermore, approximately 48% of CRC patients will die from the disease.2 However, it is particularly worrying that despite decreasing incidence and mortality rates in the older population (aged 65+), an increase in these parameters has been observed in the age group 20–49 years since 2010.3 Diagnosis of CRC is based on histological examination. Prognosis for patients with CRC has improved in the last few decades, mainly due to the implementation of bowel cancer screening programs. Despite their distinct advantages, these methods have some significant limitations.4 Colonoscopy is a more invasive procedure in comparison to blood collection and requires additional consent from the patient, as required by medical law. For such consent to be valid, two conditions are necessary. First, the patient must be able to make an informed decision that may be limited by, inter alia, his/her age or mental capacity. This is particularly important in the case of, eg, a colonoscopy combined with the removal of polyps. Secondly, the patient should be provided with the information necessary to make an informed decision. Finding substances whose determination brings valuable information regarding the pathogenesis and course of the disease gives the doctor a valuable tool not only from a medical but also from a legal viewpoint, supporting the process of patient information and thus assisting the patient in reaching the best decision concerning treatment.
At present, there are no suitable biomarkers useful in early detection of CRC and prediction of tumor behavior. In routine clinical practice, carcinoembryonic antigen (CEA) is a first-line biochemical marker used in CRC diagnosis and follow-up of patients. However, its diagnostic sensitivity and specificity remain unsatisfactory. Therefore, other non-invasive biochemical markers for CRC are necessary to improve diagnosis of this malignancy and prognosis for patients. The role of chemokines in CRC pathogenesis has been proven. However, little is known about the clinical utility of these molecules in CRC patients. Thus, the aim of our review is to highlight clinical significance of CXCL1 and CXCL8 as well as their specific receptors as potential biomarkers for CRC. We performed a comprehensive literature search using the PubMed database to identify original articles that investigated the potential application of the presented chemokines and their receptors as biomarkers in CRC diagnosis and prognosis.
It has been reported that selected chemokines and their specific receptors are able to regulate leukocyte migration in inflammation and immunity processes as well as infection, tissue injury and progression of cancers, including CRC.5–7
Chemokines in Cancer Progression
Chemokines are a group of soluble chemotactic cytokines that are produced by tissue cells and leukocytes. This family of molecules has been grouped into four classes according to the position of key cysteine residues (CXC, CX3C, CC and C).5–7 Chemokines are also classified as inflammatory, homeostatic or dual action proteins, based on their function in the immune system and inflammatory response. Homeostatic chemokines are synthesized constitutively and involved in the migration of various cells responsible for function of the immune system.8–10 Inflammatory chemokines are produced by leukocytes and might play a role in cell activation.11,12 The functional activity of chemokines is induced by binding to their specific seven-transmembrane receptors coupled to G-proteins (GPCRs) found on the surface of target cells to cause a cellular response such as migration, adhesion or chemotaxis.13 These receptors are structurally similar to chemokines and are divided into four groups: CXCR, CCR, CX3CR and XCR.14
Chemokines and their receptors play an important role in inflammation – a multi-step process associated with, eg, wound healing, tissue repair as well as defense against various pathogens. However, uncontrolled inflammation is linked to the progression of many malignancies.5–12 The proinflammatory mediator network within the tumor microenvironment facilitates cancer cell migration through the stroma.15–17 On the other hand, a growing body of evidence indicates that malignancy may promote local and systemic inflammatory responses.16 These proteins might also simplify communication between cancer and non-cancerous cells within the tumor microenvironment, promoting the infiltration and activation of neutrophils and tumor-associated macrophages.18
Since inflammation is recognized as a hallmark of cancer, chemokines, which are a key element of this process, have been found to promote tumorigenesis via various pathways including tumor necrosis factor alpha (TNF-α)/nuclear factor κB (NF-κB) and protein kinase B (PKB).19–21 Some clinical investigations have indicated that these pathways are related to tumor growth, proliferation, survival, neoangiogenesis and metastasis of malignant cells.5,22 It has been proven that cancer cells are able to express chemokine receptors and produce chemokines that promote tumor growth.23 Moreover, malignant cells acquire the ability to form new vessels to deliver oxygen and necessary nutrients to tumor cells, which is regulated by various angiogenic factors.24 The migration of neoplastic cells from the site of the primary tumor is also a crucial process in tumor progression.25 Metastatic sites start producing selected chemokines that attract circulating tumor cells to the supporting microenvironment.26,27 Therefore, selected inflammatory mediators such as chemokines may act via autocrine or paracrine mechanisms to facilitate tumor growth and dissemination at every step of metastasis including adherence of tumor cells to endothelium, proliferation, extravasation from blood vessels, angiogenesis as well as protection from the host response.5–12 The involvement of chemokines and their specific receptors in metastatic processes have been observed in various types of malignancies, including CRC.8,28,29
Growing evidence from a number of studies demonstrates that selected chemokines and their specific receptors are involved in each step of CRC development. These associations have been studied mostly in CRC tissue using immunohistochemistry (IHC) or measuring mRNA levels by a real-time polymerase chain reaction (RT-PCR).19 (Table 1). However, little is known about the concentrations of these proteins in the blood of CRC patients evaluated using non-invasive, simpler, less expensive and faster methods such as enzyme-linked immune-sorbent assay (ELISA) or multiplexing techniques.
 |
Table 1 The Significance of C-X-C Chemokine 1 (CXCL1) and C-X-C Chemokine 8 (CXCL8) and Their Specific Receptors in Colorectal Cancer |
The C-X-C family of chemokines (CXCL1 to 17) attracts neutrophils and lymphocytes and is activated by appropriate receptors (CXCR) 1 to 8. C-X-C chemokines introduce the location of the two cysteine residues near the N-terminal, with X representing any amino acid. These proteins can be subdivided according to the presence (ELR+) or absence (ELR-) of the tripeptide motif (Glu-Leu-Arg) at the NH2 terminus. ELR+ chemokines are angiogenic, while ELR- are considered to be angiostatic.19,30–33 Growth-regulated oncogene (GRO) has three different genetic variants: CXCL1 (GRO-α), CXCL2 (GRO-β) and CXCL3 (GRO-γ) which interact with CXCR2.34–42 Furthermore, CXCL8, known as interleukin-8 (IL-8), is able to activate both CXCR2 and CXCR1 and has pro-tumoral capacity via the activation of pro-tumoral neutrophils and stimulation of angiogenesis.40,42 However, only CXCR2 possesses the ability to promote neoangiogenesis, while CXCL1 binds CXCR2 with higher affinity.43 Some clinical investigations have found that these proteins are upregulated in CRC.19,44
Both CXCL1 and CXCL8 belong to the ELR+ chemokine group. For this reason, their ability to bind to the CXCR2 receptor leads to its activation and as a consequence induces various signaling cascades such as phospholipase C (PLC)/protein kinase C (PKC) or phosphatidylinositol-3-kinase (PI3K)/Akt.33 (Figure 1). These pathways result in transcription of several genes such as p38 or AKT, which plays an important role in modulating the proliferation, angiogenesis as well as migration of malignant cells.33,45,46
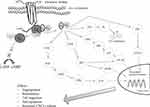 |
Figure 1 The signaling pathways of CXCL1/CXCL8 – CXCR2 axis.33,45,46 |
The CXCR1/CXCR2 axis is under intensive investigation and it appears to play a significant role in CRC development. Therefore, in our review, we focused on the importance of two CXC chemokines – CXCL1 and CXCL8 – which interact within the CXCR1/CXCR2 pathway in CRC progression. We performed a comprehensive literature search using the MEDLINE/PubMed electronic database up to March 2020 with the following search strategy (key words): ‘cancer AND CXCL1ʹ as well as ‘cancer AND CXCL8ʹ. In the next step, the search was limited to human studies conducted within the last 20 years. By adding “AND colorectal” to the key words mentioned above, we limited the search to articles regarding “CXCL1 in colorectal cancer” and “CXCL8 in colorectal cancer”. The next step involved limiting the search to full-text publications written in English and reducing the search to original articles concerning chemokine CXCL1 in colorectal cancer and CXCL8 in colorectal cancer, which were included in the study.
CXCL1 and Its Specific Receptor in Colorectal Cancer
A growing body of evidence indicates that CXCL1 promotes chemoattraction, wound healing and angiogenesis. It is well established that the chemokine enhances tumor growth via recruitment of neutrophils into the tumor microenvironment.47 However, the role of this chemokine in CRC is still poorly understood. Some clinical investigations have suggested that CXCL1 is involved in the pathophysiology of human CRC. Ogata et al found that 79% of CRC cells showed positive immunoreactivity for CXCL1.48 In addition, CXCL1 expression was higher in well and moderately differentiated adenocarcinoma in comparison to poorly differentiated CRC.48 The authors concluded that CXCL1 is closely associated with advanced tumors as well as promotes the invasive potential of colon cancer cells.48 Similar findings were presented by Baier et al who reported significantly higher CXCL1 levels in CRC tissue (2430±789.4 pg/g protein) in comparison to normal mucosa (395.7±133.6 pg/g protein).49 Moreover, the authors indicated that this chemokine contributes to tumor growth,49 which has been confirmed by Bandpalli et al.44 They also revealed that CXCL1 was overexpressed in CRC cells, while stromal cells were completely negative for CXCL1 protein, and this was related to more significant tumor growth.44 In addition, the expression of CXCL1 receptor – CXCR2 was also elevated in human CRC.50,51 Some clinical investigations have revealed that CXCL1 expression significantly correlates with clinicopathological characteristics of CRC. Overexpression of CXCL1 has been found to be significantly correlated with larger tumor size, more advanced tumor stage, greater depth of invasion and presence of lymph node metastasis.48 A study by Zhuo et al also confirmed that CXCL1 expression was higher in CRC tissue in comparison to control and that it was significantly correlated with tumor diameter, T stage, N stage, M stage, lymph vascular invasion and CEA levels.52 The authors concluded that the CXCL1/CXCR2 axis is involved in the pathophysiology of human CRC.48 Some investigators have also speculated that CXCL1 might play a role in CRC invasion and metastasis through degradation of the extracellular matrix via the activation of matrix metalloproteinases and promotion of angiogenesis within the tumor.48 Li et al53 evaluated constitutive expression of CXCL1 and its receptor – CXCR2 in human colon carcinoma cells with different metastatic potentials. Non- and low metastatic cells expressed decreased levels of CXCL1 and CXCR2 in comparison to highly metastatic colon carcinoma cells.53 Therefore, the authors concluded that the constitutive expression of CXCL1 and its receptor CXCR2 is associated with metastatic potential and modulates colon cancer cell proliferation and an invasive phenotype.53
A growing body of evidence demonstrates that primary tumor cells may initiate pre-metastatic niche formation via the secretion of proinflammatory cytokines, chemoattractants and angiogenic factors.47 A study by Wang et al demonstrated that primary CRC cells were able to secrete VEGF-A (vascular endothelial growth factor A) and stimulate tumor-associated macrophages to produce CXCL1 in the primary tumor.47 Elevated CXCL1 levels in premetastatic liver tissue recruited CXCR2-positive myeloid-derived suppressor cells to form a premetastatic niche, promoting liver metastases.47 Some clinical investigations have proven that mutant p53 binds to the promoter region of the CXCL1 gene and exerts its function by inducing CXCL1 expression in CRC cells since mutation of p53 is involved in tumorigenesis.54,55 The authors concluded that CXCL1 may play a crucial role in the presence of p53 mutation and is upregulated in a large number of human CRC cells.54 Therefore, inhibition of CXCL1/CXCR2 signaling on tumor cells may have potential utility to prevent invasion and metastasis in a large number of human CRC.49 Moreover, blockade of the CXCL1/CXCR2 pathway has been shown to decrease the frequency of liver metastasis from colon cancer.48,56 The researchers concluded that CXCL1/CXCR2 targeting could be a potentially useful new strategy in the treatment of CRC patients.48,56
Activation of the chemokine-receptor pathway in CRC may affect the prognosis of patients with this malignancy.48 Ogata et al found that patients with CXCL1-positive CRC showed significantly worse outcomes in comparison to those with CXCL1-negative CRC, which may suggest that CXCL1 is not only a potential mediator of tumor invasion, but also a significant prognostic marker in CRC patients.48 In addition, highly elevated CXCL1 expression significantly correlated with decreased overall survival in stage IV CRC patients and hence the authors confirmed that CXCL1 overexpression is a poor prognostic biomarker in metastatic CRC.57 In addition, Li et al, using the Gene Expression Omnibus database, revealed that CXCL1 gene expression was significantly correlated with disease-free survival in CRC patients. However, they failed to confirm their results using multivariate Cox regression analysis.58
A study by Divella et al indicated that serum CXCL1 levels were significantly higher in metastatic CRC patients when compared to healthy subjects. Elevated concentrations of CXCL1 were associated with the presence of lung metastases. In addition, CRC patients with CXCL1 concentrations higher than 410 pg/mL had a worse prognosis than patients with values of this cytokine below 410 pg/mL. The authors confirmed the role of circulating CXCL1 as a predictive tool in cancer prognosis and diagnosis as well as assessment of tumor sensitivity to anticancer therapy.59
CXCL8 and Its Specific Receptors in Colorectal Cancer
C-X-C motif ligand 8 (CXCL8) activates CXCR1 and CXCR2 to mediate neutrophil accumulation and activation at the site of inflammation and infection. This chemokine may act as a monomer or dimer in differential activities regulated by CXCR1 and CXCR2 receptors. CXCL8 plays an essential role in all aspects of inflammatory response, from recruiting neutrophils to the site of infection to triggering cytotoxic activities such as the release of protease and oxygen radicals to eliminate infection.60,61 Therefore, both forms of CXCL8 could interact with CXCR1 and CXCR2 with different affinities and potencies to mediate different cellular responses.19,60,62,63 CXCR1 is specific for CXCL8, whereas CXCR2 interacts with other CXC chemokines such as CXCL1 and CXCL8.54 Furthermore, CRC cells are able to regulate CXCL8 in an autocrine manner and increase the expression of CXCR1 and CXCR2, which contributes to tumor progression including cancer invasion, dissemination and metastasis. Some clinical investigations have indicated that the CXCR1/2-mediated signaling pathway is associated with inflammation. It has been found that CXCR2 promotes tumorigenesis and directs the recruitment of tumor-promoting leukocytes to tissues during tumor-inducing as well as tumor-driven inflammation.19,65,66 Moreover, antagonists of CXCR2 may have potential therapeutic significance, and treatment with inhibitors of this pathway might be a good strategy in the therapy of CRC patients.19,57,67–69 However, based on an antibody inhibition test, only CXCR1 has been found to be activated in this process.70,71
A number of studies have demonstrated CXCL8 expression on endothelial cells, tumor-associated macrophages and cancer cells, including CRC.72 CXCL8 expression, evaluated using the immunohistochemical method, has been found to be significantly higher in all CRC tissue samples in comparison to inflammatory and non-malignant samples.73,74 CXCL8 overexpression in CRC tissue correlated with more advanced stages of the disease, larger tumor size, greater depth of invasion, presence of distant metastases and tumor progression dynamics, which may indicate the potential usefulness of this protein as a marker in progression of this disease.51,72,75–78 In addition, Li et al79 reported that elevated CXCL8 mRNA and protein expression caused stronger adhesion of CRC cells to the endothelium, which may suggest the role of CXCL8 in adhesion processes of colon cancer cells to endothelial cells. Moreover, CXCL8 and its receptors play a role in the escape of CRC cells from immune surveillance.70
Some clinical investigations have also indicated significantly decreased disease-free survival and overall survival of CRC patients with high CXCL8 expression, suggesting a correlation between elevated CXCL8 expression and the poor survival rate among CRC patients.80 Moreover, absence of CXCL8 expression in epithelial cells might be a factor indicating a good prognosis.79
In our previous experimental study, we evaluate the clinical significance of serum levels of CXCL8 in CRC patients in comparison to classical tumor marker for CRC – CEA.81 We reported that serum CXCL8 concentrations were significantly higher in CRC patients in comparison to healthy controls, similarly to CEA levels, which was also confirmed in our previous studies performed on patients with pancreatic and esophageal cancer.81–83 Moreover, there were statistically significant differences in CXCL8 concentrations between tumor stages, as established by the Kruskal–Wallis test and confirmed by the post hoc Dwass-Steele-Critchlow-Fligner test. Our results are in line with the findings presented by other investigators who revealed that elevated serum CXCL8 levels are significantly correlated with advanced CRC stages.84 Moreover, in our previous study, CXCL8 concentrations were significantly elevated in CRC patients with distant metastases compared to patients without distant metastases. In addition, we assessed the diagnostic characteristics such as the percentage of elevated concentrations (diagnostic sensitivity), predictive values for negative results and area under the ROC curve of the measurement of serum CXCL8 concentrations, which were higher than those of CEA. Our findings indicate greater utility of CXCL8 in comparison to the classical tumor marker, CEA, in the diagnosis of CRC.81 Similar findings were reported by Sgourakis et al who revealed that plasma CXCL8 levels were elevated in CRC patients in comparison to healthy subjects. In addition, concentrations of CXCL8 higher than 8.83 pg/mL had sensitivity of 85%, specificity 80% and the area under ROC curve of 81% in predicting CRC.85 Our results and the findings of other authors suggest that CXCL8 might be a potential biomarker for CRC, useful in the diagnosis of CRC and evaluation of its progression.
Conclusions
Understanding the role of chemokines and their specific receptors in the pathogenesis of CRC is crucial since these molecules could have potential clinical significance in CRC. As presented in this review, the CXCR1/CXCR2 axis is the most studied pathway in cancer and its role in CRC development appears to be of profound significance and should be thoroughly investigated. Therefore, in the present review, we described the clinical utility of two ligands for these receptors – CXCL1 and CXCL8 as potential biomarkers in the diagnosis of CRC and prognosis for patients as well as their role in the pathogenesis of this malignancy. Published results indicate that increased levels of these proteins correlate with an advanced stage and clinicopathological characteristics of CRC such as larger tumor size, greater depth of invasion, presence of lymph node and/or distant metastases as well as prognosis for CRC patients. Additionally, CXCL1/CXCR2 targeting may be a potentially useful new strategy in the treatment of CRC patients. However, due to a small number of studies and contradictory results, more detailed knowledge regarding the specific role of these proteins as biomarkers for CRC is sorely needed for personalized medicine of the future.
Acknowledgments
The presented project was supported by the Medical University of Bialystok, Poland.
Author Contributions
All authors made significant contributions to conception, study design, acquisition of data, and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The sponsors had no role in the design, execution, interpretation or writing of the study. The authors report no conflicts of interest in this work.
References
1. Simon K, Simon K. Colorectal cancer development and advances in screening. Clin Interv Aging. 2016;11:967–976. doi:10.2147/CIA.S109285
2. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Gastroenterol Rev. 2019;4(2):89–103. doi:10.5114/pg.2018.81072
3. American Cancer Society. Colorectal Cancer Facts & Figures. 2017–2019. Atlanta: American Cancer Society; 2017.
4. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383(9927):1490–1502. doi:10.1016/S0140-6736(13)61649-9
5. Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256(2):137–165. doi:10.1016/j.canlet.2007.05.013
6. Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokines grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi:10.1146/annurev.immunol.22.012703.104543
7. Ransohoff RM. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity. 2009;31(5):711–721. doi:10.1016/j.immuni.2009.09.010
8. Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12(2):121–127. doi:10.1016/s1074-7613(00)80165-x
9. Mortier A, Van Damme J, Proost P. Overview of the mechanisms regulating chemokine activity and availability. Immunol Lett. 2012;145(1–2):2–9. doi:10.1016/j.imlet.2012.04.015
10. Anders HJ, Romagnani P, Mantovani A. Pathomechanisms: homeostatic chemokines in health, tissue regeneration, and progressive diseases. Trends Mol Med. 2014;20(3):154–165. doi:10.1016/j.molmed.2013.12.002
11. Shachar I, Karin N. The dual roles of inflammatory cytokines and chemokines in the regulation of autoimmune diseases and their clinical implications. J Leu Biol. 2013;93(1):51–61. doi:10.1189/jlb.0612293
12. Zaja-Milatovic S, Richmond A. CXC chemokines and their receptors: a case for a significant biological role in cutaneous wound healing. Histol Histopathol. 2008;23(11):1399–1407. doi:10.14670/hh-23.1399
13. Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. doi:10.1146/annurev-immunol-032713-120145
14. Murphy PM, Baggiolini M, Charo IF, et al. International Union of Pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52(1):145–176.
15. Vindrieux D, Escobar P, Lazennec G. Emerging roles of chemokines in prostate cancer. Endocr Relat Cancer. 2009;16(3):663–673. doi:10.1677/ERC-09-0109
16. Sethi G, Shanmugam MK, Ramachandran L, et al. Multifaceted link between cancer and inflammation. Biosci Rep. 2012;32(1):1–15. doi:10.1042/BSR20100136
17. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
18. Hartmann TN, Burger M, Burger JA. The role of adhesion molecules and chemokine receptor cxcr4 (cd184) in small cell lung cancer. J Biol Regul Homeost Agents. 2004;18(2):126–130.
19. Cabrero-de Las Heras S, Martínez-Balibrea E. CXC family of chemokines as prognostic or predictive biomarkers and possible drug targets in colorectal cancer. World J Gastroenterol. 2018;24(42):4738–4749. doi:10.3748/wjg.v24.i42.4738
20. Acharyya S, Oskarsson T, Vanharanta S, et al. CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150(1):165–178. doi:10.1016/j.cell.2012.04
21. Ruiz de Porras V, Bystrup S, Martínez-Cardús A, et al. Curcumin mediates oxaliplatin-acquired resistance reversion in colorectal cancer cell lines through modulation of CXC-chemokine/NF-κB signaling pathway. Sci Rep. 2016;6:24675. doi:10.1038/srep24675
22. Speyer CL, Ward PA. Role of endothelial chemokines and their receptors during inflammation. J Invest Surg. 2011;24(1):18–27. doi:10.3109/08941939.2010.521232
23. Chow MT, Luster AD. Chemokines in cancer. Cancer Immunol Res. 2014;2(12):1125–1131. doi:10.1158/2326-6066.CIR-14-0160
24. Viallard C, Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20(4):409–426. doi:10.1007/s10456-017-9562-9
25. Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2(8):563–572. doi:10.1038/nrc865
26. Vicari AP, Caux C. Chemokines in cancer. Cytokine Growth Factor Rev. 2002;13(2):143–154. doi:10.1016/s1359-6101(01)00033-8
27. Keeley EC, Mehrad B, Strieter RM. CXC chemokines in cancer angiogenesis and metastases. Adv Cancer Res. 2010;106:91–111. doi:10.1016/S0065-230X(10)06003-3
28. Darash-Yahana M, Pikarsky E, Abramovitch R, et al. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J. 2004;18(11):1240–1242. doi:10.1096/fj.03-0935fje
29. Murakami T, Kawada K, Iwamoto M, et al. The role of CXCR3 and CXCR4 in colorectal cancer metastasis. Int J Cancer. 2013;132(2):276–287. doi:10.1002/ijc.27670
30. Bizzarri C, Beccari AR, Bertini R, Cavicchia MR, Giorgini S, Allegretti M. ELR+ CXC chemokines and their receptors (CXC chemokine receptor 1 and CXC chemokine receptor 2) as new therapeutic targets. Pharmacol Ther. 2006;112(1):139–149. doi:10.1016/j.pharmthera.2006.04.002
31. Koizumi K, Hojo S, Akashi T, Yasumoto K, Saiki I. Chemokine receptors in cancer metastasis and cancer cell-derived chemokines in host immune response. Cancer Sci. 2007;98(11):1652–1658. doi:10.1111/j.1349-7006.2007.00606.x
32. Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267(2):226–244. doi:10.1016/j.canlet.2008.04.050
33. Cheng Y, Ma XL, Wei YQ, Wei XW. Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases. Biochim Biophys Acta Rev Cancer. 2019;1871(2):
34. Verbeke H, Struyf S, Laureys G, Van Damme J. The expression and role of CXC chemokines in colorectal cancer. Cytokine Growth Factor Rev. 2011;22(5–6):345–358. doi:10.1016/j.cytogfr.2011.09.002
35. Haskill S, Peace A, Morris J, et al. Identification of three related human GRO genes encoding cytokine functions. Proc Natl Acad Sci USA. 1990;87(19):7732–7736. doi:10.1073/pnas.87.19.7732
36. Strieter RM, Polverini PJ, Kunkel SL, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270(45):27348–27357. doi:10.1074/jbc.270.45.27348
37. Ning Y, Manegold PC, Hong YK, et al. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer. 2011;128(9):2038–2049. doi:10.1002/ijc.25562
38. Moser B, Clark-Lewis I, Zwahlen R, Baggiolini M. Neutrophil-activating properties of the melanoma growth-stimulatory activity. J Exp Med. 1990;171(5):1797–17802. doi:10.1084/jem.171.5.1797
39. Walz A, Burgener R, Car B, Baggiolini M, Kunkel SL, Strieter RM. Structure and neutrophil-activating properties of a novel inflammatory peptide (ENA-78) with homology to interleukin 8. J Exp Med. 1991;174(6):1355–1362. doi:10.1084/jem.174.6.1355
40. Wuyts A, Proost P, Lenaerts JP, Baruch A, Van Damme J, Wang JM. Differential usage of the CXC chemokine receptors 1 and 2 by interleukin-8, granulocyte chemotactic protein-2 and epithelial-cell-derived neutrophil attractant-78. Eur J Biochem. 1998;255(1):67–73. doi:10.1046/j.1432-1327.1998.2550067.x
41. Pączek S, Łukaszewicz-Zając M, Mroczko B. Chemokines-what is their role in colorectal cancer? Cancer Control. 2020;27(1):1073274820903384. doi:10.1177/1073274820903384
42. Van Damme J, Wuyts A, Froyen G, et al. Granulocyte chemotactic protein-2 and related CXC chemokines: from gene regulation to receptor usage. J Leukoc Biol. 1997;62(5):563–569. doi:10.1002/jlb.62.5.563
43. Heidemann J, Ogawa H, Dwinell MB, et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278(10):8508–8515. doi:10.1074/jbc.M208231200
44. Bandapalli OR, Ehrmann F, Ehemann V, et al. Downregulation of CXCL1 inhibits tumor growth in colorectal liver metastasis. Cytokine. 2012;57(1):46–53. doi:10.1016/j.cyto.2011.10.019
45. Xia M, Hyman BT. GROalpha/KC, a chemokine receptor CXCR2 ligand, can be a potent trigger for neuronal ERK1/2 and PI-3 kinase pathways and for tau hyperphosphorylation-a role in Alzheimer’s disease? J Neuroimmunol. 2002;122(1–2):55–64. doi:10.1016/s0165-5728(01)00463-5
46. Knall C, Worthen GS, Johnson GL. Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases. Proc Natl Acad Sci U S A. 1997;94(7):3052–3057. doi:10.1073/pnas.94.7.3052
47. Wang D, Sun H, Wei J, Cen B, DuBois RN. CXCL1 is critical for premetastatic niche formation and metastasis in colorectal cancer. Cancer Res. 2017;77(13):3655–3665. doi:10.1158/0008-5472.CAN-16-3199
48. Ogata H, Sekikawa A, Yamagishi H, et al. GRO alpha promotes invasion of colorectal cancer cells. Oncol Rep. 2010;24(6):1479–1486. doi:10.3892/or_00001008
49. Baier PK, Eggstein S, Wolff-Vorbeck G, Baumgartner U, Hopt UT. Chemokines in human colorectal carcinoma. Anticancer Res. 2005;25(5):3581.
50. Rubie C, Frick VO, Wagner M, et al. ELR+ CXC chemokine expression in benign and malignant colorectal conditions. BMC Cancer. 2008;8(1):178. doi:10.1186/1471-2407-8-178
51. Wu W, Sun C, Xu D, et al. Expression of CXCR2 and its clinical significance in human colorectal cancer. Int J Clin Exp Med. 2015;8(4):5883–5889.
52. Zhuo CH, Wu X, Li J, et al. Chemokine (C-X-C motif) ligand 1 is associated with tumor progression and poor prognosis in patients with colorectal cancer. Biosci Rep. 2018;38(4):BSR20180580. doi:10.1042/BSR20180580
53. Li A, Varney ML, Singh RK. Constitutive expression of growth regulated oncogene (gro) in human colon carcinoma cells with different metastatic potential and its role in regulating their metastatic phenotype. Clin Exp Metastasis. 2004;21(7):571–579. doi:10.1007/s10585-004-5458-3
54. Yan W, Chen X. Identification of GRO1 as a critical determinant for mutant p53 gain of function. J Biol Chem. 2009;284(18):12178–12187. doi:10.1074/jbc.M900994200
55. Soussi T. The p53 tumor suppressor gene: from molecular biology to clinical investigation. Ann NY Acad Sci. 2000;910(1):121–139. doi:10.1111/j.1749-6632.2000.tb06705.x
56. Yamamoto M, Kikuchi H, Ohta M, et al. TSU68 prevents liver metastasis of colon cancer xenografts by modulating the premetastatic niche. Cancer Res. 2008;68(23):9754–9762. doi:10.1158/0008-5472.CAN-08-1748
57. le Rolle AM, Chiu TK, Fara M, et al. The prognostic significance of CXCL1 hypersecretion by human colorectal cancer epithelia and myofibroblasts. J Transl Med. 2015;13:199. doi:10.1186/s12967-015-0555-4
58. Li X, Zhong Q, Luo D, Du Q, Liu W, Ahmad A. The prognostic value of CXC subfamily ligands in stage I-III patients with colorectal cancer. PLoS One. 2019;14(4):e0214611. doi:10.1371/journal.pone.0214611
59. Divella R, Daniele A, De Luca R, et al. Circulating levels of VEGF and CXCL1 are predictive of metastatic organotropismin in patients with colorectal cancer. Anticancer Res. 2017;37(9):4867–4871. doi:10.21873/anticanres.11895
60. Nasser MW, Raghuwanshi SK, Grant DJ, Jala VR, Rajarathnam K, Richardson R. Differential activation and regulation of CXCR1 and CXCR2 by CXCL8 monomer and dimer. J Immunol. 2009;183(5):3425–3432. doi:10.4049/jimmunol.0900305
61. Burrows SD, Doyle ML, Murphy KP, et al. Determination of the monomer-dimer equilibrium of interleukin-8 reveals it is a monomer at physiological concentrations. Biochemistry. 1994;33(43):12741–12745. doi:10.1021/bi00209a002
62. Baggiolini M. Reflections on chemokines. Immunol Rev. 2000;177:5–7. doi:10.1034/j.1600-065x.2000.17722.x
63. Liu Q, Li A, Tian Y, et al. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 2016;31:61–71. doi:10.1016/j.cytogfr.2016.08.002
64. Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi:10.1146/annurev.immunol.15.1.675
65. Triner D, Xue X, Schwartz AJ, Jung I, Colacino JA, Shah YM. Epithelial hypoxia-inducible factor 2α facilitates the progression of colon tumors through recruiting neutrophils. Mol Cell Biol. 2017;37(5):e00481–16. doi:10.1128/MCB.00481-16
66. Lee YS, Choi D, Kim NY, et al. CXCR2 inhibition enhances sulindac-mediated suppression of colon cancer development. Int J Cancer. 2014;135(1):232–237. doi:10.1002/ijc.28668
67. Ning Y, Labonte MJ, Zhang W, et al. The CXCR2 antagonist, SCH-527123, shows antitumor activity and sensitizes cells to oxaliplatin in preclinical colon cancer models. Mol Cancer Ther. 2012;11(6):1353–1364. doi:10.1158/1535-7163.MCT-11-0915
68. Kawamura M, Toiyama Y, Tanaka K, et al. CXCL5, a promoter of cell proliferation, migration and invasion, is a novel serum prognostic marker in patients with colorectal cancer. Eur J Cancer. 2012;48(14):2244–2251. doi:10.1016/j.ejca.2011.11.032
69. Lin SC, Hsiao KY, Chang N, Hou PC, Tsai SJ. Loss of dual specificity phosphatase-2 promotes angiogenesis and metastasis via up-regulation of interleukin-8 in colon cancer. J Pathol. 2017;241(5):638–648. doi:10.1002/path.4868
70. Bie Y, Ge W, Yang Z, et al. The crucial role of CXCL8 and its receptors in colorectal liver metastasis. Dis Markers. 2019;8023460. doi:10.1155/2019/8023460.
71. Bates RC, DeLeo III MJ, Mercurio AM. The epithelial-mesenchymal transition of colon carcinoma involves expression of IL-8 and CXCR-1-mediated chemotaxis. Exp Cell Res. 2004;299(2):315–324. doi:10.1016/j.yexcr.2004.05.033
72. Chang WJ, Du Y, Zhao X, Ma LY, Cao GW. Inflammation-related factors predicting prognosis of gastric cancer. World J Gastroenterol. 2014;20(16):4586–4596. doi:10.3748/wjg.v20.i16.4586
73. Oladipo O, Conlon S, O’Grady A, et al. The expression and prognostic impact of CXC-chemokines in stage II and III colorectal cancer epithelial and stromal tissue. Br J Cancer. 2011;104(3):480–487. doi:10.1038/sj.bjc.6606055
74. Rubie C, Frick VO, Pfeil S, et al. Correlation of IL-8 with induction, progression and metastatic potential of colorectal cancer. World J Gastroenterol. 2007;13(37):4996–5002. doi:10.3748/wjg.v13.i37.4996
75. Lee YS, Choi I, Ning Y, et al. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br J Cancer. 2012;106(11):1833–1841. doi:10.1038/bjc.2012.177
76. Ueda T, Shimada E, Urakawa T. Serum levels of cytokines in patients with colorectal cancer: possible involvement of interleukin-6 and interleukin-8 in hematogenous metastasis. J Gastroenterol. 1994;29(4):423–429. doi:10.1007/BF02361238
77. Csiszár A, Szentes T, Haraszti B, Balázs A, Petrányi GG, Pócsik E. The pattern of cytokine gene expression in human colorectal carcinoma. Pathol Oncol Res. 2004;10:109. doi:10.1007/BF02893465
78. Erreni M, Bianchi P, Laghi L, et al. Expression of chemokines and chemokine receptors in human colon cancer. Methods Enzymol. 2009;460:105–121. doi:10.1016/S0076-6879(09)05205-7
79. Li A, Varney ML, Singh RK. Expression of interleukin 8 and its receptors in human colon carcinoma cells with different metastatic potentials. Clin Cancer Res. 2001;7(10):3298–3304.
80. Mohammadi M, Kaghazian M, Rahmani O, et al. Overexpression of interleukins IL-17 and IL-8 with poor prognosis in colorectal cancer induces metastasis. Tumour Biol. 2018;37(6):7501–7505. doi:10.1007/s13277-015-4628-z7
81. Pączek S, Łukaszewicz-Zając M, Gryko M, Mroczko P, Kulczyńska-Przybik A, Mroczko B. CXCL-8 in preoperative colorectal cancer patients: significance for diagnosis and cancer progression. Int J Mol Sci. 2020;21(6):E2040. doi:10.3390/ijms21062040
82. Łukaszewicz-Zając M, Pączek S, Muszyński P, Kozłowski M, Mroczko B. Comparison between clinical significance of serum CXCL8 and classical tumor markers in oesophageal cancer (OC) patients. Clin Exp Med. 2019;19(2):191–199. doi:10.1007/s10238-019-00548-9
83. Litman-Zawadzka A, Łukaszewicz-Zając M, Gryko M, Kulczyńska-Przybik A, Mroczko B. Serum chemokine CXCL8 as a better biomarker for diagnosis and prediction of pancreatic cancer than its specific receptor CXCR2, C-reactive protein, and classic tumor markers CA 19–9 and CEA. Pol Arch Int Med. 2018;128(9):524–531. doi:10.20452/pamw.4307
84. Kaminska J, Nowacki MP, Kowalska M, et al. Clinical significance of serum cytokine measurements in untreated colorectal cancer patients: soluble tumor necrosis factor receptor type I – an independent prognostic factor. Tumor Biol. 2005;26:186–194. doi:10.1159/000086951
85. Sgourakis G, Papapanagiotou A, Kontovounisios C, et al. The combined use of serum neurotensin and IL-8 as screening markers for colorectal cancer. Tumor Biol. 2014;35(6):5993–6002. doi:10.1007/s13277-014-1794-3.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
